Abstract
Deep eutectic solvents (DESs) can compensate for some of the major drawbacks of traditional organic solvents and ionic liquids and meet all requirements of green chemistry. However, the potential of their use as a medium for biocatalytic reactions has not been adequately studied. In this work we used the DES betaine-glycerol with a molar ratio of 1:2 as co-solvent for enzymatic template-guided polymerization/copolymerization of aniline (ANI) and 3-aminobenzoic acid (3ABA). The laccase from the basidial fungus Trametes hirsuta and air oxygen served as catalyst and oxidant, respectively. Sodium polystyrene sulfonate (PSS) was used as template. Interpolyelectrolyte complexes of homopolymers polyaniline (PANI) and poly(3-aminobenzoic acid) (P3ABA) and copolymer poly(aniline-co-3-aminobenzoic acid) (P(ANI-3ABA)) were prepared and their physico-chemical properties were studied by UV-Vis and FTIR spectroscopy and cyclic voltammetry. According to the results obtained by atomic force microscopy, PANI/PSS had a granular shape, P(ANI-3ABA)/PSS had a spherical shape and P3ABA/PSS had a spindle-like shape. The copolymer showed a greater antimicrobial activity against Escherichia coli and Staphylcocus aureus as compared with the homopolymers. The minimal inhibitory concentration of the P(ANI-3ABA)/PSS against the gram-positive bacterium S. aureus was 0.125 mg mL−1.
1. Introduction
The term “green chemistry” implies the development of a strategy aimed at designing new products that minimize or eliminate the use and generation of hazardous substances. In the last few years, deep eutectic solvents (DESs) have drawn a great attention as an alternative to traditional organic solvents and ionic liquids. The term ‘deep eutectic solvents’ was first coined by Abbott et al. [1] in 2003, however, there is still no clear definition [2]. DESs are formed by mixing particular ratios of two or more substances. The resultant liquid has a lower melting temperature as compared to those of the DES components [1]. As a rule, the DES components—hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD)—are natural biodegradable organic substances [3,4,5]. The properties of DESs depend on the nature of HBA and HBD, their molar ratio and water content [6]. The number of HBA and HBD combinations is extremely large, which enables the formation of DESs with different properties (hydrophilic, hydrophobic, acidic, alkaline, neutral, etc.) and tailoring DESs for specific applications. DESs are similar to ionic liquids. However, they have some advantages, like ease of preparation, low preparation costs, low toxicity, biodegradability and the possibility of varying characteristics with changes of DES components and their ratio [4]. The potential of DESs results from unique properties of these solvents like nonflammability, low vapor pressure, thermal and chemical stability, a wide polarity range and high solubility of numerous compounds in them, as well as from their high extraction and stabilizing abilities [4,7,8]. Thus, DESs are promising green solvents which can find applications in many fields, including the pharmaceutical, food and cosmetics industries [9,10,11]. They can also be used for extractions and separations [10,12,13,14,15,16], biomass processing [11,17,18], dissolution of metals [11,19], in material and polymer chemistry [20,21,22,23,24] and as a reaction medium for organic reactions [11,25,26,27,28]. DESs have proved to be a good alternative to traditional organic solvents and ionic liquids in many biocatalytic processes [29]. The use of DESs as a medium for biocatalytic reactions can be promising for the biotransformation of slightly soluble substances and the design of products with novel properties.
The interest in the use of enzymes in organic synthesis has been growing in recent years [30,31,32]. In terms of the environment, enzymes are natural and renewable and, hence, belong to green catalysts [30]. In addition, enzymatic reactions proceed under ‘mild’ operating conditions, namely at pH values close to neutral, room temperature, under atmospheric pressure and in the absence of toxic organic solvents and meet the requirements of sustainable chemistry [29,33,34]. The use of DESs and water (buffer)/DES mixtures as media for enzymatic reactions is currently reported for a limited range of biocatalysts [29,33,34,35,36,37].
Of special interest as a biocatalyst for fine organic synthesis is laccase [38,39,40,41]. Laccase (p-diphenol:oxygen oxidoreductase, EC 1.10.3.2), which belongs to the blue multicopper oxidase family, catalyzes oxidation of a wide range of organic compounds with atmospheric oxygen and its reduction to water [42,43]. Laccase-catalyzed oxidation of aromatic compounds proceeds via a radical mechanism with the subsequent combination of intermediates, which results in the formation of oligomeric and polymeric products [38,44,45]. Laccase-catalyzed reactions are usually carried out in buffer solutions or, if substrates are poorly dissolved in water, in organic-water solutions.
Aniline (ANI) is a promising substrate for laccase, as its oxidation results in the formation of polyaniline (PANI), which is an important conducting polymer [46]. PANI has a great potential for technological applications due to the ease of production, low cost of the monomer, environmental stability and ability to change electrical and optical characteristics when varying the degree of protonation and oxidation [46,47,48,49,50,51]. In addition, PANI and its functionalized derivatives show antimicrobial properties [52,53,54,55,56,57], which enables their use for making film coats to protect various surfaces against bacterial contamination [58].
The properties of PANI can be varied in a wide range depending on synthesis conditions [59,60]. PANI is generally produced by electrochemical or chemical methods [46,61,62], but both methods have some drawbacks. The electrochemical method is not always suitable as monomer polymerization proceeds on electroconducting substrates of limited sizes, and the chemical synthesis requires great amounts of oxidants, whose reduction products should be disposed of. In terms of the environment, the enzymatic synthesis of PANI is a good alternative to traditional methods [63,64,65,66,67,68].
As mentioned above, the use of DES and DES/buffer mixtures in enzymatic reactions is reported for a limited range of biocatalysts. Although eutectic media have same advantages, e.g., a higher solubility of substrates and/or products, their wide application is limited by a lower stability of biocatalysts.
In this work we have investigated the influence of the DES betaine-glycerol (molar ratio 1:2) on the catalytic activity and stability of the laccase from the fungus Trametes hirsuta, performed an enzymatic template-guided polymerization/copolymerization of aniline and 3-aminobenzoic acid in a DES/buffer mixture (60/40 vol.%) and characterized the resulting products.
2. Results and Discussion
The use of deep eutectic solvents (DESs) and DES/buffer mixtures as a medium for biocatalytic reactions requires taking into account their effect on the activity and stability of enzymes. Choline chloride-based DESs are in most common use [29,34,35,36,37]. However, chloride ions inhibit the activity of fungal laccases [69]. Therefore, in our experiments we used a DES composed of betaine (HBA) and glycerol (HBD) with a molar ratio of 1:2. Betaine-glycerol (1:2) composition like most DESs is a viscous liquid. The viscosity can be markedly decreased by adding water [70]. Our studies of laccase activity in DES/buffer mixtures with a varied content of DES (Figure 1) showed that the enzyme activity increased at a DES content of 10–20 vol.%. A similar activation of laccases at a rather low DES content (up to 20 vol.%) in the reaction medium is reported in [71,72,73]. However, Hammond et al. [74] showed that DES properties were retained in the DES/H2O mixture if the water content is less than 42 wt.%, and, at higher contents of water, the DES/H2O mixture should be considered as a water solution of DES components. Therefore, we chose a DES/buffer mixture with a volume ratio of 60/40 vol.% to perform laccase-catalyzed polymerization/copolymerization of aniline (ANI) and 3-aminobenzoic acid (3ABA). At this DES/buffer ratio the mass content of water was ~35 wt.%, which enabled us to assume that the structure of DES remained intact. Besides this DES/buffer mixture was less viscous as compared to pure DES. Studies of laccase stability showed (Figure 2) that the enzyme retains ~60% of its activity after 24 h incubation in DES/buffer (60/40 vol.%) mixture.
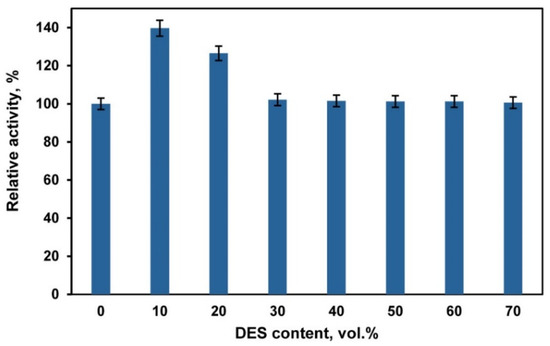
Figure 1.
Laccase activity in betaine-glycerol (1:2)/buffer mixtures with different volume ratios of the components at room temperature (21–22 °C). Laccase activity in the buffer was considered to be 100%.
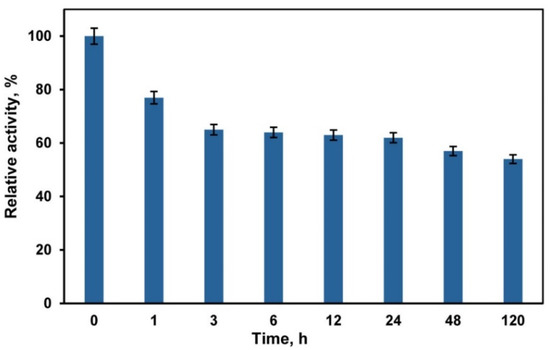
Figure 2.
Laccase stability in betaine-glycerol (1:2)/buffer (60/40 vol.%) mixture. Laccase activity in the DES/buffer mixture at the initial time was considered to be 100%.
Using enzymatic polymerization/copolymerization of ANI and 3ABA on a PSS template (Supplementary Figure S1) in DES/buffer (60/40 vol.%) mixture (pH 3.5), we obtained PANI/PSS, P3ABA/PSS and P(ANI-3ABA)/PSS interpolyelectrolite complexes, whose UV–visible spectra are shown in Figure 3. It is noteworthy that the PANI/PSS homopolymer and copolymer had green color typical of conducting polyanilines, while the P3ABA/PSS homopolymer was of light brown color.
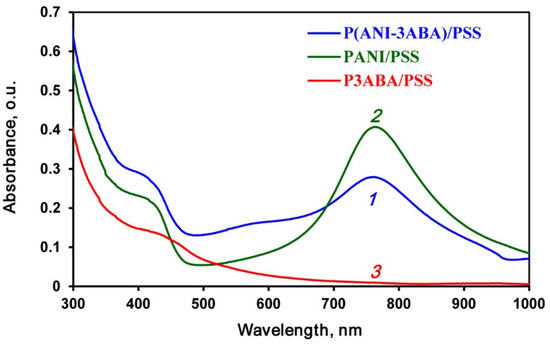
Figure 3.
UV–visible spectra of the P(ANI-3ABA)/PSS copolymer (1), and PANI/PSS (2) and P3ABA/PSS (3) homopolymers. The samples were diluted with buffer (1:10).
The PANI/PSS spectrum (Figure 3, line 2) has two wide absorbance bands with maxima at 762 and 405 nm, which indicates the formation of a polaron in emeraldine salt of PANI. It should be mentioned that the spectrum is no different from the spectrum of PANI/PSS synthesized in the aqua medium using laccase [75]. The absorbance band attributed to the polaron in the spectrum of P(ANI-3ABA)/PSS are less distinct, which is due to the side electron acceptor (carboxyl) group of aminobenzoic acid in the main chain of the copolymer. The spectrum of P3ABA/PSS has no bands assigned to the polaron, which seems to be related to the fact that the charge carriers are more localized on the nitrogen atoms. This effect can be explained by the formation of a five-member ring caused by electrostatic interaction between COO− groups and the cationic radical nitrogen atoms [76]. Very low conductivity of P3ABA/PSS (~ 10−10 S cm−1) seems to be due to this effect. At the same time, the conductivity of PANI/PSS and P(ANI-3ABA)/PSS was much higher (2.2 × 10−3 and 1.3 × 10−5 S cm−1, respectively).
The molecular structures of the interpolyelectrolite complexes PANI/PSS, P3ABA/PSS and P(ANI-3ABA)/PSS were studied using FTIR-spectroscopy, which enabled us to get additional information about the polymers synthesized in the DES/buffer mixture (Figure 4). The bands at 970–1200 cm−1 in the spectra of all the polymers are assigned to the S=O bonds stretching in the PSS template, which indicates the formation of durable polymer/PSS complexes. The characteristic peaks at 1500 and 1600 cm−1 are assigned to the C–C aromatic ring stretching of the benzenoid diamine units and to the stretching of both C=N and C=C of the quinoid diimine units, respectively. In addition, an additional band is observed in the spectra of P(ANI-3ABA)/PSS (1705 cm−1) and homopolymer P3ABA/PSS (1690 cm−1). This can be explained by C=O stretching in the carboxyl groups which is absent in the PANI/PSS spectrum. The vibrations in the range of 650–850 cm−1 are attributed to the C–H in-plane bending vibration of the benzene rings. The homopolymer P3ABA/PSS spectrum has a band at 900 cm−1, which corresponds to the C–H out-of-plane bending vibration of the trisubstituted benzene ring.
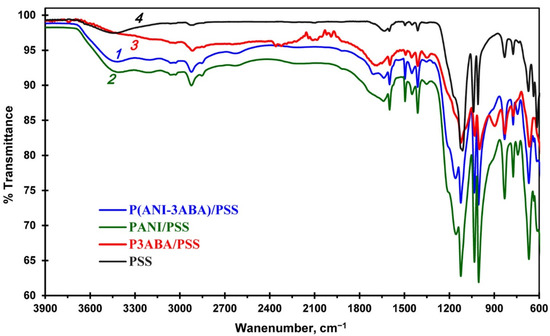
Figure 4.
ATR-FTIR spectra of the P(ANI-3ABA)/PSS copolymer (1), PANI/PSS (2) and P3ABA/PSS (3) homopolymers, and PSS template (4).
The homopolymers and copolymer were tested by cyclic voltammetry (CV) on a spectroscopic graphite (SG) electrode. The studies showed that they are all electrochemically active (Figure 5). Redox conversions of the benzenoid and quinoid units are marked with couples of pseudoreversible peaks, whose characteristics are given in Table 1, where ∆E is the difference in potentials for the maximum of anodic (Ea) and cathodic (Ec) peaks, and Emp is the middle point potential. No redox conversions were registered on the bare SG electrode.
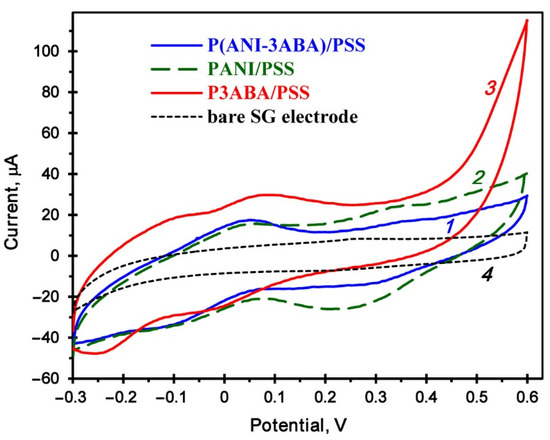
Figure 5.
Cyclic voltammograms of the copolymer P(ANI-3ABA)/PSS (1), homopolymers PANI/PSS (2) and P3ABA/PSS (3), and the bare SG electrode (4) recorded in 0.1 M Na-citrate-phosphate buffer, pH 3.5 at a scan rate of 50 mV s−1.

Table 1.
Characteristics of the redox conversions of the polymer/PSS complexes *.
Studies of the films of the polymer/PSS complexes formed on the surface of highly oriented pyrolytic graphite (Figure 6) showed significant morphological differences. PANI/PSS has a morphology with small, aggregated granules with a diameter of 20–50 nm. P(ANI-3ABA)/PSS nanoparticles have a spherical shape with a diameter varying from 60 to 180 nm, while P3ABA/PSS particles have a spindle-like shape with a diameter of 60–90 nm and length of 300–500 nm.
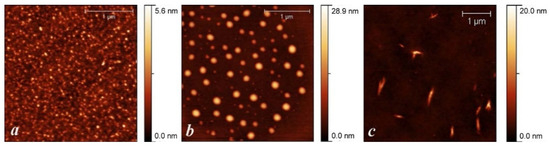
Figure 6.
AFM-images PANI/PSS (a), P(ANI-3ABA)/PSS (b), P3ABA/PSS (c).
The synthesized polymers were tested against microbial activities of the bacteria E. coli and S. aureus and the yeast Yarrowia lipolytica. The PSS template had no effect on the growth of the microbes. The PANI/PSS homopolymer at a maximum concentration obtained in the study (2.9 mg mL−1) significantly but not completely suppressed the growth of E. coli and S. aureus. The minimal inhibitory concentration (MIC) of the P(ANI-3ABA)/PSS copolymer against the gram-positive bacterium S. aureus was 0.125 mg mL−1. In addition, the copolymer essentially suppressed growth of the gram-negative bacterium E. coli at a concentration of 0.5 mg mL−1. MIC of the P3ABA/PSS homopolymer against both S. aureus and E. coli was 2.93 mg mL−1. In addition, at a concentration of 5.85 mg mL−1 P3ABA/PSS suppressed growth of the yeast Y. lipolytica, whereas PANI/PSS and P(ANI-3ABA)/PSS showed no effect on the yeast growth.
It is noteworthy that MIC values for polyaniline, poly(3-aminobenzoic acid) and poly(aniline-co-3-aminobenzoic acid) synthesized by the traditional chemical method [54] were 10, 2.5 and 1.25 mg mL−1 against E. coli and >10, 2.5 and 1.2 mg mL−1 against S. aureus, respectively, which makes it clear that polymer/PSS complexes enzymatically synthesized in the DES/buffer mixture are more efficient as growth inhibitors of gram-positive and gram-negative bacteria.
Conducting polymers, in particular polyaniline and its derivatives, are rather new antibacterial agents, whose mechanism of action has not been adequately studied yet, but several possible antibacterial mechanisms have been proposed. Sheshardi & Bhat [52], who were the first to show the antibacterial action of chemically synthesized PANI against the bacteria S. aureus and E. coli, and the fungus Candida albicans, attributed it to the destruction of cell walls caused by electrostatic interaction between the polymer and bacteria. Recently, other mechanisms were reported in [77], according to which the antimicrobial action of PANI is related to the production of hydrogen peroxide that causes the formation of hydroxyl radicals, while P3ABA disrupts metabolic and respiratory machinery by targeting ATP synthase and causes acid stress. Higher antimicrobial activity of P(ANI-3ABA)/PSS as compared to homopolymers can probably be explained by involving all the three mechanisms.
3. Materials and Methods
3.1. Materials
KH2PO4, NaOH, citric acid, 2,2-azino-bis(3-ethyl-benzthiazoline-6-sulfonate (ABTS), betaine, aniline (ANI), poly(sodium 4-styrenesulfonate) solution (PSS, 30 wt.% in H2O) (Sigma-Aldrich, Saint Louis, MO, USA), glycerol (99%) (Panreac Quimica SA, Barselona, Spain), 3-aminobenzoic acid (3ABA) (Acros organics, Geel, Belgium) were used without further purification. Aniline (ANI) was distilled in vacuo before use. All the solutions were prepared using water purified with a Simplicity® Water Purification System (Merck KGaA, Darmstadt, Germany).
3.2. Enzyme
A laccase from the fungus Trametes hirsuta (Wulfen) Pilát CF-28 was purified to homogeneity as described previously [78]. The laccase activity was measured spectrophotometrically using 1 mM ABTS as chromogenic substrate (λ = 420 nm, ε = 36,000 M−1cm−1) at 24 °C in 0.1 M Na-citrate-phosphate buffer, pH 4.5. One unit of activity is defined as the amount of laccase oxidizing 1 µM of substrate per min. The specific activity of the enzyme stock solution was about 145 U mg−1 of protein. Protein concentration was determined as described in [79] by the difference in the optical density of the protein solution at 228.5 and 234.5 nm using a bovine serum albumin (BSA) as standard. The protein concentration was ca. 7.8 mg mL−1.
3.3. Preparation of DES and DES/Buffer Mixture
DES betaine/glycerol (molar ratio 1:2) was obtained using a thermal mixing procedure at 40 °C with 300 rpm agitation. DES/buffer binary mixture was obtained by adding 4 mL of 0.1 M Na-citrate-phosphate buffer (pH 3.5) to 6 mL of DES.
3.4. Enzymatic Polymerization/Copolymerization in DES/Buffer Mixture
The enzymatic copolymerization of ANI and 3ABA (molar ratio 1:1) in DES/buffer mixture on PSS template was carried out as follows: 0.152 g of a 30% aqueous solution of PSS (concentration 20 mM based on the monomeric repeat unit) was added to 10 mL of DES/buffer mixture (60/40 vol.%) and stirred for 30 min. The 10 µL of ANI and 0.016 g of 3ABA were added and the resultant solution was stirred for another 30 min and its pH was adjusted to 3.5 with phosphoric acid. Polymerization was initiated by adding a laccase stock solution. The specific activity of the enzyme in the reaction mixture was ~1.0 U mL−1. The reaction was performed at room temperature (21–22 °C) under aerobic conditions and with constant stirring on a magnetic stirrer for 24 h. The resulting P(ANI-3ABA)/PSS copolymer was purified by dialysis against acidified deionized water (pH 3.5) in order to remove excess monomers and DES components.
The synthesis of PANI/PSS and P3ABA/PSS homopolymers was performed under the same conditions with the appropriate monomers (20 µL ANI or 0.032 g 3ABA).
3.5. Characterization of Products
The absorption spectra were recorded with a Shimadzu UV 1240 mini spectrophotometer (Japan) in a quartz cuvette with an optical path length of 1 cm. ATR FTIR-spectra were recorded on a Spectrum Two™ FT-IR spectrometer (PerkinElmer Inc., Waltham, MA, USA).
The morphology of the products was studied using a SmartSPM 1000 Scanning Probe Microscope (AIST-NT, Moscow, Russia) on the surface of highly oriented pyrolytic graphite (Advanced Technologies Centre, Russia). For AFM studies, samples were diluted 10 times with deionized water.
Four-point conductivity measurements were carried out with a Loresta GP MCP-T610 resistivity meter (Mitsubishi Chemical Analytech Co., Chigasaki, Kanagawa, Japan) using a MCP-TP06P probe (inter-pin distance 1.5 mm, pin points 0.26 R, spring pressure 70 g pin−1). Electrochemical measurements were performed using a BAS CV-50W voltammetric analyzer (Bioanalytical Systems Inc., West Lafayette, IN, USA) and a single-compartment three-electrode cell. A platinum wire and an Ag/AgCl electrode (BAS) served as counter and reference electrodes, respectively. The rod of spectroscopic graphite (SG) electrode with an outer diameter of 3.05 mm (type RW001, Ringsdorff Werke GmbH, Germany) coated with a synthesized polymer/PSS served as a working electrode.
3.6. Determination of Minimal Inhibitory Concentration (MIC)
The minimal inhibitory concentration (MIC) of the polymer/PSS complexes was determined by the standard serial two-fold dilution method in LB nutrient medium (Luria-Bertani, Miller, Sigma). The gram-positive bacterium Staphylococcus aureus 209P, gram-negative bacterium E. coli K 12 and the yeast Yarrowia lipolytica 367-2 were used as test microorganisms. 200 µL of earlier prepared sterile media containing various concentrations of polymer/PSS was placed in the wells of a 96-well microplate in 3 replicates for each concentration. Next, each well was inoculated with 4 µL of the test culture during the stationary growth phase (1 day) and incubated at 28 °C with 150 rpm agitation for 24 h. Then the optical density of each well was measured relative to controls without inoculum at 540 nm using an Ao Absorbance Microplate Reader (Azure biosystems, Dublin, CA, USA). The growth of microorganisms was assessed by change in optical density values compared to the initial values (immediately after medium inoculation). MIC was defined as the lowest concentration of a compound that inhibited bacterial growth within 24 h. Each strain was tested in triplicate.
4. Conclusions
The present work has demonstrated the possibility of using betain-glycerol DES as a co-solvent for effective laccase catalyzed polymerization/copolymerization of aniline (ANI) and 3-aminobenzoic acid (3ABA) to produce conducting polyanilines. The composition of the reaction medium meets the requirements of sustainable chemistry. The synthesized polymer/PSS complexes are different in morphology and electrochemical activity, and their conductivity decreases in the following way: PANI/PSS > P(ANI-3ABA)/PSS > P3ABA. P(ANI-3ABA)/PSS strongly inhibits the growth of S. aureus and E.coli. The laccase from the fungus T. hirsuta used for polymerization preserves about 50% of activity after incubation for 120 h in the DES/buffer mixture (60/40 vol.%). Thus, betaine-based deep eutectic solvents are promising media for biotransformation of various substrates of laccase, including poorly soluble compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231911409/s1.
Author Contributions
Conceptualization, A.Y.; synthesis, I.V.; physicochemical experiments, G.S.; microbiological experiments, O.M.; writing, reviewing and editing, A.Y. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Russian Foundation for Basic Research, grant number 20-08-00104.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbott, A.P.; Capper, G.; Davies, D.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; de Oliveira Vigier, K.; Royer, S.; Jéróme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C. R. Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of deep eutectic solvents related to health, synthesis, and extraction of natural based chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Redha, A.A. Review on extraction of phenolic compounds from natural sources using green deep eutectic solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining acid-based deep eutectic solvents and microwave irradiation for improved chestnut shell waste valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; González-Rivera, J.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting deep eutectic solvents and ionic liquids for the valorization of chestnut shell waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Li, X.; Binnemans, K. Oxidative dissolution of metals in organic solvents. Chem. Rev. 2021, 121, 4506–4530. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of deep eutectic solvents in polymer chemistry—A review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Matias, A.A.; Paiva, A.; Duarte, A.R.C. Polymer science and engineering using deep eutectic solvents. Polymers 2019, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The benefits of deep eutectic solvents in polymer and materials science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Wazeer, I.; Hayyan, M.; Hadj-Kali, M.K. Deep eutectic solvents: Designer fluids for chemical processes. J. Chem. Technol. Biotechnol. 2018, 93, 945–958. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.W.; Zong, M.H.; Li, N.; Lou, W.Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 34. [Google Scholar] [CrossRef]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.; Amzazi, S.; Lavandera, I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules 2019, 24, 2190. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Paul, C. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef]
- Erol, Ö.; Hollmann, F. Natural deep eutectic solvents as performance additives for biocatalysis. Adv. Bot. Res. 2021, 97, 95–132. [Google Scholar] [CrossRef]
- Witayakran, S.; Ragauskas, A. Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Baez, A.; Torres, E. Dioxygen activation by laccases: Green chemistry for fine chemical synthesis. Catalysts 2018, 8, 223. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with laccases: An updated overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- Chaurasia, P.K.; Bharati, S.L.; Kumar, S.; Singh, S.; Mani, A. Potential involvement of laccases as efficient biocatalysts in the field of organic synthesis: An editorial presenting a short overview on functional applicability and fate. Mini-Rev. Org. Chem. 2022, 19, 676–680. [Google Scholar] [CrossRef]
- Solomon, E.; Sundaram, U.; Machonkin, T. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” Laccases. Biochemistry 2007, 72, 1136–1150. [Google Scholar] [CrossRef]
- Walde, P.; Guo, Z. Enzyme-catalyzed chemical structure-controlling template polymerization. Soft Matter 2011, 7, 316–331. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I. Enzyme initiated radical polymerizations. Polymers 2012, 4, 759–793. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Nautiyal, A.; Liu, Z.; Zhang, X.Y. Recent progress on nanostructured conducting polymers and composites: Synthesis, application and future aspects. Sci. China Mater. 2018, 61, 303–352. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, S.K. Advances in polyaniline-based nanocomposites. J. Mater. Sci. 2020, 55, 1331–1365. [Google Scholar] [CrossRef]
- Gao, F.J.; Mu, J.; Bi, Z.X.; Wang, S.; Li, Z.L. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E. Advances in polyaniline for biomedical applications. Curr. Med. Chem. 2022, 29, 329–357. [Google Scholar] [CrossRef] [PubMed]
- Sheshardi, D.T.; Bhat, N. Use of polyaniline as an antimicrobial agent in textiles. Indian J. Fibre Text. Res. 2005, 30, 204–206. Available online: http://nopr.niscair.res.in/bitstream/123456789/24677/1/IJFTR%2030(2)%20204-206.pdf (accessed on 26 September 2022).
- Shi, N.L.; Guo, X.M.; Jing, H.M.; Gong, J.; Sun, C.; Yang, K. Antibacterial effect of the conducting polyaniline. J. Mater. Sci. Technol. 2006, 22, 289–290. Available online: https://www.jmst.org/CN/Y2006/V22/I03/289 (accessed on 26 September 2022).
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
- Kucekova, Z.; Humpolicek, P.; Kasparkova, V.; Perecko, T.; Lehocký, M.; Hauerlandová, I.; Sáha, P.; Stejskal, J. Colloidal polyaniline dispersions: Antibacterial activity, cytotoxicity and neutrophil oxidative burst. Colloids Surf. B 2014, 116, 411–417. [Google Scholar] [CrossRef]
- Shalini, A.; Nishanthi, R.; Palani, P.; Jaisankar, V. One pot synthesis, characterization of polyaniline and cellulose/polyaniline nanocomposites: Application towards in vitro measurements of antibacterial activity. Mater. Today Proc. 2016, 3, 1633–1642. [Google Scholar] [CrossRef]
- Lashkenari, M.S.; Eisazadeh, H. Chemical copolymerization and characterization of colloidal poly(aniline-co-3-aminobenzoic acid) as a high-performance antibacterial polymer. Adv. Polym. Technol. 2017, 33, 21466. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Pagnon, J.C.; Ali, N.; Sum, R.; Davies, N.; Roddam, L.F.; Ambrose, M. Functionalized polyanilines disrupt Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Colloids Surf. B 2015, 136, 666–673. [Google Scholar] [CrossRef]
- Anand, J.; Palaniappan, S.; Sathyanarayana, D.N. Conducting polyaniline blends and composites. Prog. Polym. Sci. 1998, 23, 993–1018. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a novel conducting polymer—Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J. Chem. Soc., Faraday Trans. 1 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Syed, A.A.; Dinesan, M.K. Review: Polyaniline—A novel polymeric material. Talanta 1991, 38, 815–837. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically synthesized conducting polyaniline. J. Am. Chem. Soc. 1999, 121, 71–78. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Romero-García, J.; Angulo-Sánchez, J.L.; Ledezma-Pérez, A.; Arias-Marín, E.; Moggio, I.; Flores-Loyola, E. Template-free enzymatic synthesis of electrically conducting polyaniline using soybean peroxidase. Eur. Polym. J. 2005, 41, 1129–1135. [Google Scholar] [CrossRef]
- Streltsov, A.V.; Morozova, O.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Staroverova, I.N.; Shumakovich, G.P.; Yaropolov. Synthesis and characterization of conducting polyaniline prepared by laccase-catalyzed method in sodium dodecylbenzenesulfonate micellar solutions. J. Appl. Polym. Sci. 2009, 114, 928–934. [Google Scholar] [CrossRef]
- Shumakovich, G.P.; Vasil’eva, I.S.; Morozova, O.V.; Khomenkov, V.G.; Staroverova, I.N.; Budashov, I.A.; Kurochkin, I.N.; Boyeva, J.A.; Sergeyev, V.G.; Yaropolov, A.I. A comparative study of water dispersible polyaniline nanocomposites prepared by laccase-catalyzed and chemical methods. J. Appl. Polym. Sci. 2010, 117, 1544–1550. [Google Scholar] [CrossRef]
- Otrokhov, G.V.; Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Khlupova, M.E.; Yaropolov, A.I. Biocatalytic synthesis of conducting polymers and prospects for its application. Biochemistry 2013, 78, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Kashima, K.; Ćirić-Marjanović, G. Synthesizing polyaniline with laccase/O2 as catalyst. Front. Bioeng. Biotechnol. 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Naki, A.; Varfolomeev, S.D. Mechanism of the inhibition of laccase activity from Polyporus versicolor by halide-ions. Biochemistry 1981, 46, 1344–1350. Available online: https://pubmed.ncbi.nlm.nih.gov/7295828/ (accessed on 4 August 2022).
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-moghadam, E.; Khajeh, K.; Ghazic, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Pereira, M.; Freire, M.; Silva, J.; Coutinho, J.; Tavares, A. Laccase activation in deep eutectic solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814. [Google Scholar] [CrossRef]
- Delorme, A.E.; Andanson, J.M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. Int. J. Biol. Macromol. 2020, 163, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Karamyshev, A.V.; Shleev, S.V.; Koroleva, O.V.; Yaropolov, A.I.; Sakharov, I.Y. Laccase-catalyzed synthesis of conducting polyaniline. Enz. Microb. Technol. 2003, 33, 556–564. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Acevedo, D.F.; Miras, M.C.; Motheo, A.J.; Barbero, C.A. Comparative study of 2-amino and 3-aminobenzoic acid copolymerization with aniline synthesis and copolymer properties. J. Polym. Sci. Part A-1 Polym. Chem. 2004, 42, 5587–5599. [Google Scholar] [CrossRef]
- Robertson, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Swift, S. The antimicrobial action of polyaniline involves production of oxidative stress while functionalisation of polyaniline introduces additional mechanisms. PeerJ 2018, 6, e5135. [Google Scholar] [CrossRef] [PubMed]
- Gorshina, E.S.; Rusinova, T.V.; Biryukov, V.V.; Morozova, O.V.; Shleev, S.V.; Yaropolov, A.I. The dynamics of oxidase activity during cultivation of basidiomycetes from the genus Trametes Fr. Appl. Biochem. Microbiol. 2006, 42, 558–563. [Google Scholar] [CrossRef]
- Ehresmann, B.; Imbault, P.; Well, J.H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA’s and rRNA’s. Anal. Biochem. 1973, 54, 454–463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).