Sono-Enzymatically Embedded Antibacterial Silver-Lignin Nanoparticles on Cork Filter Material for Water Disinfection
Abstract
1. Introduction
2. Results
2.1. Characterization of AgLNP
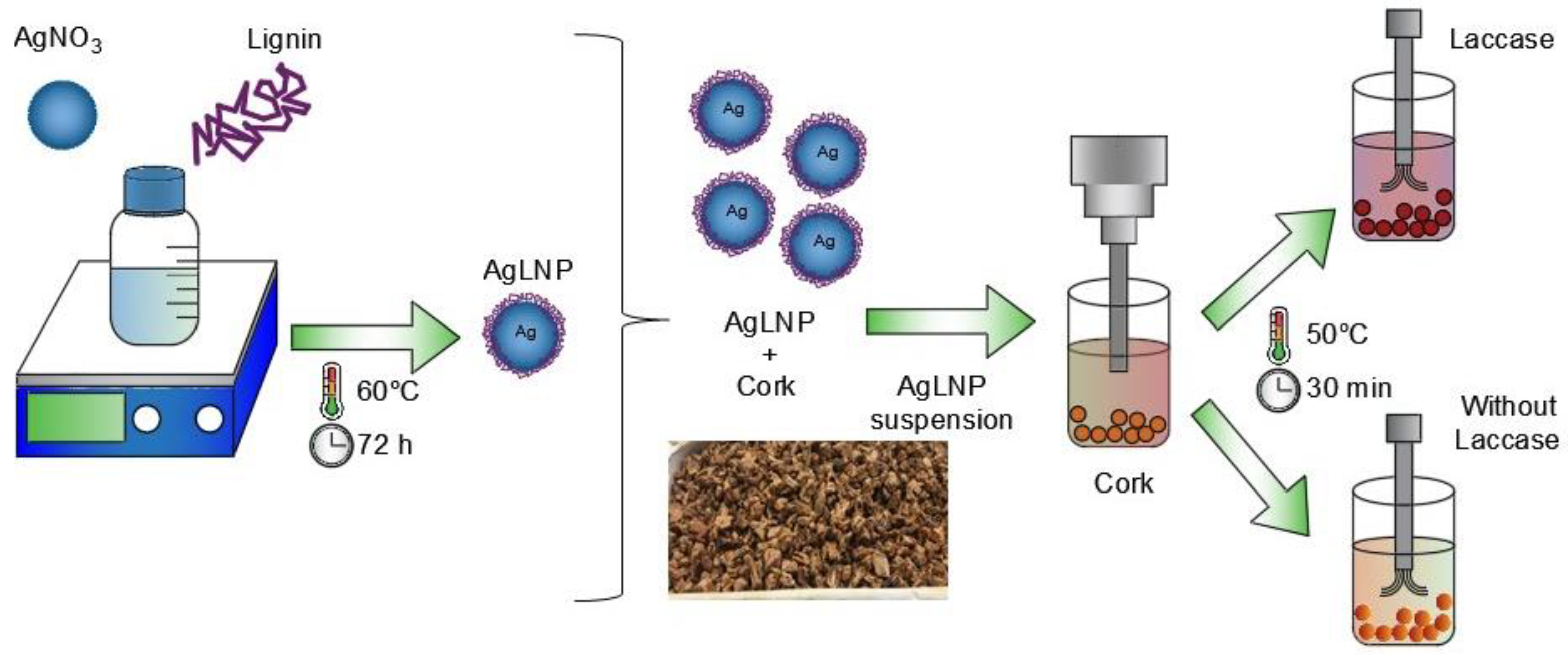
2.2. Sonochemical Coating of Cork with AgLNP
2.3. Characterization of the AgLNP-Coated Cork Nanocomposite Material
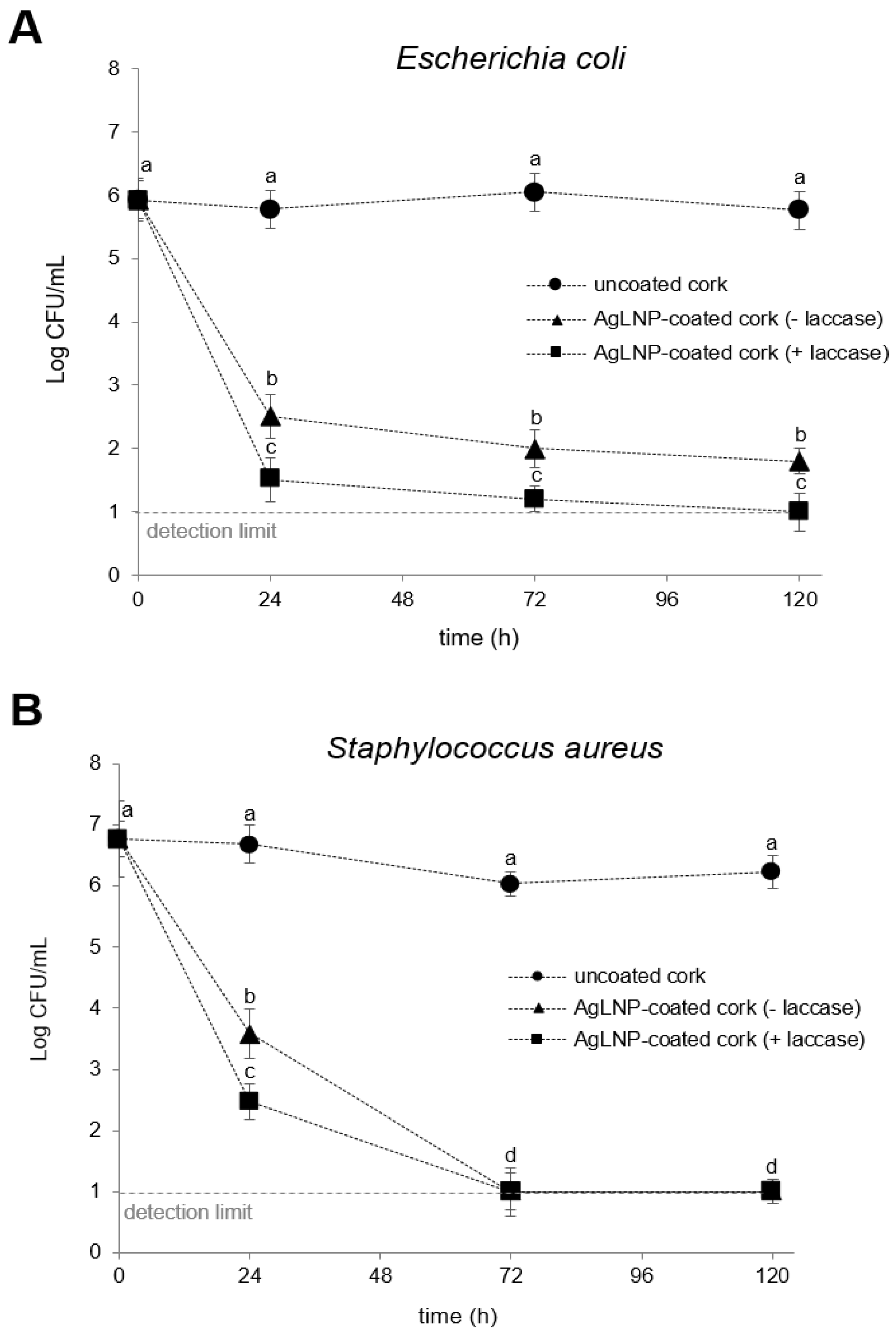
2.4. Determination of Disinfection Efficiency of AgLNP Coated-Cork in a Biofiltration System
3. Materials and Methods
3.1. Materials, Reagents, and Test Bacteria
3.2. Preparation and Characterization of AgLNP
3.3. Sonochemical Coating of Cork with AgLNP
3.4. Physicochemical and Antimicrobial Characterization of the AgLNP−Cork Nanocomposite
3.5. Determination of AgLNP-Cork Nanocomposite Disinfection Efficiency in a Water Filtration System
3.6. Scanning Electron Microscopy
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/detail/12-07-2017-2-1-billion-people-lack-safe-drinking-water-at-home-more-than-twice-as-many-lack-safe-sanitation (accessed on 24 May 2020).
- Cabral, J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef] [PubMed]
- Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 16 August 2022).
- Grunert, A.; Frohnert, A.; Selinka, H.C.; Szewzyk, R. A New Approach to Testing the Efficacy of Drinking Water Disinfectants. Int. J. Hyg. Environ. Health 2018, 221, 1124–1132. [Google Scholar] [CrossRef]
- Fawell, J.; Nieuwenhuijsen, M.J. Contaminants in drinking water: Environmental pollution and health. Br. Med. Bull. 2003, 68, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Castro Medeiros, L.; Alencar, F.L.S.; Navoni, J.A.; Araujo, A.L.C.; Amaral, V.S. Toxicological Aspects of Trihalomethanes: A Systematic Review. Environ. Sci. Pollut. Res. 2019, 26, 5316–5332. [Google Scholar] [CrossRef] [PubMed]
- Styskal, O.; Ishchenko, V.; Petruk, R.; Pohrebennyk, V.; Kochanek, A. Assessment of Chlorinated Water Impact on Phytoplankton. In Proceedings of the 16th International Multidisciplinary Geoconference SGEM 2016, SGEM Vienna GREEN Extended Scientific Sessions, Vienna, Austria, 2–5 November 2016; Volume 3, pp. 373–380. [Google Scholar]
- Cohl, M.; Lazar, L.; Cretescu, I.; Balasanian, I. Trihalomethanes Issues Drinking Water after Chlorination Treatment. Rev. De Chim. 2015, 66, 1282–1287. [Google Scholar]
- Charrois, J.W.A. Analysis of Emerging Disinfection By-Products in Drinking Water. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–16. [Google Scholar] [CrossRef]
- Amjad, H.; Hashmi, I.; Rehman, M.S.U.; Ali Awan, M.; Ghaffar, S.; Khan, Z. Cancer and Non-Cancer Risk Assessment of Trihalomethanes in Urban Drinking Water Supplies of Pakistan. Ecotoxicol. Environ. Saf. 2013, 91, 25–31. [Google Scholar] [CrossRef]
- Aslani, H.; Hosseini, M.S.; Mohammadi, S.; Naghavi-Behzad, M. Drinking Water Disinfection By-Products and Their Carcinogenicity; a Review of an Unseen Crisis. Int. J. Cancer Manag. 2019, 12, 88930. [Google Scholar] [CrossRef]
- Benmarhnia, T.; Delpla, I.; Schwarz, L.; Rodriguez, M.; Levallois, P. Heterogeneity in the Relationship between Disinfection By-Products in Drinking Water and Cancer: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 979. [Google Scholar] [CrossRef]
- Jones, R.R.; Weyer, P.J.; DellaValle, C.T.; Robien, K.; Cantor, K.P.; Krasner, S.; Beane Freeman, L.E.; Ward, M.H. Ingested Nitrate, Disinfection By-Products, and Kidney Cancer Risk in Older Women. Epidemiology 2017, 28, 703–711. [Google Scholar] [CrossRef]
- Cotruvo, J.A.; Amato, H. National Trends of Bladder Cancer and Trihalomethanes in Drinking Water: A Review and Multicountry Ecological Study. Dose-Response 2019, 17, 1559325818807781. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Hwang, C.; Liu, W.T.; Boon, N.; Köster, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. A Microbiology-Based Multi-Parametric Approach towards Assessing Biological Stability in Drinking Water Distribution Networks. Water Res. 2013, 47, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zeng, S.; Gu, A.Z.; He, M.; Shi, H. Inactivation, Reactivation and Regrowth of Indigenous Bacteria in Reclaimed Water after Chlorine Disinfection of a Municipal Wastewater Treatment Plant. J. Environ. Sci. 2013, 25, 1319–1325. [Google Scholar] [CrossRef]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A Study on Prevalence and Characterization of Bacillus Cereus in Ready-to-Eat Foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Rosell, C.M.; Martinez, A. Risk of Bacillus Cereus in Relation to Rice and Derivatives. Foods 2021, 10, 302. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Setlow, B.; Setlow, P. Ultraviolet Irradiation of DNA Complexed with α/β-Type Small, Acid-Soluble Proteins from Spores of Bacillus or Clostridium Species Makes Spore Photoproduct but Not Thymine Dimers. Proc. Natl. Acad. Sci. USA 1991, 88, 8288–8292. [Google Scholar] [CrossRef]
- Riesenman, P.J.; Nicholson, W.L. Role of the Spore Coat Layers in Bacillus Subtilis Spore Resistance to Hydrogen Peroxide, Artificial UV-C, UV-B, and Solar UV Radiation. Appl. Environ. Microbiol. 2000, 66, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.; Somborn-schulz, A. Innovations in Nanotechnology for Water Treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Kwan, S.; Nirmala, A.; Bhatnagar, A. Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Sankar, M.U.; Aigal, S.; Maliyekkal, S.M.; Chaudhary, A.; Kumar, A.A.; Chaudhari, K.; Pradeep, T. Biopolymer-Reinforced Synthetic Granular Nanocomposites for Affordable Point-of-Use Water Puri Fi Cation. Proc. Natl. Acad. Sci. USA 2013, 110, 8459–8464. [Google Scholar] [CrossRef]
- Hanif, Z.; Khan, Z.A.; Siddiqui, M.F.; Tariq, M.Z.; Park, S.; Park, S.J. Tannic Acid-Mediated Rapid Layer-by-Layer Deposited Non-Leaching Silver Nanoparticles Hybridized Cellulose Membranes for Point-of-Use Water Disinfection. Carbohydr. Polym. 2020, 231, 115746. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO Nanoparticles to Selected Environmentally Relevant Test Organisms and Mammalian Cells in Vitro: A Critical Review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Croteau, M.N.; Misra, S.K.; Luoma, S.N.; Valsami-Jones, E. Silver Bioaccumulation Dynamics in a Freshwater Invertebrate after Aqueous and Dietary Exposures to Nanosized and Ionic Ag. Environ. Sci. Technol. 2011, 45, 6600–6607. [Google Scholar] [CrossRef]
- Hoyo, J.; Ivanova, K.; Torrent-Burgues, J.; Tzanov, T. Interaction of Silver-Lignin Nanoparticles with Mammalian Mimetic Membranes. Front. Bioeng. Biotechnol. 2020, 8, 439. [Google Scholar] [CrossRef]
- Francesko, A.; Fossas, M.C.; Petkova, P.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Sonochemical Synthesis and Stabilization of Concentrated Antimicrobial Silver-Chitosan Nanoparticle Dispersions. J. Appl. Polym. Sci. 2017, 134, 45136. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.Y.; Lebihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An Environmentally Benign Antimicrobial Nanoparticle Based on a Silver-Infused Lignin Core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an Antifouling Zwitterionic Coating on Urinary Catheters Using an Enzymatically Triggered Bottom-up Approach. ACS Appl. Mater. Interfaces 2014, 6, 11385–11393. [Google Scholar] [CrossRef]
- Aracri, E.; Díaz Blanco, C.; Tzanov, T. An Enzymatic Approach to Develop a Lignin-Based Adhesive for Wool Floor Coverings. Green Chem. 2014, 16, 2597–2603. [Google Scholar] [CrossRef]
- Hadzhiyska, H.; Calafell, M.; Gibert, J.M.; Dagà, J.M.; Tzanov, T. Laccase-Assisted Dyeing of Cotton. Biotechnol. Lett. 2006, 28, 755–759. [Google Scholar] [CrossRef]
- Rocasalbas, G.; Francesko, A.; Touriño, S.; Fernández-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-Assisted Formation of Bioactive Chitosan/Gelatin Hydrogel Stabilized with Plant Polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Simultaneous Ultrasound-Assisted Hybrid Polyzwitterion/Antimicrobial Peptide Nanoparticles Synthesis and Deposition on Silicone Urinary Catheters for Prevention of Biofilm-Associated Infections. Nanomaterials 2021, 11, 3143. [Google Scholar] [CrossRef] [PubMed]
- Salat, M.; Petkova, P.; Hoyo, J.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Durable Antimicrobial Cotton Textiles Coated Sonochemically with ZnO Nanoparticles Embedded in an In-Situ Enzymatically Generated Bioadhesive. Carbohydr. Polym. 2018, 189, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Ivanova, K.; Perelshtein, I.; Gedanken, A.; Todorova, K.; Milcheva, R.; Dimitrov, P.; Popova, T.; Tzanov, T. Sonochemically Engineered Nano-Enabled Zinc Oxide/Amylase Coatings Prevent the Occurrence of Catheter-Associated Urinary Tract Infections. Mater. Sci. Eng. C 2021, 131, 112518. [Google Scholar] [CrossRef]
- Vanesa, L.; Pen, G.; Petkova, P.; Margalef-marti, R.; Vives, M.; Aguilar, L.; Gallegos, A.; Francesko, A.; Perelshtein, I.; Gedanken, A.; et al. Hybrid Chitosan − Silver Nanoparticles Enzymatically Embedded on Cork Filter Material for Water Disinfection. Ind. Eng. Chem. Res. 2017, 56, 3599–3606. [Google Scholar] [CrossRef]
- Zeng, F.; Cao, S.; Jin, W.; Zhou, X.; Ding, W.; Tu, R.; Han, S.F.; Wang, C.; Jiang, Q.; Huang, H.; et al. Inactivation of Chlorine-Resistant Bacterial Spores in Drinking Water Using UV Irradiation, UV/Hydrogen Peroxide and UV/Peroxymonosulfate: Efficiency and Mechanism. J. Clean. Prod. 2020, 243, 118666. [Google Scholar] [CrossRef]
- Ding, W.; Jin, W.; Cao, S.; Zhou, X.; Wang, C.; Jiang, Q.; Huang, H.; Tu, R.; Han, S.F.; Wang, Q. Ozone Disinfection of Chlorine-Resistant Bacteria in Drinking Water. Water Res. 2019, 160, 339–349. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y. Lo Silver Nanoparticle Synthesis Using Lignin as Reducing and Capping Agents: A Kinetic and Mechanistic Study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef]
- Francesko, A.; Blando, L.; Va, M.; Petkova, P.; Morato, J.; Pfeifer, A.; Heinze, T.; Mendoza, E.; Tzanov, T. Enzymatic Functionalization of Cork Surface with Antimicrobial Hybrid Biopolymer/Silver Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9792–9799. [Google Scholar] [CrossRef]
- Infante, N.; Rodríguez, R.; Bartolo, Y.; Sánchez, O.; Sanz, I.; Bermeo, L.; Morató, J. Biofunctionalization of Cork with Moringa Oleifera Seeds and Use of PMA Staining and qPCR to Detect Viability of Escherichia coli. Water 2021, 13, 2731. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Shi, J. Activation of Cellulosic Ethanol Lignin by Laccase and Its Application as Plywood Adhesive. BioResources 2018, 13, 3005–3016. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Abdullah, S. Bacillus Cereus Food Poisoning: International and Indian Perspective. J. Food Sci. Technol. 2014, 52, 2500–2511. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermeo, L.; Ivanova, K.; Pérez, L.M.; Forés, E.; Pérez-Rafael, S.; Casas-Zapata, J.C.; Morató, J.; Tzanov, T. Sono-Enzymatically Embedded Antibacterial Silver-Lignin Nanoparticles on Cork Filter Material for Water Disinfection. Int. J. Mol. Sci. 2022, 23, 11679. https://doi.org/10.3390/ijms231911679
Bermeo L, Ivanova K, Pérez LM, Forés E, Pérez-Rafael S, Casas-Zapata JC, Morató J, Tzanov T. Sono-Enzymatically Embedded Antibacterial Silver-Lignin Nanoparticles on Cork Filter Material for Water Disinfection. International Journal of Molecular Sciences. 2022; 23(19):11679. https://doi.org/10.3390/ijms231911679
Chicago/Turabian StyleBermeo, Lizeth, Kristina Ivanova, Leonardo Martín Pérez, Eva Forés, Sílvia Pérez-Rafael, Juan C. Casas-Zapata, Jordi Morató, and Tzanko Tzanov. 2022. "Sono-Enzymatically Embedded Antibacterial Silver-Lignin Nanoparticles on Cork Filter Material for Water Disinfection" International Journal of Molecular Sciences 23, no. 19: 11679. https://doi.org/10.3390/ijms231911679
APA StyleBermeo, L., Ivanova, K., Pérez, L. M., Forés, E., Pérez-Rafael, S., Casas-Zapata, J. C., Morató, J., & Tzanov, T. (2022). Sono-Enzymatically Embedded Antibacterial Silver-Lignin Nanoparticles on Cork Filter Material for Water Disinfection. International Journal of Molecular Sciences, 23(19), 11679. https://doi.org/10.3390/ijms231911679







