Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging
Abstract
1. Introduction
2. Results
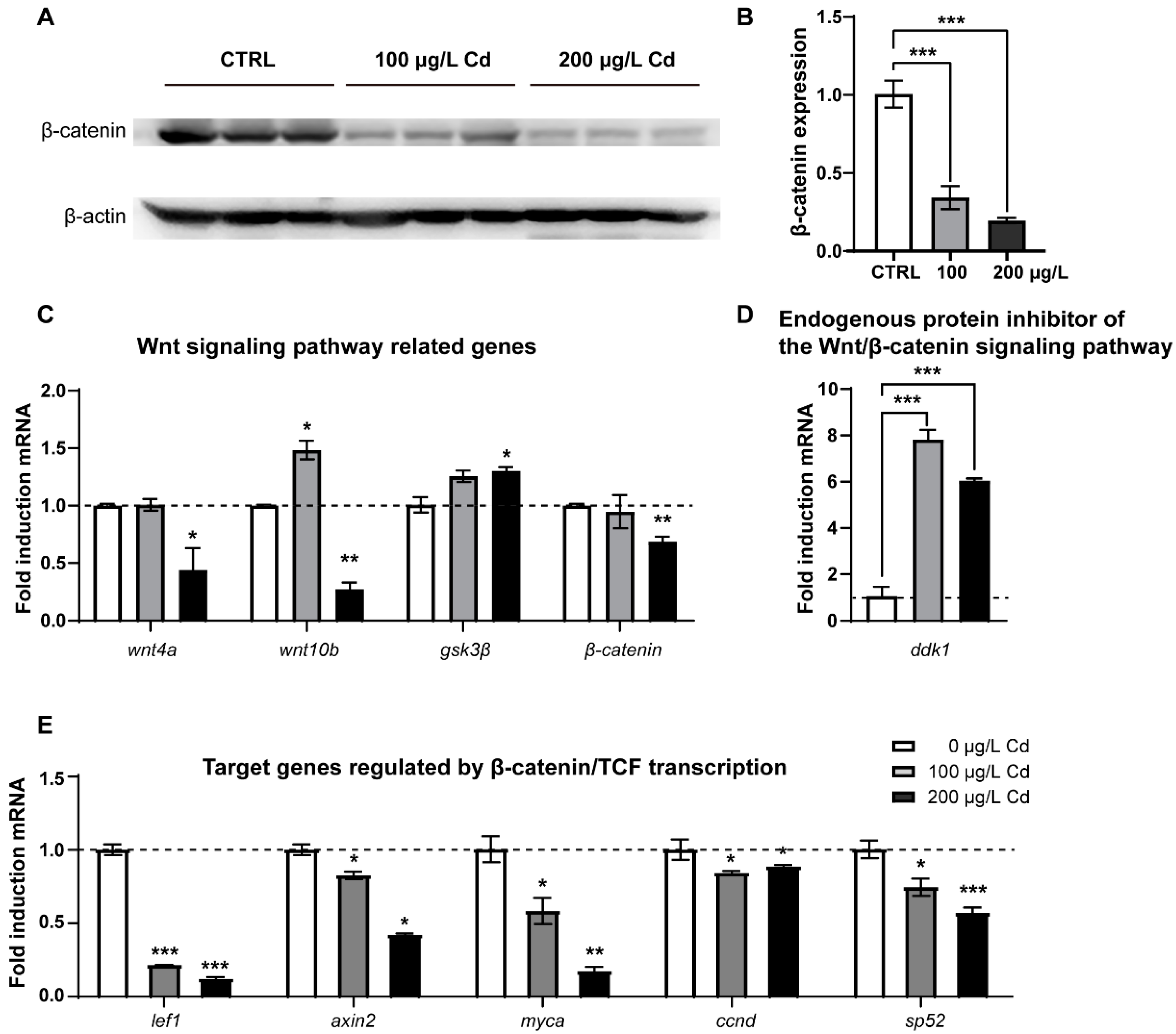
2.1. Wnt/β-Catenin Signaling Is Declined upon Cd2+ Exposure in Zebrafish Larvae
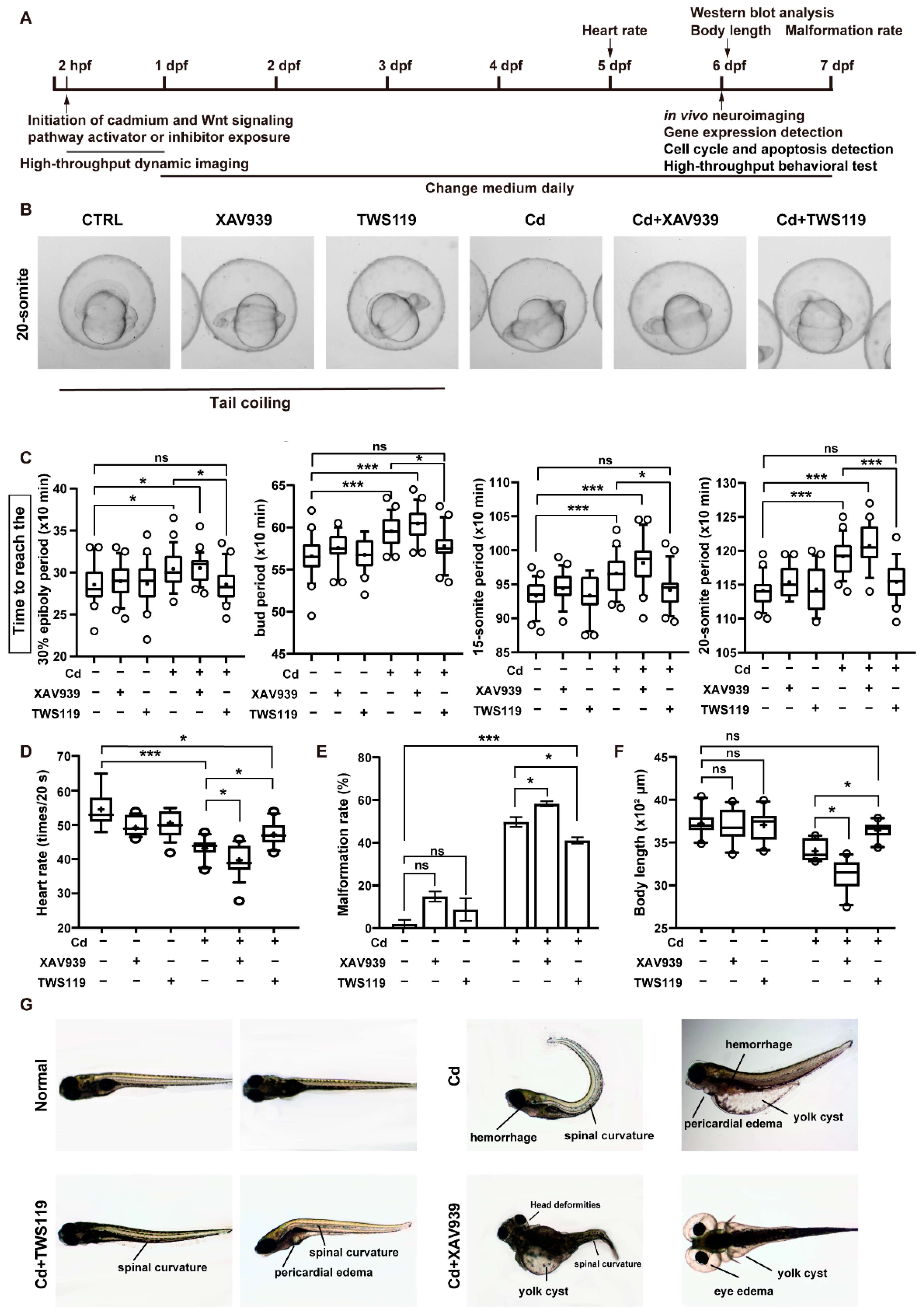
2.2. Activation of Wnt Signaling Pathway can Attenuate the Damaging Effects of Cd2+ on Zebrafish Early Development
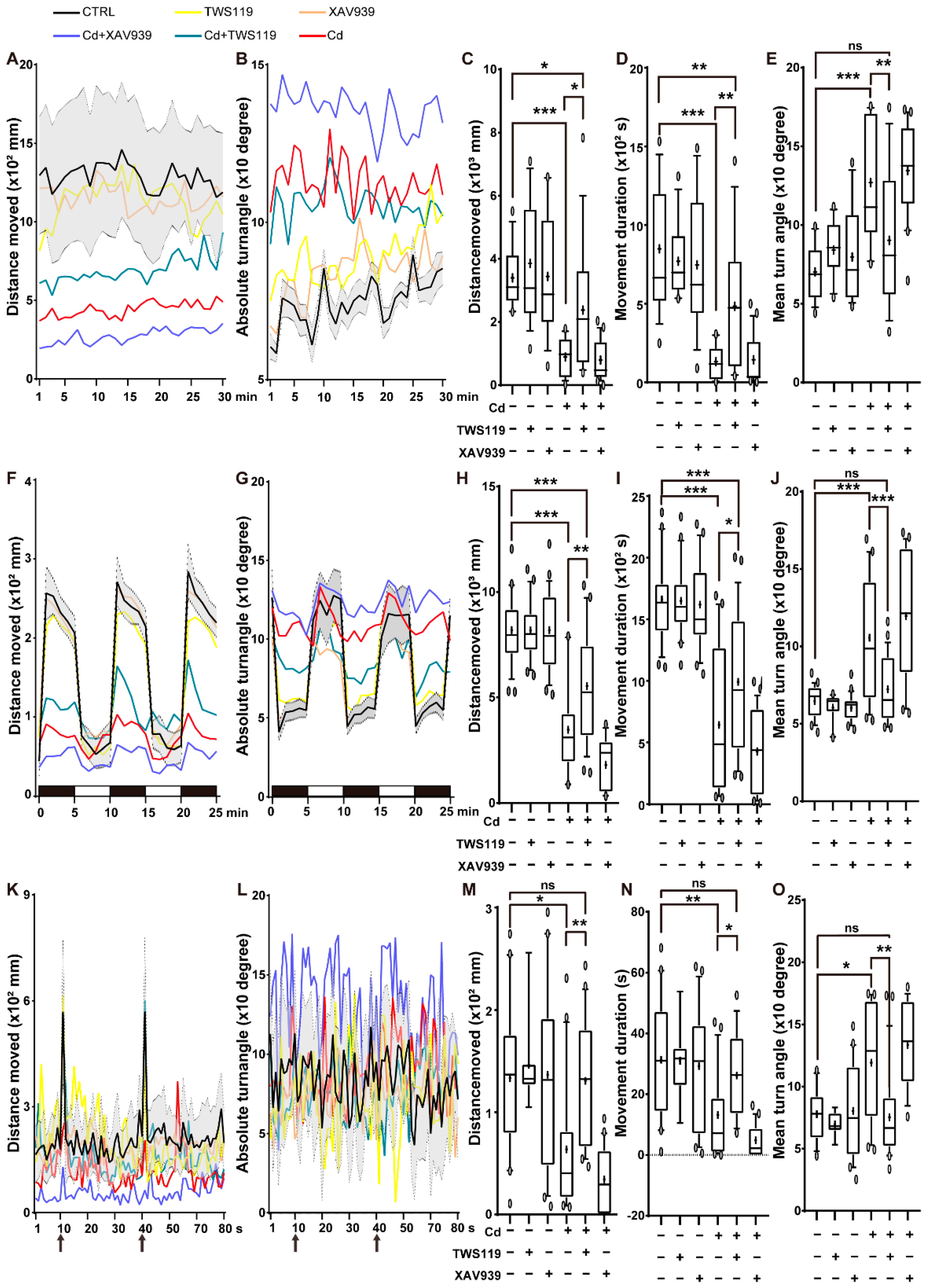
2.3. Activation of Wnt Signaling Pathway Alleviates the Inhibitory Effects of Cd2+ on Zebrafish Activity and Reactivity
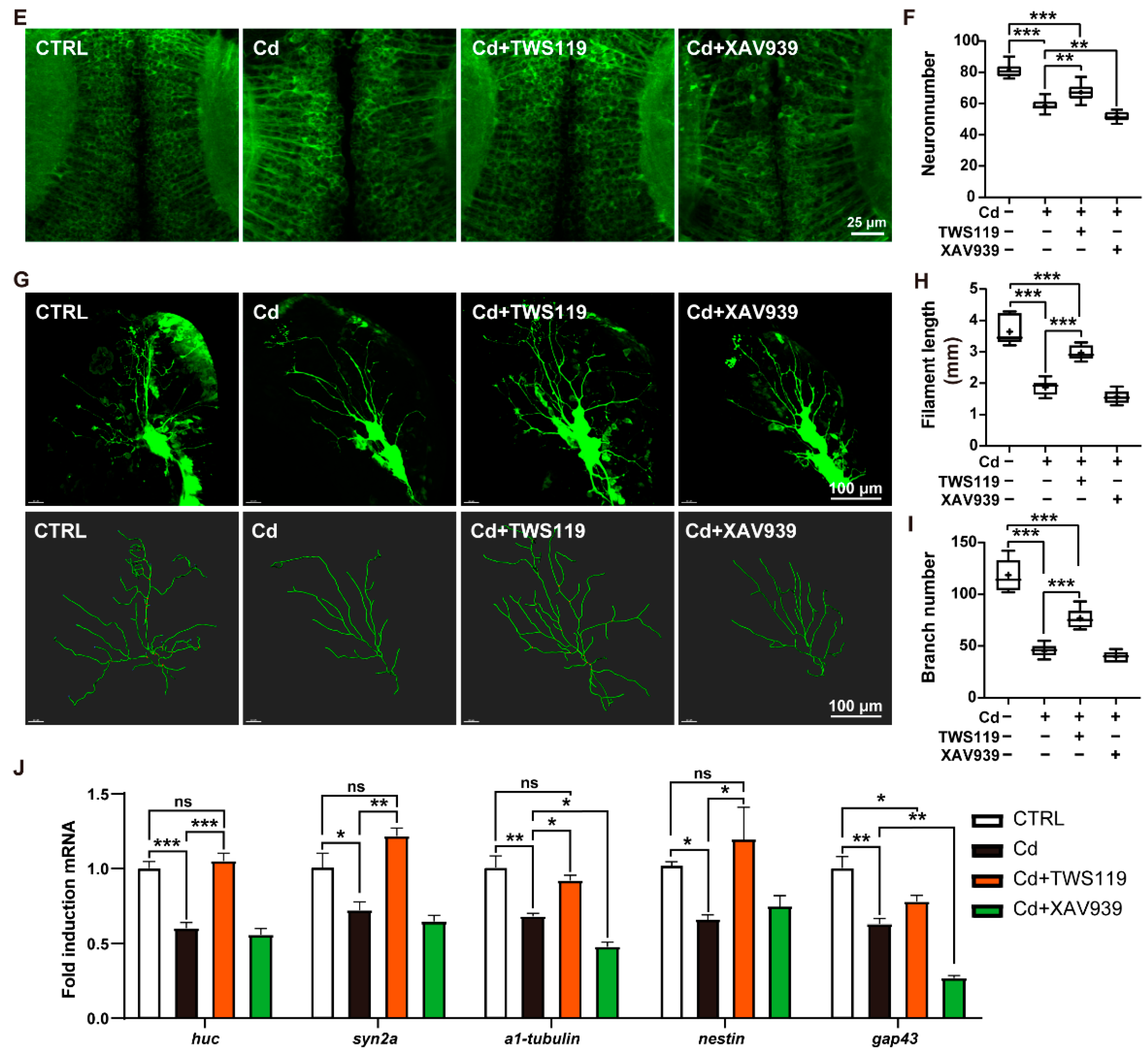
2.4. Activation of Wnt Signaling Pathway Alleviates Cd2+-Induced Neurodevelopmental Disorders
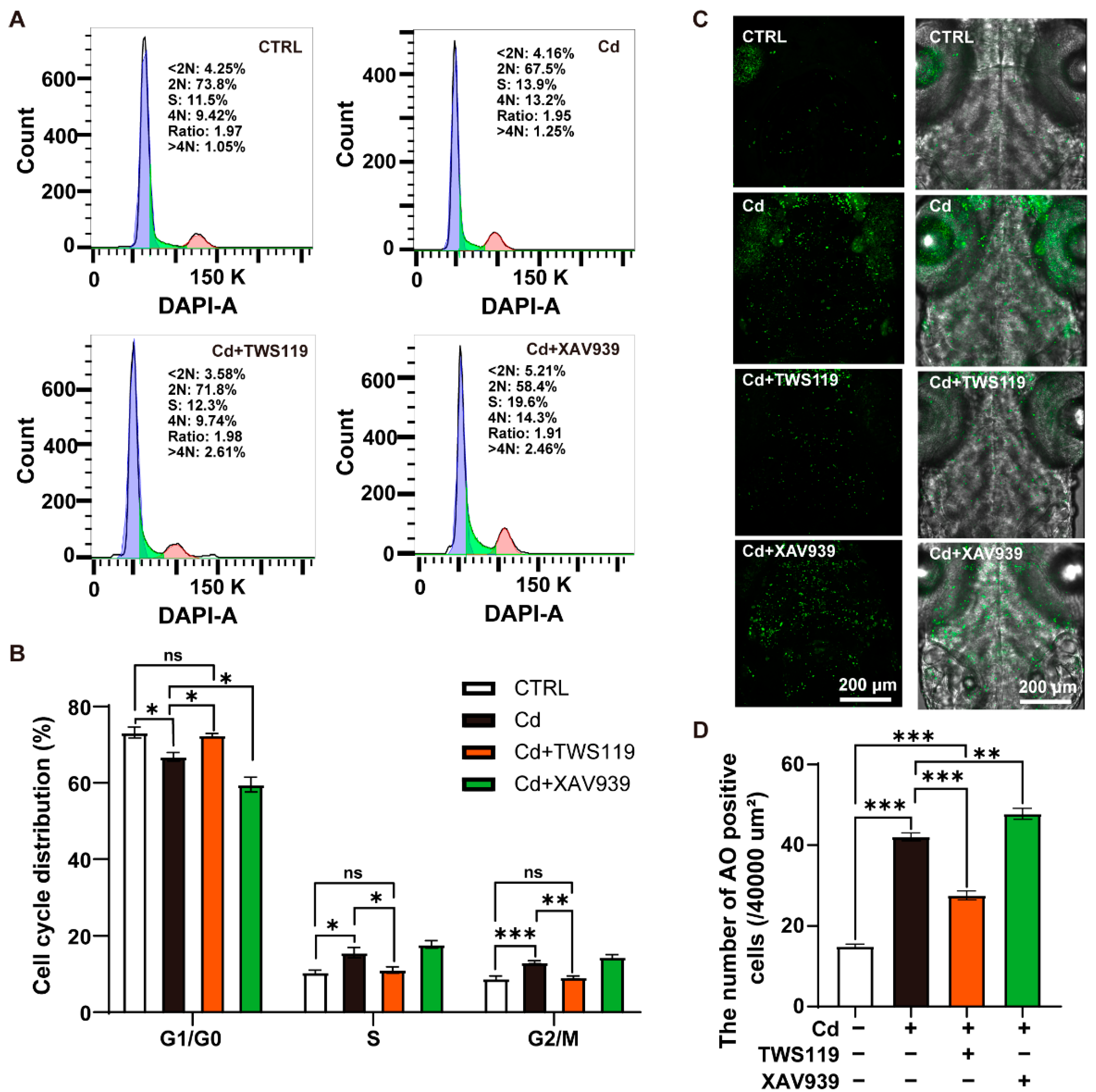
2.5. Activation of Wnt Signaling Pathway Restores Cd2+-Induced Neuronal Apoptosis and Cell Cycle Arrest
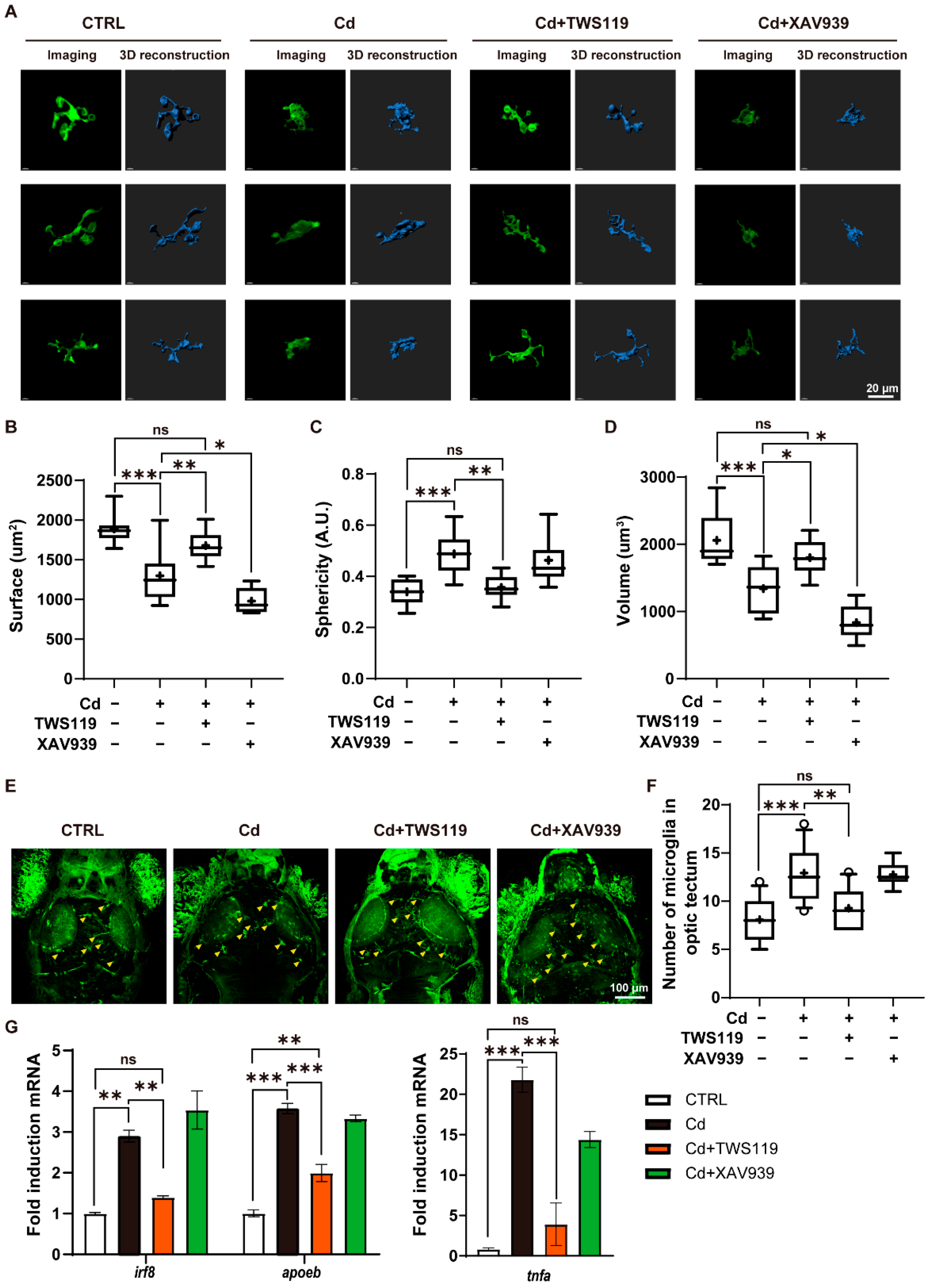
2.6. Activation of Wnt Signaling Pathway Inhibites Cd2+-Induced Microgliosis and Neuroinflammation
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Ethics Statement and Zebrafish Maintenance
4.3. Cd2+ Treatment and Developmental Toxicity Assays on Zebrafish Model
4.4. Neurobehavioral Tests in Zebrafish Larvae
4.5. Western Blot (WB) Analysis
4.6. In Vivo Imaging of the Central Nervous System of Zebrafish Larvae
4.7. Acridine Orange (AO) Staining and Apoptosis Analysis
4.8. Flow Cytometry: Analysis of Cell Cycle Distribution
4.9. qRT-PCR: Analysis of Relative Gene Expression
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, and Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Wter Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- GB 5749-2006; National Standard of the People’s republic of China: Standard for Drinking Water Quality. Ministry of Health of China; Standardization Administration of China: Beijing, China, 2006.
- UNEP. Final Review of Scientific Information on Cadmium; United Nations Environment Programme: Nairobi, Kenya, 2010. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qiu, Z.; Zhang, J.; Liu, W.; Liu, C.; Zeng, G. Investigation, Pollution Mapping and Simulative Leakage Health Risk Assessment for Heavy Metals and Metalloids in Groundwater from a Typical Brownfield, Middle China. Int. J. Environ. Res. Public Health 2017, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Welbourn, P.M. Cadmium in the aquatic environment: A review of ecological, physiological, and toxicological effects on biota. Environ. Rev. 1994, 2, 187–214. [Google Scholar] [CrossRef]
- Campoy-Diaz, A.D.; Escobar-Correas, S.; Canizo, B.V.; Wuilloud, R.G.; Vega, I.A. A Freshwater Symbiosis as Sensitive Bioindicator of Cadmium. Environ. Sci. Pollut. Res. Int. 2020, 27, 2580–2587. [Google Scholar] [CrossRef]
- Tripathy, A.P.; Dixit, P.K.; Panigrahi, A.K. Impact of effluent of Pulp & Paper industry on the flora of river basin at Jaykaypur, Odisha, India and its ecological implications. Environ. Res. 2022, 204, 111769. [Google Scholar] [CrossRef]
- European Chemicals Bureau, Institute for Health and Consumer Protection (Joint Research Centre), Toxicology and Chemical Substances. European Union Risk Assessment Report—Cadmium Oxide and Cadmium Metal Part I—Environment; Office for Official Publications of the European Communities: Luxembourg, 2007. [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry; ATSDR: Atlanta, GA, USA, 2012. [Google Scholar]
- Järup, L.; Akesson, A. Current Status of Cadmium as an Environmental Health Problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Morucci, G.; Pacini, A. Cadmium-Induced Neurotoxicity: Still Much Ado. Neural Regen. Res. 2018, 13, 1879–1882. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef]
- Chow, E.S.H.; Hui, M.N.Y.; Lin, C.C.; Cheng, S.H. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat. Toxicol. 2008, 87, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; Liu, D.; Yin, J.; Chen, M.; Zhou, L.; Yin, H. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 2021, 81, 103545. [Google Scholar] [CrossRef]
- Jiang, J.H.; Ge, G.; Gao, K.; Pang, Y.; Chai, R.C.; Jia, X.H.; Kong, J.G.; Yu, A.C.H. Calcium Signaling Involvement in Cadmium-Induced Astrocyte Cytotoxicity and Cell Death Through Activation of MAPK and PI3K/Akt Signaling Pathways. Neurochem. Res. 2015, 40, 1929–1944. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, Q.; Xu, H.; Du, X.; Shi, L.; Jia, F.; Jiang, H. Biometal dyshomeostasis and toxic metal accumulations in the development of alzheimer’s disease. Front. Mol. Neurosci. 2017, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental Pollutants as Risk Factors for Neurodegenerative Disorders: Alzheimer and Parkinson Diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, Z. The Electrophysiological Effects of Cadmium on Retzius Nerve Cells of the Leech Haemopis Sanguisuga. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2021, 247, 109062. [Google Scholar] [CrossRef]
- Graniel-Amador, M.A.; Torres-Rodríguez, H.F.; Jiménez-Andrade, J.M.; Hernández-Rodríguez, J.; Arteaga-Silva, M.; Montes, S. Cadmium Exposure Negatively Affects the Microarchitecture of Trabecular Bone and Decreases the Density of a Subset of Sympathetic Nerve Fibers Innervating the Developing Rat Femur. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021, 34, 87–96. [Google Scholar] [CrossRef]
- Tsentsevitsky, A.N.; Zakyrjanova, G.F.; Petrov, A.M. Cadmium Desynchronizes Neurotransmitter Release in the Neuromuscular Junction: Key Role of ROS. Free Radic. Biol. Med. 2020, 155, 19–28. [Google Scholar] [CrossRef]
- Cooper, G.P.; Manalis, R.S. Influence of Heavy Metals on Synaptic Transmission: A Review. Neurotoxicology 1983, 4, 69–83. [Google Scholar]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.-M. Cadmium Toxicity in Animal Cells by Interference with Essential Metals. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Wen, L.; Lei, D.; Li, S.; Wang, J.; Huang, J.; Wang, N.; Durkan, C.; Liao, X.; et al. Cadmium-induced dysfunction of the blood-brain barrier depends on ROS-mediated inhibition of PTPase activity in zebrafish. J. Hazard. Mater. 2021, 412, 125198. [Google Scholar] [CrossRef] [PubMed]
- Nemmiche, S. Oxidative signaling response to cadmium exposure. Toxicol. Sci. 2017, 156, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Hartenstein, V.; Stollewerk, A. The Evolution of Early Neurogenesis. Dev. Cell 2015, 32, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt Signaling in Development and Tissue Homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Behrens, J.; von Kries, J.P.; Kühl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382, 638–642. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Jordens, I.; Kirchner, K.; Slootstra, J.W.; Kruitwagen, T.; Bouwman, B.A.M.; Noutsou, M.; Rüdiger, S.G.D.; Schwamborn, K.; Schambony, A.; et al. Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. USA 2012, 109, E812–E820. [Google Scholar] [CrossRef]
- Taciak, B.; Pruszynska, I.; Kiraga, L.; Bialasek, M.; Krol, M. Wnt Signaling Pathway in Development and Cancer. J. Physiol. Pharmacol. 2018, 69, 185–196. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-Catenin Signalling in Liver Development, Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-β-Catenin Signalling—A Versatile Player in Kidney Injury and Repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Salinas, P.C.; Zou, Y. Wnt Signaling in Neural Circuit Assembly. Annu. Rev. Neurosci. 2008, 31, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Beebe, T.; Jen, N.; Yu, F.; Takabe, W.; Harrison, M.; Cao, H.; Lee, J.; Yang, H.; Han, P.; et al. Shear Stress-Activated Wnt-Angiopoietin-2 Signaling Recapitulates Vascular Repair in Zebrafish Embryos. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Spranger, S.; Fuchs, E.; López-Soto, A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019, 29, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.-A.; Schitcu, V.; Gurzau, E.; Stavaru, C.; Manda, G.; Szedlacsek, S.; Berindan-Neagoe, I. Biological and Molecular Modifications Induced by Cadmium and Arsenic during Breast and Prostate Cancer Development. Environ. Res. 2019, 178, 108700. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Schang, A.-L.; Krishnan, M.L.; Degos, V.; Delahaye-Duriez, A.; Bokobza, C.; Csaba, Z.; Verdonk, F.; Montané, A.; Sigaut, S.; et al. Decreased Microglial Wnt/β-Catenin Signalling Drives Microglial pro-Inflammatory Activation in the Developing Brain. Brain 2019, 142, 3806–3833. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Kurita, K.; Burgess, S.M.; Sakai, N. Transgenic zebrafish produced by retroviral infection of in vitro-cultured sperm. Proc. Natl. Acad. Sci. USA 2004, 101, 1263–1267. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Wragg, J.; Müller, F. Transcriptional Regulation During Zygotic Genome Activation in Zebrafish and Other Anamniote Embryos. Adv. Genet. 2016, 95, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, H.; Wang, Z.; Gao, H.; Liu, J.; Li, K.; Song, Z.; Yuan, C.; Lan, X.; Pan, C.; et al. Developmental Exposure to Environmental Levels of Cadmium Induces Neurotoxicity and Activates Microglia in Zebrafish Larvae: From the Perspectives of Neurobehavior and Neuroimaging. Chemosphere 2021, 291, 132802. [Google Scholar] [CrossRef] [PubMed]
- Madden, M.E.; Suminaite, D.; Ortiz, E.; Early, J.J.; Koudelka, S.; Livesey, M.R.; Bianco, I.H.; Granato, M.; Lyons, D.A. CNS Hypomyelination Disrupts Axonal Conduction and Behavior in Larval Zebrafish. J. Neurosci. 2021, 41, 9099–9111. [Google Scholar] [CrossRef]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Z.; Yang, F.; Zhu, M.; Cao, J.; Chen, J.; Lin, Y.; Guo, S.; Li, J.; Liu, Z. Cadmium Disturbs Epigenetic Modification and Induces DNA Damage in Mouse Preimplantation Embryos. Ecotoxicol. Environ. Saf. 2021, 219, 112306. [Google Scholar] [CrossRef]
- Yiin, S.J.; Chern, C.L.; Sheu, J.Y.; Lin, T.H. Cadmium Induced Lipid Peroxidation in Rat Testes and Protection by Selenium. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 1999, 12, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Peng, C.; Wang, P.; Fan, S.; Yan, L.; Qiu, L. Identification of Co-Chaperone Cdc37 in Penaeus Monodon: Coordination with Hsp90 Can Reduce Cadmium Stress-Induced Lipid Peroxidation. Ecotoxicol. Environ. Saf. 2021, 209, 111800. [Google Scholar] [CrossRef]
- López, E.; Arce, C.; Oset-Gasque, M.J.; Cañadas, S.; González, M.P. Cadmium Induces Reactive Oxygen Species Generation and Lipid Peroxidation in Cortical Neurons in Culture. Free Radic. Biol. Med. 2006, 40, 940–951. [Google Scholar] [CrossRef]
- Laan, M.; Richmond, H.; He, C.M.; Campbell, R.K. Zebrafish as a Model for Vertebrate Reproduction: Characterization of the First Functional Zebrafish (Danio Rerio) Gonadotropin Receptor. Gen. Comp. Endocrinol. 2002, 125, 349–364. [Google Scholar] [CrossRef]
- Dawid, I.B. Developmental Biology of Zebrafish. Understanding and Optimizing Human Development: From Cells to Patients to Populations. Ann. N. Y. Acad. Sci. 2004, 1038, 88–93. [Google Scholar] [CrossRef]
- Nagpal, J.; Cryan, J.F. Microbiota-Brain Interactions: Moving toward Mechanisms in Model Organisms. Neuron 2021, 109, 3930–3953. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.-M.; Zhou, F.-Q. GSK3 Signalling in Neural Development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Bielen, H.; Houart, C. The Wnt Cries Many: Wnt Regulation of Neurogenesis Through Tissue Patterning, Proliferation, and Asymmetric Cell Division. Dev. Neurobiol. 2014, 74, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Aniol, V.A.; Tishkina, A.O.; Gulyaeva, N.V. Neurogenesis and Neuroinflammation: The Role of Wnt Proteins. Neurochem. J. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt Signaling Regulates Organ-Specific Assembly and Differentiation of CNS Vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/Beta-Catenin Signaling Is Required for CNS, but Not Non-CNS, Angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Tabar, V.; Studer, L. Pluripotent Stem Cells in Regenerative Medicine: Challenges and Recent Progress. Nat. Rev. Genet. 2014, 15, 82–92. [Google Scholar] [CrossRef]
- Davidson, G.; Shen, J.; Huang, Y.-L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell Cycle Control of Wnt Receptor Activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef]
- Cerpa, W.; Godoy, J.A.; Alfaro, I.; Farías, G.G.; Metcalfe, M.J.; Fuentealba, R.; Bonansco, C.; Inestrosa, N.C. Wnt-7a Modulates the Synaptic Vesicle Cycle and Synaptic Transmission in Hippocampal Neurons. J. Biol. Chem. 2008, 283, 5918–5927. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Moon, R.T. The Ups and Downs of Wnt Signaling in Prevalent Neurological Disorders. Oncogene 2006, 25, 7545–7553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.K.; Lee, W.-K.; Molitor, M.; Wolff, N.A.; Thévenod, F. Cadmium Induces Wnt Signaling to Upregulate Proliferation and Survival Genes in Sub-Confluent Kidney Proximal Tubule Cells. Mol. Cancer 2010, 9, 102. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Scharner, B.; Jurasovic, J.; Messner, B.; Bernhard, D.; Thévenod, F. Chronic Cadmium Exposure Induces Transcriptional Activation of the Wnt Pathway and Upregulation of Epithelial-to-Mesenchymal Transition Markers in Mouse Kidney. Toxicol. Lett. 2010, 198, 69–76. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Yang, Z.; Shao, Y.; Xue, P.; Qu, W.; Jia, X.; Cheng, L.; He, M.; He, R.; et al. Cadmium Activates Noncanonical Wnt Signaling to Impair Hematopoietic Stem Cell Function in Mice. Toxicol. Sci. 2018, 165, 254–266. [Google Scholar] [CrossRef]
- Knani, L.; Venditti, M.; Kechiche, S.; Banni, M.; Messaoudi, I.; Minucci, S. Melatonin Protects Bone against Cadmium-Induced Toxicity via Activation of Wnt/β-Catenin Signaling Pathway. Toxicol. Mech. Methods 2020, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.A.; Yi, H.; Nakamura, R.E.I.; Hackam, A.S. The Wnt Signaling Pathway Protects Retinal Ganglion Cell 5 (RGC-5) Cells from Elevated Pressure. Cell. Mol. Neurobiol. 2011, 31, 163–173. [Google Scholar] [CrossRef]
- Hoseth, E.Z.; Krull, F.; Dieset, I.; Morch, R.H.; Hope, S.; Gardsjord, E.S.; Steen, N.E.; Melle, I.; Brattbakk, H.R.; Steen, V.M.; et al. Exploring the Wnt Signaling Pathway in Schizophrenia and Bipolar Disorder. Transl. Psychiatry 2018, 8, 55. [Google Scholar] [CrossRef]
- Cotter, D.; Kerwin, R.; Al-Sarraji, S.; Brion, J.P.; Chadwich, A.; Lovestone, S.; Anderton, B.; Everall, I. Abnormalities of Wnt Signalling in Schizophrenia—Evidence for Neurodevelopmental Abnormality. Neuroreport 1998, 9, 1379–1383. [Google Scholar] [CrossRef]
- Panaccione, I.; Napoletano, F.; Forte, A.M.; Kotzalidis, G.D.; Del Casale, A.; Rapinesi, C.; Brugnoli, C.; Serata, D.; Caccia, F.; Cuomo, I.; et al. Neurodevelopment in Schizophrenia: The Role of the Wnt Pathways. Curr. Neuropharmacol. 2013, 11, 535–558. [Google Scholar] [CrossRef]
- Park, H.C.; Hong, S.K.; Kim, H.S.; Kim, S.H.; Yoon, E.J.; Kim, C.H.; Miki, N.; Huh, T.L. Structural Comparison of Zebrafish Elav/Hu and Their Differential Expressions during Neurogenesis. Neurosci. Lett. 2000, 279, 81–84. [Google Scholar] [CrossRef]
- Peri, F.; Nüsslein-Volhard, C. Live Imaging of Neuronal Degradation by Microglia Reveals a Role for v0-ATPase a1 in Phagosomal Fusion In Vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio); University of Oregon: Eugene, OR, USA, 1989. [Google Scholar]
- Zhao, H.; Di Mauro, G.; Lungu-Mitea, S.; Negrini, P.; Guarino, A.M.; Frigato, E.; Braunbeck, T.; Ma, H.; Lamparter, T.; Vallone, D.; et al. Modulation of DNA Repair Systems in Blind Cavefish during Evolution in Constant Darkness. Curr. Biol. 2018, 28, 3229–3243. [Google Scholar] [CrossRef] [PubMed]
- Swinburne, I.A.; Mosaliganti, K.R.; Green, A.A.; Megason, S.G. Improved Long-Term Imaging of Embryos with Genetically Encoded α-Bungarotoxin. PLoS ONE 2015, 10, e0134005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, H.; Xu, Y.; Zhao, J.; Song, Z.; Bi, Y.; Li, Y.; Lan, X.; Pan, C.; Foulkes, N.S.; et al. Early-Life Lead Exposure Induces Long-Term Toxicity in the Central Nervous System: From Zebrafish Larvae to Juveniles and Adults. Sci. Total Environ. 2022, 804, 150185. [Google Scholar] [CrossRef]
- Kaur, J.; Bachhawat, A.K. A Modified Western Blot Protocol for Enhanced Sensitivity in the Detection of a Membrane Protein. Anal. Biochem. 2009, 384, 348–349. [Google Scholar] [CrossRef]
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in zebrafish embryos and adults after cadmium exposure. Eur. J. Histochem. 2017, 61, 276–279. [Google Scholar] [CrossRef]
- Allan, K.; DiCicco, R.; Ramos, M.; Asosingh, K.; Yuan, A. Preparing a Single Cell Suspension from Zebrafish Retinal Tissue for Flow Cytometric Cell Sorting of Müller Glia. Cytometry A 2020, 97, 638–646. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, J.; Tian, Y.; Wang, Z.; Song, Z.; Li, K.; Zhang, S.; Zhao, H. Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging. Int. J. Mol. Sci. 2022, 23, 11434. https://doi.org/10.3390/ijms231911434
Xu Y, Liu J, Tian Y, Wang Z, Song Z, Li K, Zhang S, Zhao H. Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging. International Journal of Molecular Sciences. 2022; 23(19):11434. https://doi.org/10.3390/ijms231911434
Chicago/Turabian StyleXu, Yanyi, Junru Liu, Yonghui Tian, Zuo Wang, Zan Song, Kemin Li, Shengxiang Zhang, and Haiyu Zhao. 2022. "Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging" International Journal of Molecular Sciences 23, no. 19: 11434. https://doi.org/10.3390/ijms231911434
APA StyleXu, Y., Liu, J., Tian, Y., Wang, Z., Song, Z., Li, K., Zhang, S., & Zhao, H. (2022). Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging. International Journal of Molecular Sciences, 23(19), 11434. https://doi.org/10.3390/ijms231911434





