WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate
Abstract
1. Introduction
2. Results
2.1. Effect of WNT1 Inducible Signaling Pathway Protein 1 (WISP1) on the Proliferation of Human Prostate Fibroblast HPrF Cells
2.2. WISP1 Enhances the Migration of Prostate Fibroblast HPrF Cells
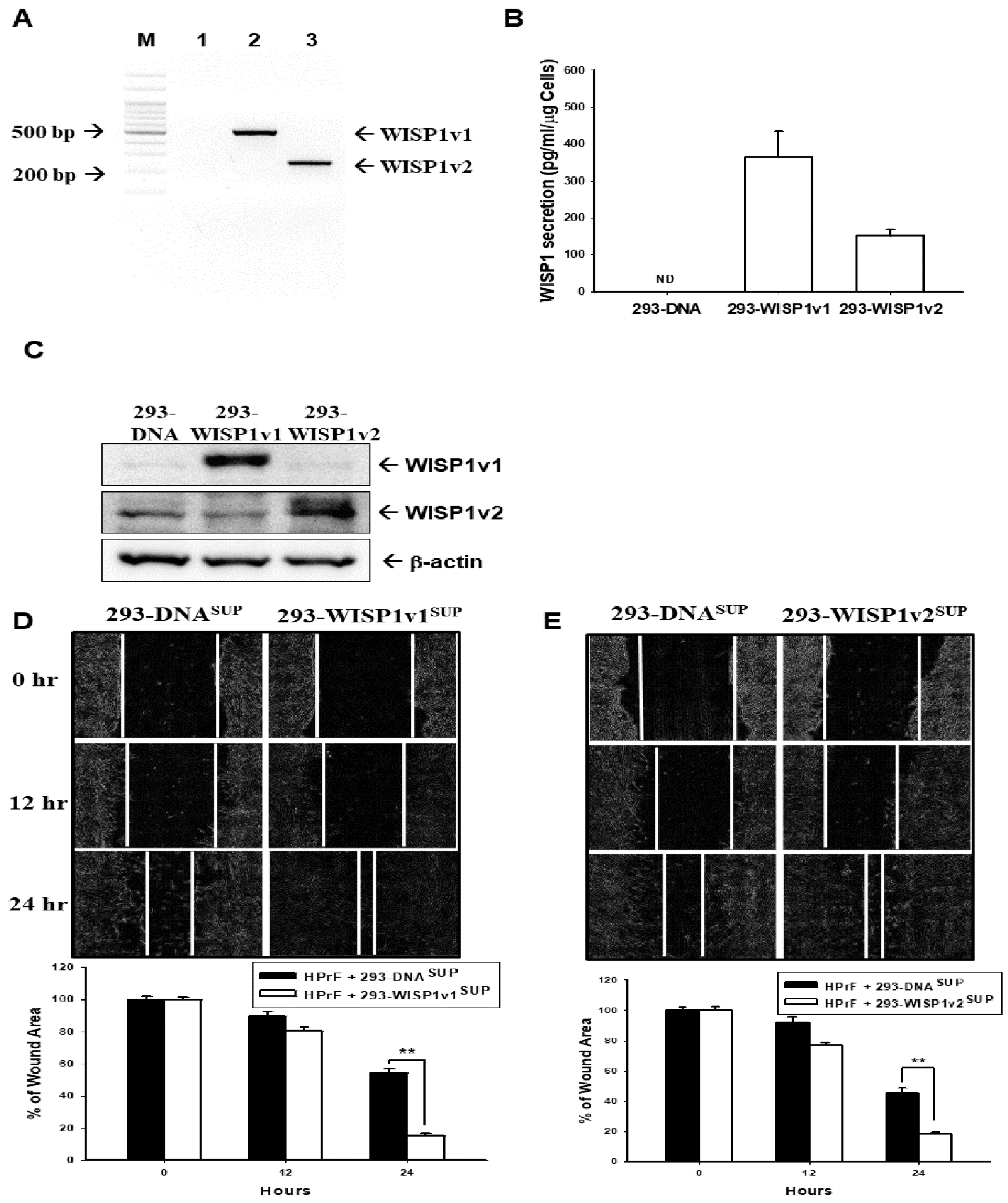
2.3. TNFα Induces the WISP1 Secretion to Enhance the Contraction of Human Prostate Fibroblast HPrF Cells
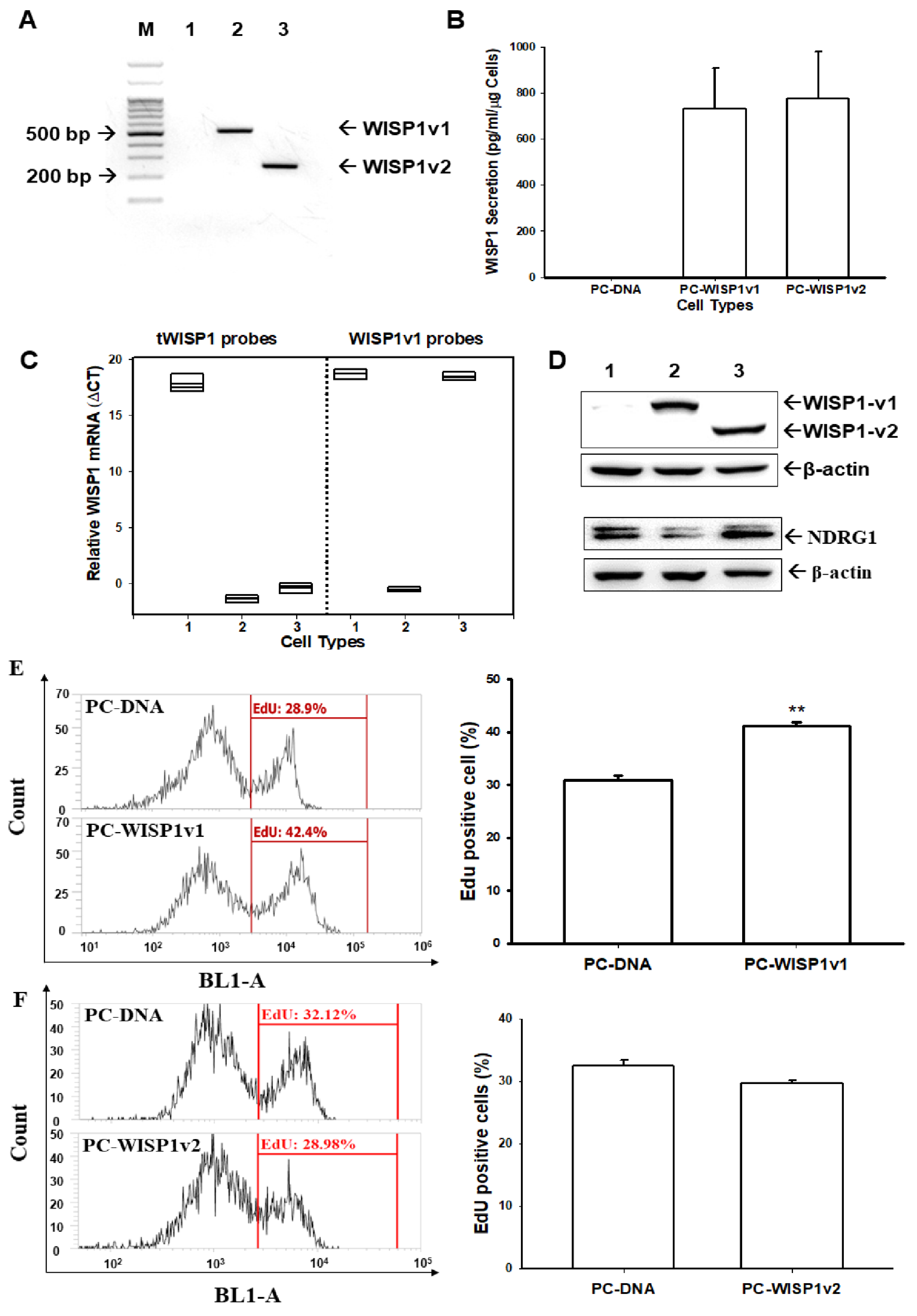
2.4. Ectopic Overexpression of WISP1v1 but Not WISP1v2 Enhances Cell Proliferation
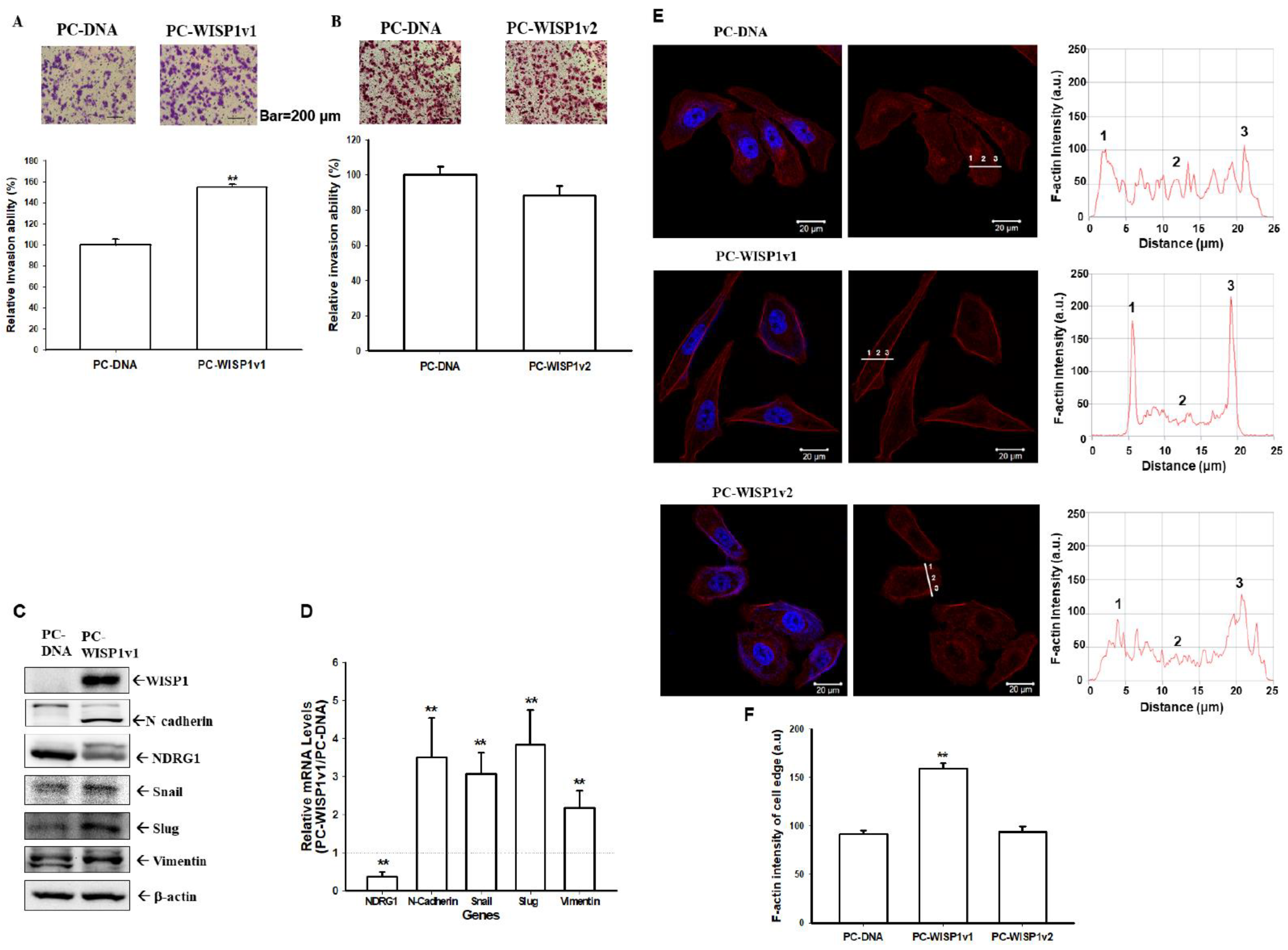
2.5. Ectopic Overexpression of WISP1v1 but Not WISP1v2 Enhances the Cell Migration via the Induction of the Epithelial-Mesenchymal Transition
2.6. Ectopic Overexpression of WISP1v1 Enhances Tumor Growth in a Xenograft Animal Model
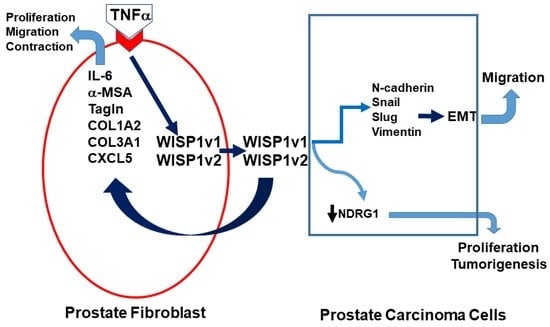
3. Discussion
4. Materials and Methods
4.1. Materials, Cell Lines, and Cell Cultures
4.2. Gene Knock-down
4.3. Expression Vector Constructs and the Stable Transfection
4.4. Cell Proliferation Assay
4.5. Cell Contraction Assay
4.6. Cell Migration
4.7. Immunoblot Assays
4.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and the Quantitative RT-qPCR
4.9. Immunofluorescence F-Actin Staining
4.10. Enzyme Linked Immunosorbent Assay
4.11. Xenograft Animal Model
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiese, K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr. Neurovasc. Res. 2014, 11, 378–389. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, B.; Zhang, Y.; Ali, A.; Liao, X. CCN family proteins in cancer: Insight into their structures and coordination role in tumor microenvironment. Front. Genet. 2021, 12, 649387. [Google Scholar] [CrossRef]
- Lau, L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal. 2016, 10, 121–127. [Google Scholar] [CrossRef]
- Murahovschi, V.; Pivovarova, O.; Ilkavets, I.; Dmitrieva, R.M.; Döcke, S.; Keyhani-Nejad, F.; Gögebakan, Ö.; Oterhoff, M.; Kemper, M.; Hornemann, S. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes 2015, 64, 856–866. [Google Scholar] [CrossRef]
- Maiese, K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neur. Regener. Res. 2015, 10, 518–528. [Google Scholar] [CrossRef]
- Maiese, K. Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J. Translat. Sci. 2016, 1, 83–85. [Google Scholar] [CrossRef]
- Lu, S.; Liu, H.; Lu, L.; Wan, H.; Lin, Z.; Qian, K.; Yao, X.; Chen, Q.; Liu, W.; Yan, J.; et al. WISP1 overexpression promotes proliferation and migration of human vascular smooth muscle cells via AKT signaling pathway. Euro. J. Pharmacol. 2016, 788, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, P.O.; Clairefond, S.; Class, C.A.; Boulay, P.L.; Chrobak, P.; Allard, B.; Azzi, F.; Pommey, S.; DoK, A.; Saad, F.; et al. WISP1 is associated to advance disease, EMT and an inflamed tumor microenvironment in multiple solid tumors. OncoImmunology 2019, 8, e1581545. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sugimachi, K.; Saeki, H.; Kinoshita, J.; Ohga, T.; Shimada, M.; Maehara, Y. A novel variant of WISP1 lacking a Von Wille brand type C module overexpressed in scirrhous gastric carcinoma. Oncogene 2001, 20, 5525–5532. [Google Scholar] [CrossRef][Green Version]
- Pennica, D.; Swanson, T.A.; Welsh, J.W.; Roy, M.A.; Lawrence, D.A.; Lee, J.; Brush, J.; Taneyhill, L.A.; Deuel, B.; Lew, M.; et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. USA 1998, 95, 14717–14722. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sugimachi, K.; Kameyama, T.; Maehara, S.; Shirabe, K.; Shimada, M.; Wands, J.R.; Maehara, Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology 2003, 37, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Cervello, M.; Giannitrapani, L.; Labbozzetta, M.; Notarbartolo, M.; D’Alessandro, N.; Lampiasi, N.; Azzolina, A.; Montalto, G. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann. N. Y. Acad. Sci. 2004, 1028, 432–439. [Google Scholar] [CrossRef]
- Yanagita, T.; Kubota, S.; Kawaki, H.; Kawata, K.; Kondo, S.; Takano-Yamamoto, T.; Tanaka, S.; Takigawa, M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007, 274, 1655–1665. [Google Scholar] [CrossRef]
- Tian, C.; Zhou, Z.G.; Meng, W.J.; Sun, X.F.; Yu, Y.Y.; Li, L.; Luo, H.Z.; Yang, L.; Zhou, B.; Gu, J. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J. Gastroenterol. 2007, 13, 3878–3882. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.K.; Kim, S.A.; Yoon, T.M.; Lee, K.H.; Kim, H.K.; Lee, D.H.; Lee, J.K.; Chung, I.J.; Joo, Y.E.; Lim, S.C. WNT1-inducible signaling pathway protein-1 contributes to tumor progression and treatment failure in oral squamous cell carcinoma. Oncol. Lett. 2017, 14, 1719–1724. [Google Scholar] [CrossRef][Green Version]
- Polovic, M.; Dittmar, S.; Hennemeier, I.; Humpf, H.U.; Seliger, B.; Fornara, P.; Theil, G.; Azinovic, P.; Nolze, A.; Köhn, M.; et al. Identification of a novel lncRNA induced by the nephrotoxin ochratoxin A and expressed in human renal tumor tissue. Cell. Mol. Life Sci. 2018, 75, 2241–2256. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, I.; Chiquet-Ehrismann, R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): A focus on its role in cancer. Int. J. Biochem. Cell Biol. 2015, 62, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Huang, P.; Lin, L.; Ye, H.; Lin, D.; Koeffler, H.P.; Wang, J.; Yin, D. Expression of CCN family members correlates with the clinical features of hepatocellular carcinoma. Oncol. Rep. 2015, 33, 1481–1492. [Google Scholar] [CrossRef]
- Clausen, M.J.A.M.; Melchers, L.J.; Mastik, M.F.; Slagter-Menkema, L.; Groen, H.J.M.; van der Laan, B.F.A.M.; van Criekinge, W.; de Meyer, T.; Denil, S.; Wisman, G.B.A.; et al. Identification and validation of WISP1 as an epigenetic regulator of metastasis in oral squamous cell carcinoma. Genes Chromo. Cancer 2016, 55, 45–59. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, C.N.; Chung, L.C.H.; Feng, T.H.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; Yeh, T.S.; Chen, S.C.; Juang, H.H. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci. Rep. 2015, 5, 8686. [Google Scholar] [CrossRef]
- Wu, J.L.; Cai, H.; Du, C.; Liu, X.; Yu, S.; Wang, Y. High expression of WISP1 in colon cancer is associated with apoptosis, invasion and poor prognosis. Oncotarget 2016, 7, 49834–49847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jing, D.; Zhang, Q.; Yu, H.; Zhao, Y.; Shen, L. Identification of WISP1 as a novel oncogene in glioblastoma. Int. J. Oncol. 2017, 51, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Ji, C.B.; Li, S.B.; Yan, F.; Gu, Q.S.; Balic, J.J.; Yu, L.; Li, J.K. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. Int. Immunopharmacol. 2018, 59, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, X.; Wei, L.; Li, T.; Zang, Y.; Qian, Y.; Bai, T.; Li, J.; Xie, M.; Zhu, Y.; et al. Tp53 Mutation inhibits ubiquitination and degradation of WISP1 via down-regulation of Siah1 in pancreatic carcinogenesis. Front. Pharmacol. 2018, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Hennemeier, I.; Humpf, H.U.; Gekle, M.; Schwerdt, G. The food contaminant and nephrotoxin ochratoxin A enhances Wnt1 inducible signaling protein 1 and tumor necrosis factor-α expression in human primary proximal tubule cells. Mol. Nutr. Food Res. 2012, 56, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Chiou, H.L.; Wang, S.S.; Hung, S.C.; Chou, M.C.; Yang, S.F.; Hsieh, M.J.; Chou, Y.E. WISP1 genetic variants as predictors of tumor development with urothelial cell carcinoma. Urol. Oncol. 2018, 36, 160.e15–160.e21. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Inkson, C.A.; Sonn, R.; Kilts, T.M.; de Castro, L.F.; Maeda, A.; Fisher, L.W.; Robey, P.G.; Berendsen, A.D.; Li, L.; et al. WISP1/CCN4: A potential target for inhibiting prostate cancer growth and spread to bone. PLoS ONE 2013, 8, e71709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Z.; Hou, X.; Sun, Y. LncRNA AFAP1-AS1 promotes the progression of colorectal cancer through miR-195-5p and WISP1. J. Oncol. 2021, 2021, 6242798. [Google Scholar] [CrossRef] [PubMed]
- Sluka, P.; Davis, I.D. Cell mates: Paracrine and stromal targets for prostate cancer therapy. Nat. Rev. Urol. 2013, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Königshoff, M.; Kramer, M.; Balsara, N.; Wilhelm, J.; Amarie, O.V.; Jahn, A.; Rose, F.; Fink, L.; Seeger, W.; Schaefer, L.; et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Investig. 2009, 119, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Masaki, A.; Maeda, A.; Kilts, T.M.; Hara, E.S.; Komori, T.; Pham, H.; Kuboki, T.; Young, M.F. WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol. 2018, 68–69, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Lin, D.; Takhar, M.; Rammarine, V.R.; Dong, X.; Bell, R.H.; Volik, S.V.; Wang, K.; Xue, H.; Wang, Y.; et al. Stromal gene expression is predictive for metastatic primary prostate cancer. Eur. Urol. 2018, 73, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Klee, S.; Lehmann, M.; Wagner, D.E.; Baarsma, H.A.; Königshoff, M. WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts. Sci. Rep. 2016, 6, 20547. [Google Scholar] [CrossRef] [PubMed]
- Elasfadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, 2321. [Google Scholar] [CrossRef]
- Hu, B.; Wu, Z.; Pham, S.H. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am. J. Respir. Cell Mol. Biol. 2003, 29, 397–404. [Google Scholar] [CrossRef]
- Gharaee-Kermani, M.; Kasina, S.; Moore, B.B.; Thomas, D.; Mehra, R.; Macoska, J.A. CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis. PLoS ONE 2012, 7, e49278. [Google Scholar] [CrossRef]
- Pivovarova, O.; Loske, J.; Homemann, S.; Markova, M.; Seebeck, N.; Rosenthal, A.; Klauschen, F.; Castro, J.P.; Buschow, R.; Grune, T.; et al. Hepatic Wnt1 inducible signaling pathway protein 1(WISP-1/CCN4) associates with markers of liver fibrosis in severe obesity. Cells 2021, 10, 1048. [Google Scholar] [CrossRef]
- Berschneider, B.; Königshoff, M. WNT1 inducible signaling pathway protein 1 (WISP1): A novel mediator linking development and disease. Int. J. Biochem. Cell Biol. 2011, 43, 306–309. [Google Scholar] [CrossRef]
- Yang, M.; Du, Y.; Xu, Z.; Jiang, Y. Functional Effects of WNT1-inducible signaling pathway protein-1 on bronchial smooth muscle cell migration and proliferation in OVA-induced airway remodeling. Inflammation 2016, 39, 16–29. [Google Scholar] [CrossRef]
- Li, L.; Lv, S.; Li, X.; Liu, J. Wnt-induced secreted proteins-1 play an important role in paraquat-induced pulmonary fibrosis. BMC Pharmacol. Toxicol. 2022, 23, 21. [Google Scholar] [CrossRef]
- Berschneider, B.; Ellwanger, D.C.; Baarsma, H.A.; Thiel CShimbori, C.; White, E.S.; Kolb, M.; Neth, P.; Königshoff, M. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2014, 53, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, X.; Liu, Y.; Tian, Y.; Ran, X.; Jiang, Y. A role for WNT1-inducible signaling protein-1 in airway remodeling in a rat asthma model. Int. Immunopharmacol. 2013, 17, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Li, X.; Yu, Y.; Liang, K.; Shan, X.; Zhao, K.; Niu, Q.; Tian, Z. WISP1 is increased in intestinal mucosa and contributes to inflammatory cascades in inflammatory bowel disease. Dis. Markers 2016, 2016, 3547096. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.D.; Watt, M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Levesque, C.; Nelson, P.C. Cellular constituents of the prostate stroma: Key contributors to prostate cancer progression and therapy resistance. Cold Spring Harb. Perspect. Med. 2018, 8, a030510. [Google Scholar] [CrossRef] [PubMed]
- Tyekucheva, S.; Bowden, M.; Bango, C.; Giunchi, F.; Huang, Y.; Zhou, C.; Bondi, A.; Lis, R.; Van Hemelrijck, M.; Andren, O.; et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat. Commun. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.C.; Chang, A.C.; Yu, H.J.; Huang, C.Y.; Tsai, Y.C.; Lai, Y.W.; Sun, H.L.; Tang, C.H.; Wang, S.-W. Osteoblast-derived WISP-1 increase VCAM-1 expression and enhances prostate cancer metastasis by down-regulating miR-126. Oncotarget 2014, 5, 7589–7598. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Hsu, S.Y.; Lin, S.J.; Yeh, C.N.; Pang, J.H.S.; Wang, S.Y.; Hsu, J.T.; Yeh, T.S.; Chen, L.W.; Kuo, S.F.; et al. PTEN insufficiency increases breast cancer cell metastasis in vitro and in vivo in a xenograft zebrafish model. Anticancer Res. 2016, 36, 3997–4005. [Google Scholar]
- Chang, A.C.; Lien, M.Y.; Tsai, M.H.; Hua, C.H.; Tang, C.H. WISP1 promotes epithelial-mesenchymal transition in oral squamous cell carcinoma cells via the muir-153/Snail axis. Cancers 2019, 11, 1903. [Google Scholar] [CrossRef]
- Deng, W.; Fernandez, A.; McLaughlin, S.L.; Klinke, D.J., II. WNT1-inducible signaling pathway protein 1 (WISP1) stimulates melanoma cell invasion and metastasis by promoting epithelial-mesenchymal transition. J. Biol. Chem. 2019, 294, 5261–5280. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Chang, K.S.; Sung, H.C.; Hsu, S.Y.; Lin, Y.H.; Hou, C.P.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. Mucosa-associated lymphoid tissue 1 is an oncogene inducing cell proliferation, invasion, and tumor growth via the upregulation of NF-κB activity in human prostate carcinoma cells. Biomedicines 2021, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.P.; Tsui, K.H.; Chang, K.S.; Sung, H.C.; Hsu, S.Y.; Lin, Y.H.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. Caffeic acid phenethyl ester inhibits the growth of bladder carcinoma cells by upregulating growth differentiation factor 15. Biomed. J. 2022, in press. [CrossRef]
- Chang, K.S.; Tsui, K.H.; Lin, Y.H.; Hou, C.P.; Feng, T.H.; Juang, H.H. Migration and invasion enhancer 1 is a NF-ĸB-inducing gene enhancing cell proliferation and invasion ability of human prostate carcinoma cells in vitro and in vivo. Cancers 2019, 11, 1486. [Google Scholar] [CrossRef]
- Chang, K.S.; Tsui, K.H.; Hsu, S.Y.; Sung, H.C.; Lin, Y.H.; Hou, C.P.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. The antitumor effect of caffeic acid phenethyl ester by downregulating mucosa-associated lymphoid tissue 1 via AR/p53/NF-κB signaling in prostate carcinoma cells. Cancers 2022, 14, 274. [Google Scholar] [CrossRef]
- Chiang, K.C.; Chang, K.S.; Hsu, S.Y.; Sung, H.C.; Feng, T.H.; Chao, M.; Juang, H.H. Human heme oxygenase-1 induced by interleukin-6 via JAK/STAT3 pthways is a tumor suppressor gene in hepatoma cells. Antioxidants 2020, 9, 251. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-S.; Chen, S.-T.; Sung, H.-C.; Hsu, S.-Y.; Lin, W.-Y.; Hou, C.-P.; Lin, Y.-H.; Feng, T.-H.; Tsui, K.-H.; Juang, H.-H. WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate. Int. J. Mol. Sci. 2022, 23, 11437. https://doi.org/10.3390/ijms231911437
Chang K-S, Chen S-T, Sung H-C, Hsu S-Y, Lin W-Y, Hou C-P, Lin Y-H, Feng T-H, Tsui K-H, Juang H-H. WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate. International Journal of Molecular Sciences. 2022; 23(19):11437. https://doi.org/10.3390/ijms231911437
Chicago/Turabian StyleChang, Kang-Shuo, Syue-Ting Chen, Hsin-Ching Sung, Shu-Yuan Hsu, Wei-Yin Lin, Chen-Pang Hou, Yu-Hsiang Lin, Tsui-Hsia Feng, Ke-Hung Tsui, and Horng-Heng Juang. 2022. "WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate" International Journal of Molecular Sciences 23, no. 19: 11437. https://doi.org/10.3390/ijms231911437
APA StyleChang, K.-S., Chen, S.-T., Sung, H.-C., Hsu, S.-Y., Lin, W.-Y., Hou, C.-P., Lin, Y.-H., Feng, T.-H., Tsui, K.-H., & Juang, H.-H. (2022). WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate. International Journal of Molecular Sciences, 23(19), 11437. https://doi.org/10.3390/ijms231911437