Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte
Abstract
1. Introduction
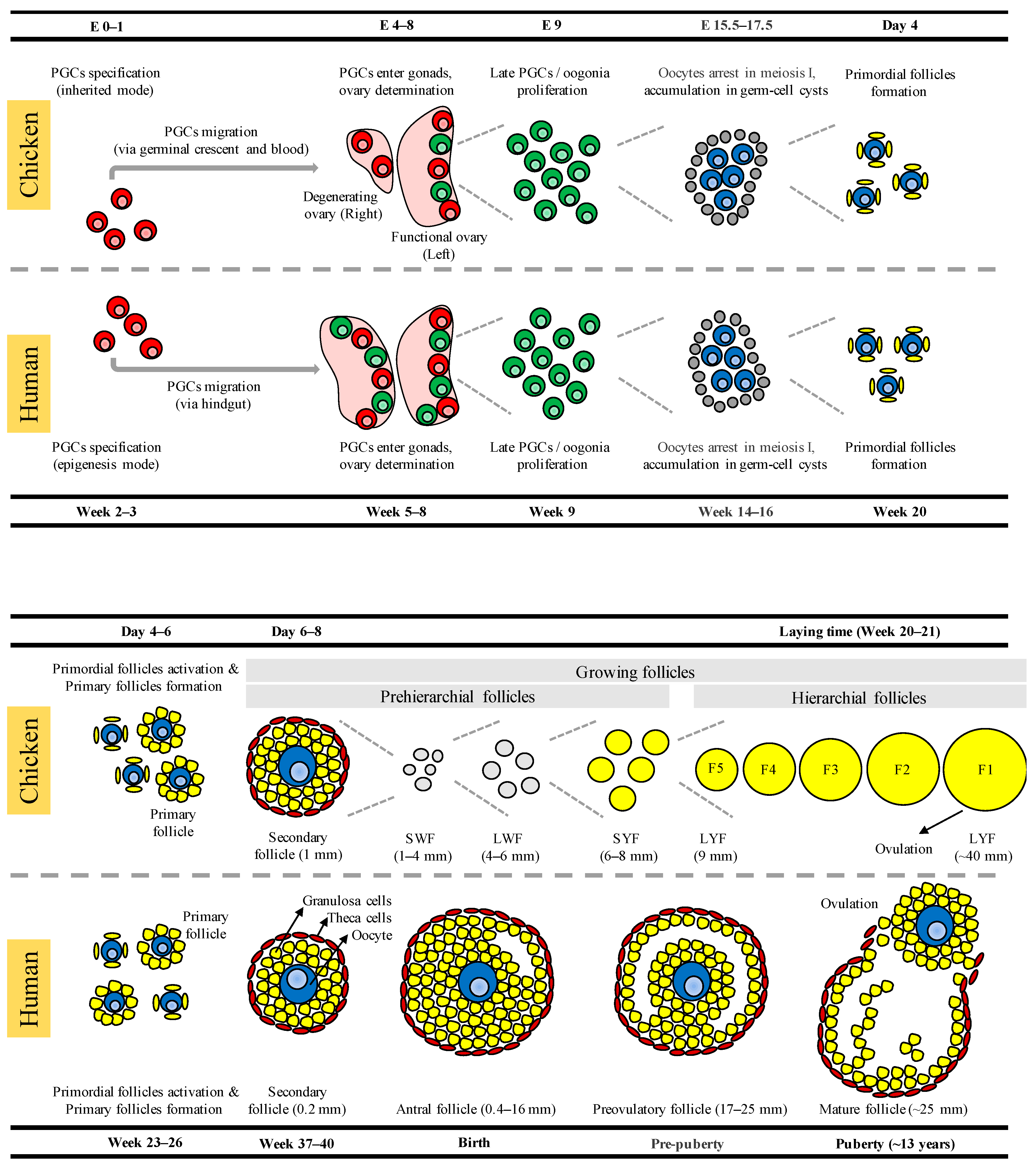
2. Development of Germ Cells, Oocytes, and Ovarian Follicles in Chickens
3. Development of Germ Cells, Oocytes, and Ovarian Follicles in Humans
4. Ovarian Follicles at Young and Old Ages
5. Transcriptome-Based Conservation of Female Germ Cell Development in Chickens and Humans
6. Chicken Oocyte Expressed Genes Convergent with the Human Oocyte
7. Chicken Oocyte Expressed Genes Divergent from the Human Oocyte
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamieson-Lucy, A.; Mullins, M.C. The vertebrate Balbiani body, germ plasm, and oocyte polarity. Curr. Top. Dev. Biol. 2019, 135, 1–34. [Google Scholar] [PubMed]
- Rengaraj, D.; Lee, B.R.; Lee, S.I.; Seo, H.W.; Han, J.Y. Expression patterns and miRNA regulation of DNA methyltransferases in chicken primordial germ cells. PLoS ONE 2011, 6, e19524. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Miyauchi, H. Gametogenesis from pluripotent stem cells. Cell Stem Cell 2016, 18, 721–735. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, K.M.; Rengaraj, D.; Lee, K.Y.; Yoo, E.; Kim, T.H.; Han, J.Y. Single-cell RNA sequencing of mitotic-arrested prospermatogonia with DAZL::GFP chickens and revealing unique epigenetic reprogramming of chickens. J. Anim. Sci. Biotechnol. 2022, 13, 64. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of dietary vitamin E on fertility functions in poultry species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef]
- Nitta, H.; Mason, J.I.; Bahr, J.M. Localization of 3 beta-hydroxysteroid dehydrogenase in the chicken ovarian follicle shifts from the theca layer to granulosa layer with follicular maturation. Biol. Reprod. 1993, 48, 110–116. [Google Scholar] [CrossRef]
- Lovell, T.M.; Gladwell, R.T.; Groome, N.P.; Knight, P.G. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers. J. Endocrinol. 2003, 177, 45–55. [Google Scholar] [CrossRef]
- Okumura, H. Avian egg and egg coat. Adv. Exp. Med. Biol. 2017, 1001, 75–90. [Google Scholar]
- Hamazaki, N.; Kyogoku, H.; Araki, H.; Miura, F.; Horikawa, C.; Hamada, N.; Shimamoto, S.; Hikabe, O.; Nakashima, K.; Kitajima, T.S.; et al. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 2021, 589, 264–269. [Google Scholar] [CrossRef]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic genome activation in vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hwang, Y.S.; Lee, H.C.; Han, J.Y. Zygotic genome activation in the chicken: A comparative review. Cell. Mol. Life Sci. 2020, 77, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, M.; Crichton, J.H.; Playfoot, C.J.; Adams, I.R. Oocyte development, meiosis and aneuploidy. Semin. Cell Dev. Biol. 2015, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chang, L.; Wang, Y.; Wang, R.; Hu, L.; Zhao, Z.; Geng, L.; Liu, Z.; Gong, Y.; Li, J.; et al. Genome-wide differential expression of long noncoding RNAs and mRNAs in ovarian follicles of two different chicken breeds. Genomics 2019, 111, 1395–1403. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Li, C.; Wu, X.; Si, S.J.; Yang, P.K.; Li, W.T.; Han, R.L.; Li, G.X.; Liu, X.J.; et al. Transcriptome identification and characterization of long non-coding RNAs in the ovary of hens at four stages. Anim. Biotechnol. 2022, 1–12, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, N.; Naito, M.; Sakai, Y.; Nishida, T.; Noce, T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000, 127, 2741–2750. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, H.C.; Kim, H.J.; Lee, H.G.; Kim, Y.M.; Lee, H.J.; Park, Y.H.; Yang, S.Y.; Rengaraj, D.; Park, T.S.; et al. Spatial and temporal action of chicken primordial germ cells during initial migration. Reproduction 2015, 149, 179–187. [Google Scholar] [CrossRef]
- Lee, H.C.; Choi, H.J.; Lee, H.G.; Lim, J.M.; Ono, T.; Han, J.Y. DAZL expression explains origin and central formation of primordial germ cells in chickens. Stem Cells Dev. 2016, 25, 68–79. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, J.Y. The early development of germ cells in chicken. Int. J. Dev. Biol. 2018, 62, 145–152. [Google Scholar] [CrossRef]
- Stebler, J.; Spieler, D.; Slanchev, K.; Molyneaux, K.A.; Richter, U.; Cojocaru, V.; Tarabykin, V.; Wylie, C.; Kessel, M.; Raz, E. Primordial germ cell migration in the chick and mouse embryo: The role of the chemokine SDF-1/CXCL12. Dev. Biol. 2004, 272, 351–361. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Rengaraj, D.; Choi, J.W.; Park, K.J.; Lee, S.I.; Han, J.Y. Expression pattern of meiosis associated SYCP family members during germline development in chickens. Reproduction 2009, 138, 483–492. [Google Scholar] [CrossRef]
- Rengaraj, D.; Lee, B.R.; Park, K.J.; Lee, S.I.; Kang, K.S.; Choi, J.W.; Kang, S.J.; Song, G.; Han, J.Y. The distribution of neuron-specific gene family member 1 in brain and germ cells: Implications for the regulation of germ-line development by brain. Dev. Dyn. 2011, 240, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lee, H.J.; Lee, H.C.; Hwang, Y.S.; Park, Y.H.; Ono, T.; Han, J.Y. The dynamic development of germ cells during chicken embryogenesis. Poult. Sci. 2018, 97, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.N.; Yan, L.Y.; Chen, Z.; Li, H.; Ying, S.J.; Zhu, H.X.; Shi, Z.D. Investigating right ovary degeneration in chick embryos by transcriptome sequencing. J. Reprod. Dev. 2017, 63, 295–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, C.A.; Roeszler, K.N.; Bowles, J.; Koopman, P.; Sinclair, A.H. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev. Biol. 2008, 8, 85. [Google Scholar] [CrossRef]
- Rengaraj, D.; Cha, D.G.; Lee, H.J.; Lee, K.Y.; Ha Choi, Y.; Jung, K.M.; Kim, Y.M.; Choi, H.J.; Choi, H.J.; Yoo, E.; et al. Dissecting chicken germ cell dynamics by combining a germ cell tracing transgenic chicken model with single-cell RNA sequencing. Comput. Struct. Biotecnol. J. 2022, 20, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, L.; Pigozzi, M.I. Chromosomal axis formation and meiotic progression in chicken oocytes: A quantitative analysis. Cytogenet. Genome Res. 2012, 137, 15–21. [Google Scholar] [CrossRef]
- Rengaraj, D.; Lee, S.I.; Yoo, M.; Kim, T.H.; Song, G.; Han, J.Y. Expression and knockdown analysis of glucose phosphate isomerase in chicken primordial germ cells. Biol. Reprod. 2012, 87, 57. [Google Scholar] [CrossRef]
- Guo, C.Q.; Liu, G.; Zhao, D.; Mi, Y.L.; Zhang, C.Q.; Li, J. Interaction of follicle-stimulating hormone and stem cell factor to promote primordial follicle assembly in the chicken. Front. Endocrinol. 2019, 10, 91. [Google Scholar] [CrossRef]
- Johnson, P.A. Follicle selection in the avian ovary. Reprod. Domest. Anim. 2012, 47, 283–287. [Google Scholar] [CrossRef]
- Nie, R.X.; Zheng, X.T.; Zhang, W.H.; Zhang, B.; Ling, Y.; Zhang, H.; Wu, C.X. Morphological characteristics and transcriptome landscapes of chicken follicles during selective development. Animals 2022, 12, 713. [Google Scholar] [CrossRef]
- Perry, M.M. Nuclear events from fertilization to the early cleavage stages in the domestic fowl (Gallus domesticus). J. Anat. 1987, 150, 99–109. [Google Scholar] [PubMed]
- Tang, W.W.C.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Campos, R.; Aguilar, E.; Cibelli, J.B. Progress towards human primordial germ cell specification in vitro. Mol. Hum. Reprod. 2017, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, N.; Hou, L.; Kim, R.; Faith, J.; Aslanyan, M.; Tao, Y.; Zheng, Y.; Fu, J.; Liu, W.; et al. Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. 2019, 29, 4568–4582.e5. [Google Scholar] [CrossRef] [PubMed]
- Sybirna, A.; Tang, W.W.C.; Smela, M.P.; Dietmann, S.; Gruhn, W.H.; Brosh, R.; Surani, M.A. A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat. Commun. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Singersam, J.; Goldstein, L.; Dai, A.H.; Gartler, S.M.; Riggs, A.D. A potentially critical Hpa-II site of the X-chromosome-linked PGK1 gene is unmethylated prior to the onset of meiosis of human oogenic cells. Proc. Natl. Acad. Sci. USA 1992, 89, 1413–1417. [Google Scholar] [CrossRef]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A comparative analysis of oocyte development in mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef]
- Jones, K.T. Meiosis in oocytes: Predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 2008, 14, 143–158. [Google Scholar] [CrossRef]
- Baudat, F.; Buard, J.; Grey, C.; Fledel-Alon, A.; Ober, C.; Przeworski, M.; Coop, G.; de Massy, B. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 2010, 327, 836–840. [Google Scholar] [CrossRef]
- Hertig, A.T. The primary human oocyte: Some observations on the fine structure of Balbiani’s vitelline body and the origin of the annulate lamellae. Am. J. Anat. 1968, 122, 107–137. [Google Scholar] [CrossRef]
- Pepling, M.E.; Wilhelm, J.E.; O’Hara, A.L.; Gephardt, G.W.; Spradling, A.C. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl. Acad. Sci. USA 2007, 104, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [CrossRef]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.L.; Wang, P.; Xie, B.T.; Yang, M.; Li, S.; Cui, Z.K.; Fan, Y.; Li, M.; Xiong, B. BRCA2 deficiency is a potential driver for human primary ovarian insufficiency. Cell Death Dis. 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Mcnatty, K.P. Hormonal correlates of follicular development in the human-ovary. Aust. J. Biol. Sci. 1981, 34, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variables. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- Mfoundou, J.D.L.; Guo, Y.J.; Liu, M.M.; Ran, X.R.; Fu, D.H.; Yan, Z.Q.; Li, M.N.; Wang, X.R. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult. Sci. 2021, 100, 101191. [Google Scholar] [CrossRef]
- Joyner, C.J.; Peddie, M.J.; Taylor, T.G. The effect of age on egg-production in the domestic hen. Gen. Comp. Endocr. 1987, 65, 331–336. [Google Scholar] [CrossRef]
- Cheng, S.B.; Li, X.Q.; Wang, J.X.; Wu, Y.; Li, P.; Pi, J.S. The effect of light on follicular development in laying hens. Anim. Biosci. 2021, 34, 1766–1775. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ansari-Pirsaraei, Z. Follicle Diameters, Egg weight, and egg production performance in old laying hens injected with growth hormone and testosterone. J. Agr. Sci. Tech. 2016, 18, 949–959. [Google Scholar]
- Amevor, F.K.; Cui, Z.F.; Du, X.X.; Ning, Z.F.; Shu, G.; Jin, N.N.; Deng, X.; Tian, Y.F.; Zhang, Z.C.; Kang, X.C.; et al. Combination of quercetin and vitamin E supplementation promotes yolk precursor synthesis and follicle development in aging breeder hens via liver-blood-ovary signal axis. Animals 2021, 11, 1915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Jia, R.; Gong, H.J.; Celi, P.; Zhuo, Y.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Yin, H.D.; Xu, S.Y.; et al. The effect of oxidative stress on the chicken ovary: Involvement of microbiota and melatonin interventions. Antioxidants 2021, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Ovarian follicular growth in humans: Ovarian ageing and population of growing follicles. Maturitas 1998, 30, 137–142. [Google Scholar] [CrossRef]
- Zhou, J.W.; Peng, X.W.; Mei, S.Q. Autophagy in ovarian follicular development and atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020, 19, e13259. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef]
- Bukovsky, A.; Caudle, M.R.; Svetlikova, M.; Wimalasena, J.; Ayala, M.E.; Dominguez, R. Oogenesis in adult mammals, including humans: A review. Endocrine 2005, 26, 301–316. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakamura, T.; Okamoto, K.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Seita, Y.; Nakamura, S.; Shiraki, N.; Takakuwa, T.; et al. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev. Cell 2016, 39, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Seo, M.; Lee, B.R.; Lee, H.J.; Park, Y.H.; Kim, S.K.; Lee, H.C.; Choi, H.J.; Yoon, J.; Kim, H.; et al. The transcriptome of early chicken embryos reveals signaling pathways governing rapid asymmetric cellularization and lineage segregation. Development 2018, 145, dev157453. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell 2017, 20, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.; Baird, D.D.; Weinberg, C.R.; McConnaughey, D.R.; Wilcox, A.J. Length of human pregnancy and contributors to its natural variation. Hum. Reprod. 2013, 28, 2848–2855. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Sheng, G.J. Day-1 chick development. Dev. Dyn. 2014, 243, 357–367. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Pollok, S.; Bauerschmidt, C.; Sanger, J.; Nasheuer, H.P.; Grosse, F. Human Cdc45 is a proliferation-associated antigen. FEBS J. 2007, 274, 3669–3684. [Google Scholar] [CrossRef]
- Porter, L.A.; Dellinger, R.W.; Tynan, J.A.; Barnes, E.A.; Kong, M.; Lenormand, J.L.; Donoghue, D.J. Human Speedy: A novel cell cycle regulator that enhances proliferation through activation of Cdk2. J. Cell Biol. 2002, 157, 357–366. [Google Scholar] [CrossRef]
- Romanienko, P.J.; Camerini-Otero, R.D. Cloning, characterization, and localization of mouse and human SPO11. Genomics 1999, 61, 156–169. [Google Scholar] [CrossRef]
- Bellani, M.A.; Boateng, K.A.; McLeod, D.; Camerini-Otero, R.D. The expression profile of the major mouse SPO11 isoforms indicates that SPO11 beta introduces double strand breaks and suggests that SPO11 alpha has an additional role in prophase in both spermatocytes and oocytes. Mol. Cell Biol. 2010, 30, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, J.; Chen, B.; Du, J.; Kuang, Y.; Sun, X.; Fu, J.; Li, B.; Mu, J.; Zhang, Z.; et al. Homozygous mutations in REC114 cause female infertility characterised by multiple pronuclei formation and early embryonic arrest. J. Med. Genet. 2020, 57, 187–194. [Google Scholar] [CrossRef]
- Ribeiro, J.; Dupaigne, P.; Petrillo, C.; Duquenne, C.; Veaute, X.; Saintome, C.; Busso, D.; Guerois, R.; Martini, E.; Livera, G. The meiosis-specific MEIOB-SPATA22 complex cooperates with RPA to form a compacted mixed MEIOB/SPATA22/RPA/ssDNA complex. DNA Repair 2021, 102, 103097. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.C.; Hou, D.; Ji, Z.Y.; Pang, D.M.; Li, P.; Tian, R.H.; Zhang, Y.X.; Ou, N.J.; Bai, H.W.; Zhi, E.L.; et al. Bi-allelic SPATA22 variants cause premature ovarian insufficiency and nonobstructive azoospermia due to meiotic arrest. Clin. Genet. 2022, 101, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cruz, R.; Brieno, M.A.; Roig, I.; Grossmann, M.; Velilla, E.; Pujol, A.; Cabero, L.; Pessarrodona, A.; Barbero, J.L.; Garcia Caldes, M. Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum. Reprod. 2010, 25, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.R.; Maman, J.D.; Pellegrini, L. Structural analysis of the human SYCE2-TEX12 complex provides molecular insights into synaptonemal complex assembly. Open Biol. 2012, 2, 120099. [Google Scholar] [CrossRef] [PubMed]
- Sciurano, R.B.; Pigozzi, M.I.; Benavente, R. Disassembly of the synaptonemal complex in chicken oocytes analyzed by super-resolution microscopy. Chromosoma 2019, 128, 443–451. [Google Scholar] [CrossRef]
- Kurahashi, H.; Kogo, H.; Tsutsumi, M.; Inagaki, H.; Ohye, T. Failure of homologous synapsis and sex-specific reproduction problems. Front. Genet. 2012, 3, 112. [Google Scholar] [CrossRef]
- Homer, H.A.; McDougall, A.; Levasseur, M.; Murdoch, A.P.; Herbert, M. RNA interference in meiosis I human oocytes: Towards an understanding of human aneuploidy. Mol. Hum. Reprod. 2005, 11, 397–404. [Google Scholar] [CrossRef]
- Gromley, A.; Jurczyk, A.; Sillibourne, J.; Halilovic, E.; Mogensen, M.; Groisman, I.; Blomberg, M.; Doxsey, S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 2003, 161, 535–545. [Google Scholar] [CrossRef]
- Sun, T.Y.; Wang, H.Y.; Kwon, J.W.; Yuan, B.; Lee, I.W.; Cui, X.S.; Kim, N.H. Centriolin, a centriole-appendage protein, regulates peripheral spindle migration and asymmetric division in mouse meiotic oocytes. Cell Cycle 2017, 16, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, X.; Huang, J.; Gao, Y.; Wang, D.; Feng, Z.; Zhai, G.; Lou, Q.; He, J.; Wang, Z.; et al. Rbm46, a novel germ cell-specific factor, modulates meiotic progression and spermatogenesis. Biol. Reprod. 2021, 104, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.; Yang, Q.; Marzluff, W.F.; Clarke, H.J. The stem-loop binding protein regulates translation of histone mRNA during mammalian oogenesis. Dev. Biol. 2005, 286, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Leu, N.A.; Eckardt, S.; McLaughlin, K.J.; Wang, P.J. STK31/TDRD8, a germ cell-specific factor, is dispensable for reproduction in mice. PLoS ONE 2014, 9, e89471. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, F.; Gutierrez, C.G.; Hartshorne, G.M. Apoptosis in mouse fetal and neonatal oocytes during meiotic prophase one. BMC Dev. Biol. 2007, 7, 87. [Google Scholar] [CrossRef]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- Pepling, M.E.; Spradling, A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001, 234, 339–351. [Google Scholar] [CrossRef]
- Higgins, J.; Midgley, C.; Bergh, A.M.; Bell, S.M.; Askham, J.M.; Roberts, E.; Binns, R.K.; Sharif, S.M.; Bennett, C.; Glover, D.M.; et al. Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol. 2010, 11, 85. [Google Scholar] [CrossRef]
- Raemaekers, T.; Ribbeck, K.; Beaudouin, J.; Annaert, W.; Van Camp, M.; Stockmans, I.; Smets, N.; Bouillon, R.; Ellenberg, J.; Carmeliet, G. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J. Cell Biol. 2003, 162, 1017–1029. [Google Scholar] [CrossRef]
- Koffa, M.D.; Casanova, C.M.; Santarella, R.; Kocher, T.; Wilm, M.; Mattaj, I.W. HURP is part of a ran-dependent complex involved in spindle formation. Curr. Biol. 2006, 16, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Yu, S.Q.; Sun, X.W.; Yin, M.C.; Fei, J.; Zhou, J.W. Identification of key biomarkers associated with development and prognosis in patients with ovarian carcinoma: Evidence from bioinformatic analysis. J. Ovarian Res. 2019, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Dou, X.L.; You, X.; Shi, Q.M.; Ke, M.J.; Liu, G.D. Pan-cancer analysis of the oncogenic role of discs large homolog associated protein 5 (DLGAP5) in human tumors. Cancer Cell Int. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Bendre, S.; Rondelet, A.; Hall, C.; Schmidt, N.; Lin, Y.C.; Brouhard, G.J.; Bird, A.W. GTSE1 tunes microtubule stability for chromosome alignment and segregation by inhibiting the microtubule depolymerase MCAK. J. Cell Biol. 2016, 215, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Desai, A.; Cleveland, D.W. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. 2005, 170, 873–880. [Google Scholar] [CrossRef]
- Chen, R.H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 2002, 158, 487–496. [Google Scholar] [CrossRef]
- Watanabe, R.; Hara, M.; Okumura, E.I.; Herve, S.; Fachinetti, D.; Ariyoshi, M.; Fukagawa, T. CDK1-mediated CENP-C phosphorylation modulates CENP-A binding and mitotic kinetochore localization. J. Cell Biol. 2019, 218, 4042–4062. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.; Lee, S.; Park, B.; Koh, W.; Lee, D.J.; Lim, D.S.; Lee, S. Cancer-upregulated gene 2 (CUG2), a new component of centromere complex, is required for kinetochore function. Mol. Cells 2009, 27, 697–701. [Google Scholar] [CrossRef]
- Sun, S.C.; Lee, S.E.; Xu, Y.N.; Kim, N.H. Perturbation of Spc25 expression affects meiotic spindle organization, chromosome alignment and spindle assembly checkpoint in mouse oocytes. Cell Cycle 2010, 9, 4552–4559. [Google Scholar] [CrossRef]
- Crane, R.; Gadea, B.; Littlepage, L.; Wu, H.; Ruderman, J.V. Aurora A, meiosis and mitosis. Biol. Cell 2004, 96, 215–229. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Schindler, K. Specialize and divide (twice): Functions of three aurora kinase homologs in mammalian oocyte meiotic maturation. Trends Genet. 2017, 33, 349–363. [Google Scholar] [CrossRef]
- Murphy, L.A.; Sarge, K.D. Phosphorylation of CAP-G is required for its chromosomal DNA localization during mitosis. Biochem. Biophys. Res. Commun. 2008, 377, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Trinkle-Mulcahy, L.; Andersen, J.; Lam, Y.W.; Moorhead, G.; Mann, M.; Lamond, A.I. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J. Cell Biol. 2006, 172, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Vagnarelli, P.; Hudson, D.F.; Ribeiro, S.A.; Trinkle-Mulcahy, L.; Spence, J.M.; Lai, F.; Farr, C.J.; Lamond, A.I.; Earnshaw, W.C. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat. Cell Biol. 2006, 8, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, L.; Huang, Y.; Zhao, H.; Luo, Y. PBK/TOPK: A therapeutic target worthy of attention. Cells 2021, 10, 371. [Google Scholar] [CrossRef]
- Assou, S.; Cerecedo, D.; Tondeur, S.; Pantesco, V.; Hovatta, O.; Klein, B.; Hamamah, S.; De Vos, J. A gene expression signature shared by human mature oocytes and embryonic stem cells. BMC Genomics 2009, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Balakier, H.; Xiao, R.; Zhao, J.; Zaver, S.; Dziak, E.; Szczepanska, K.; Opas, M.; Yie, S.; Librach, C. Expression of survivin in human oocytes and preimplantation embryos. Fertil. Steril. 2013, 99, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Rungaldier, S.; Umlauf, E.; Mairhofer, M.; Salzer, U.; Thiele, C.; Prohaska, R. Structure-function analysis of human stomatin: A mutation study. PLoS ONE 2017, 12, e0178646. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef]
- Sauvageau, E.; Rochdi, M.D.; Oueslati, M.; Hamdan, F.F.; Percherancier, Y.; Simpson, J.C.; Pepperkok, R.; Bouvier, M. CNIH4 interacts with newly synthesized GPCR and controls their export from the endoplasmic reticulum. Traffic 2014, 15, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Zahraoui, A.; Touchot, N.; Chardin, P.; Tavitian, A. The human Rab genes encode a family of GTP-binding proteins related to yeast Ypt1 and Sec4 products involved in secretion. J. Biol. Chem. 1989, 264, 12394–12401. [Google Scholar] [CrossRef]
- Tsunedomi, R.; Yoshimura, K.; Kimura, Y.; Nishiyama, M.; Fujiwara, N.; Matsukuma, S.; Kanekiyo, S.; Matsui, H.; Shindo, Y.; Watanabe, Y.; et al. Elevated expression of RAB3B plays important roles in chemoresistance and metastatic potential of hepatoma cells. BMC Cancer 2022, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.L.; Liu, Y.P.; Yuan, Z.L.; Huang, L.S.; Diao, B. Expression of Rab3b in human glioma: Influence on cell proliferation and apoptosis. Curr. Pharm. Design 2021, 27, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Ji, C.; Gu, S.; Lv, Y.; Li, S.; Xu, Y.; Xie, Y.; Mao, Y. Cloning, expression and characterization of human glutathione S-transferase Omega 2. Int. J. Mol. Med. 2005, 16, 19–27. [Google Scholar] [CrossRef]
- Markovich, D.; Regeer, R.R. Expression of membrane transporters in cane toad Bufo marinus oocytes. J. Exp. Biol. 1999, 202, 2217–2223. [Google Scholar] [CrossRef]
- Hallak, J.; Sharma, R.K.; Pasqualotto, F.F.; Ranganathan, P.; Thomas, A.J.; Agarwal, A. Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology 2001, 58, 446–451. [Google Scholar] [CrossRef]
- Das, S.C.; Isobe, N.; Yoshimura, Y. Changes in the expression of interleukin-1beta and lipopolysaccharide-induced TNF factor in the oviduct of laying hens in response to artificial insemination. Reproduction 2009, 137, 527–536. [Google Scholar] [CrossRef]
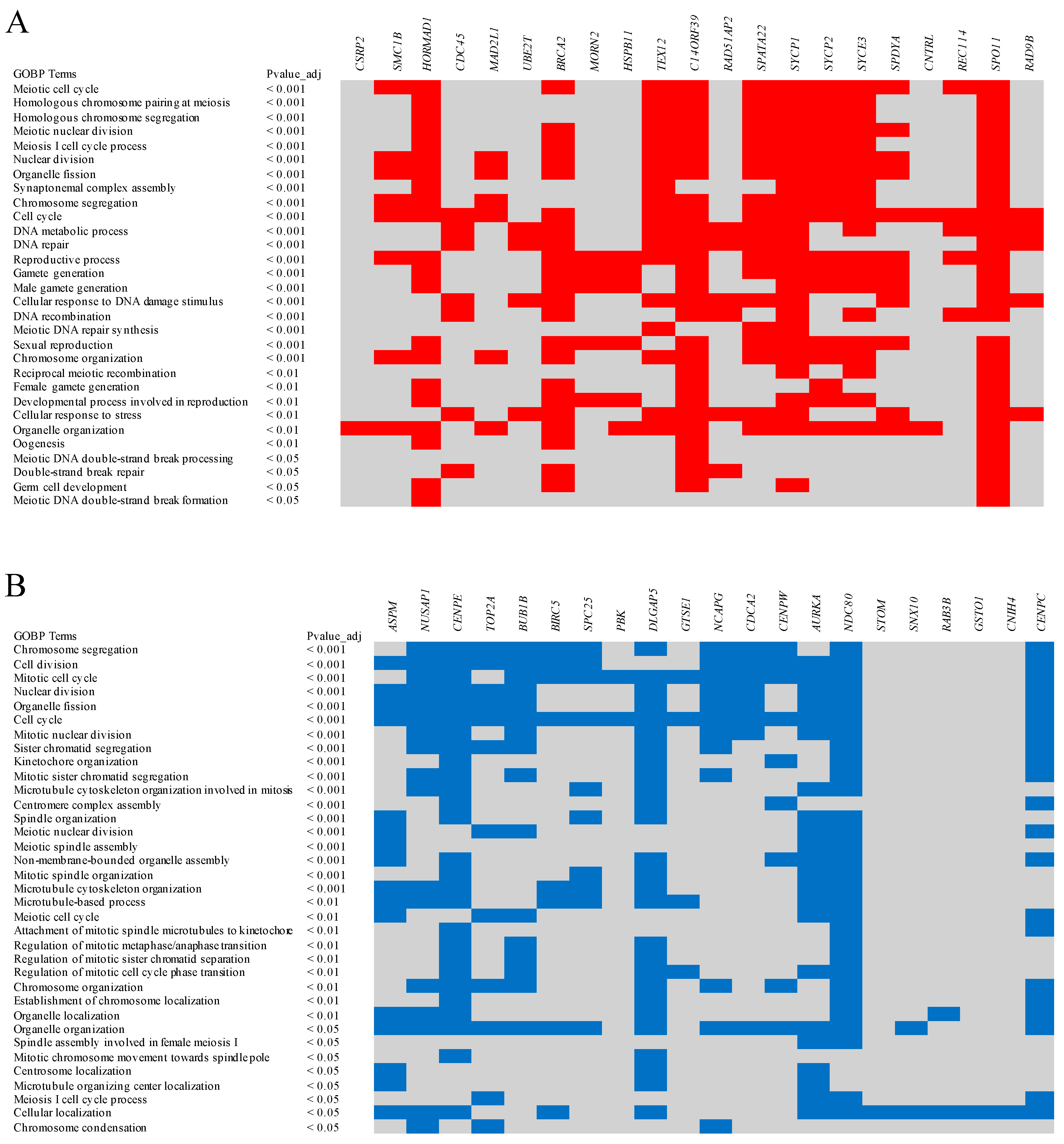

| Gene | Description | Enriched in Chicken | Enriched in Human | Chromosome in Chicken | Chromosome in Human |
|---|---|---|---|---|---|
| Convergent genes | |||||
| CSRP2 | Cysteine and glycine rich protein 2 | Oocytes | Oocytes | Chr.1 | Chr.12 |
| SMC1B | Structural maintenance of chromosomes 1B | Oocytes | Oocytes | Chr.1 | Chr.22 |
| HORMAD1 | HORMA domain containing 1 | Oocytes | Oocytes | Chr.25 | Chr.1 |
| RBM46 | RNA binding motif protein 46 | Oocytes | Oocytes | Chr.4 | Chr.4 |
| SLBP | Stem-loop binding protein | Oocytes | Oocytes | Chr.4 | Chr.4 |
| DEPDC1 | DEP domain containing 1 | Oocytes | Oocytes | Chr.8 | Chr.1 |
| CDC45 | Cell division cycle 45 | Oocytes | Oocytes | Chr.15 | Chr.22 |
| MAD2L1 | Mitotic arrest deficient 2 like 1 | Oocytes | Oocytes | Chr.4 | Chr.4 |
| UBE2T | Ubiquitin conjugating enzyme E2 T | Oocytes | Oocytes | Chr.26 | Chr.1 |
| BRCA2 | BRCA2, DNA repair associated | Oocytes | Oocytes | Chr.1 | Chr.13 |
| STK31 | Serine/threonine kinase 31 | Oocytes | Oocytes | Chr.2 | Chr.7 |
| GLCCI1 | Glucocorticoid induced 1 | Oocytes | Oocytes | Chr.2 | Chr.7 |
| ZMAT1 | Zinc finger matrin-type 1 | Oocytes | Oocytes | Chr.4 | Chr.X |
| MORN2 | MORN repeat containing 2 | Oocytes | Oocytes | Chr.3 | Chr.2 |
| HSPB11 | Heat shock protein family B (small) member 11 | Oocytes | Oocytes | Chr.8 | Chr.1 |
| TEX12 | Testis expressed 12 | Oocytes | Oocytes | Chr.24 | Chr.11 |
| C14ORF39 | Chromosome 14 open reading frame 39 | Oocytes | Oocytes | Chr.5 | Chr.14 |
| RAD51AP2 | RAD51 associated protein 2 | Oocytes | Oocytes | Chr.3 | Chr.2 |
| SPATA22 | Spermatogenesis associated 22 | Oocytes | Oocytes | Chr.19 | Chr.17 |
| CCDC73 | Coiled-coil domain containing 73 | Oocytes | Oocytes | Chr.5 | Chr.11 |
| SYCP1 | Synaptonemal complex protein 1 | Oocytes | Oocytes | Chr.26 | Chr.1 |
| SYCP2 | Synaptonemal complex protein 2 | Oocytes | Oocytes | Chr.20 | Chr.20 |
| SYCE3 | Synaptonemal complex central element protein 3 | Oocytes | Oocytes | Chr.1 | Chr.22 |
| C18ORF63 | Chromosome 18 open reading frame 63 | Oocytes | Oocytes | Chr.2 | Chr.18 |
| SPDYA | Speedy/RINGO cell cycle regulator family member A | Oocytes | Oocytes | Chr.3 | Chr.2 |
| CNTRL | Centriolin | Oocytes | Oocytes | Chr.17 | Chr.9 |
| REC114 | REC114 meiotic recombination protein | Oocytes | Oocytes | Chr.10 | Chr.15 |
| SPO11 | SPO11, initiator of meiotic double stranded breaks | Oocytes | Oocytes | Chr.20 | Chr.20 |
| RAD9B | RAD9 checkpoint clamp component B | Oocytes | Oocytes | Chr.15 | Chr.12 |
| Divergent genes | |||||
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 | Oocytes | Mitotic GCs | Chr.1 | Chr.X |
| CKB | Creatine kinase B | Oocytes | Mitotic GCs | Chr.5 | Chr.14 |
| ASPM | Abnormal spindle microtubule assembly | Oocytes | Mitotic GCs | Chr.8 | Chr.1 |
| NUSAP1 | Nucleolar and spindle associated protein 1 | Oocytes | Mitotic GCs | Chr.5 | Chr.15 |
| CENPE | Centromere protein E | Oocytes | Mitotic GCs | Chr.4 | Chr.4 |
| TOP2A | Topoisomerase (DNA) II alpha | Oocytes | Mitotic GCs | Chr.27 | Chr.17 |
| BUB1B | BUB1 mitotic checkpoint serine/threonine kinase B | Oocytes | Mitotic GCs | Chr.5 | Chr.15 |
| BIRC5 | Baculoviral IAP repeat containing 5 | Oocytes | Mitotic GCs | Chr.3 | Chr.17 |
| SPC25 | SPC25, NDC80 kinetochore complex component | Oocytes | Mitotic GCs | Chr.7 | Chr.2 |
| PBK | PDZ binding kinase | Oocytes | Mitotic GCs | Chr.3 | Chr.8 |
| DLGAP5 | DLG associated protein | Oocytes | Mitotic GCs | Chr.5 | Chr.14 |
| GTSE1 | G2 and S-phase expressed 1 | Oocytes | Mitotic GCs | Chr.1 | Chr.22 |
| NCAPG | Non-SMC condensin I complex subunit G | Oocytes | Mitotic GCs | Chr.4 | Chr.4 |
| CDCA2 | Cell division cycle associated 2 | Oocytes | Mitotic GCs | Chr.22 | Chr.8 |
| CENPW | Centromere protein W | Oocytes | Mitotic GCs | Chr.3 | Chr.6 |
| AURKA | Aurora kinase A | Oocytes | Mitotic GCs | Chr.20 | Chr.20 |
| NDC80 | NDC80, kinetochore complex component | Oocytes | Mitotic GCs | Chr.2 | Chr.18 |
| STOM | Stomatin | Oocytes | Mitotic GCs | Chr.17 | Chr.9 |
| MGST3 | Microsomal glutathione S-transferase 3 | Oocytes | Mitotic GCs | Chr.8 | Chr.1 |
| SNX10 | Sorting nexin 10 | Oocytes | Mitotic GCs | Chr.2 | Chr.7 |
| COA7 | Cytochrome c oxidase assembly factor 7 | Oocytes | Mitotic GCs | Chr.8 | Chr.1 |
| RAB3B | RAB3B, member RAS oncogene family | Oocytes | Mitotic GCs | Chr.8 | Chr.1 |
| LITAF | Lipopolysaccharide induced TNF factor | Oocytes | Mitotic GCs | Chr.14 | Chr.16 |
| GSTO1 | Glutathione S-transferase omega 1 | Oocytes | Mitotic GCs | Chr.6 | Chr.10 |
| SERPINI1 | Serpin family I member 1 | Oocytes | Mitotic GCs | Chr.9 | Chr.3 |
| CNIH4 | Cornichon family AMPA receptor auxiliary protein 4 | Oocytes | Mitotic GCs | Chr.3 | Chr.1 |
| CENPC | Centromere protein C | Oocytes | Mitotic GCs | Chr.4 | Chr.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengaraj, D.; Han, J.Y. Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte. Int. J. Mol. Sci. 2022, 23, 11412. https://doi.org/10.3390/ijms231911412
Rengaraj D, Han JY. Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte. International Journal of Molecular Sciences. 2022; 23(19):11412. https://doi.org/10.3390/ijms231911412
Chicago/Turabian StyleRengaraj, Deivendran, and Jae Yong Han. 2022. "Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte" International Journal of Molecular Sciences 23, no. 19: 11412. https://doi.org/10.3390/ijms231911412
APA StyleRengaraj, D., & Han, J. Y. (2022). Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte. International Journal of Molecular Sciences, 23(19), 11412. https://doi.org/10.3390/ijms231911412






