Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction
Abstract
1. Introduction
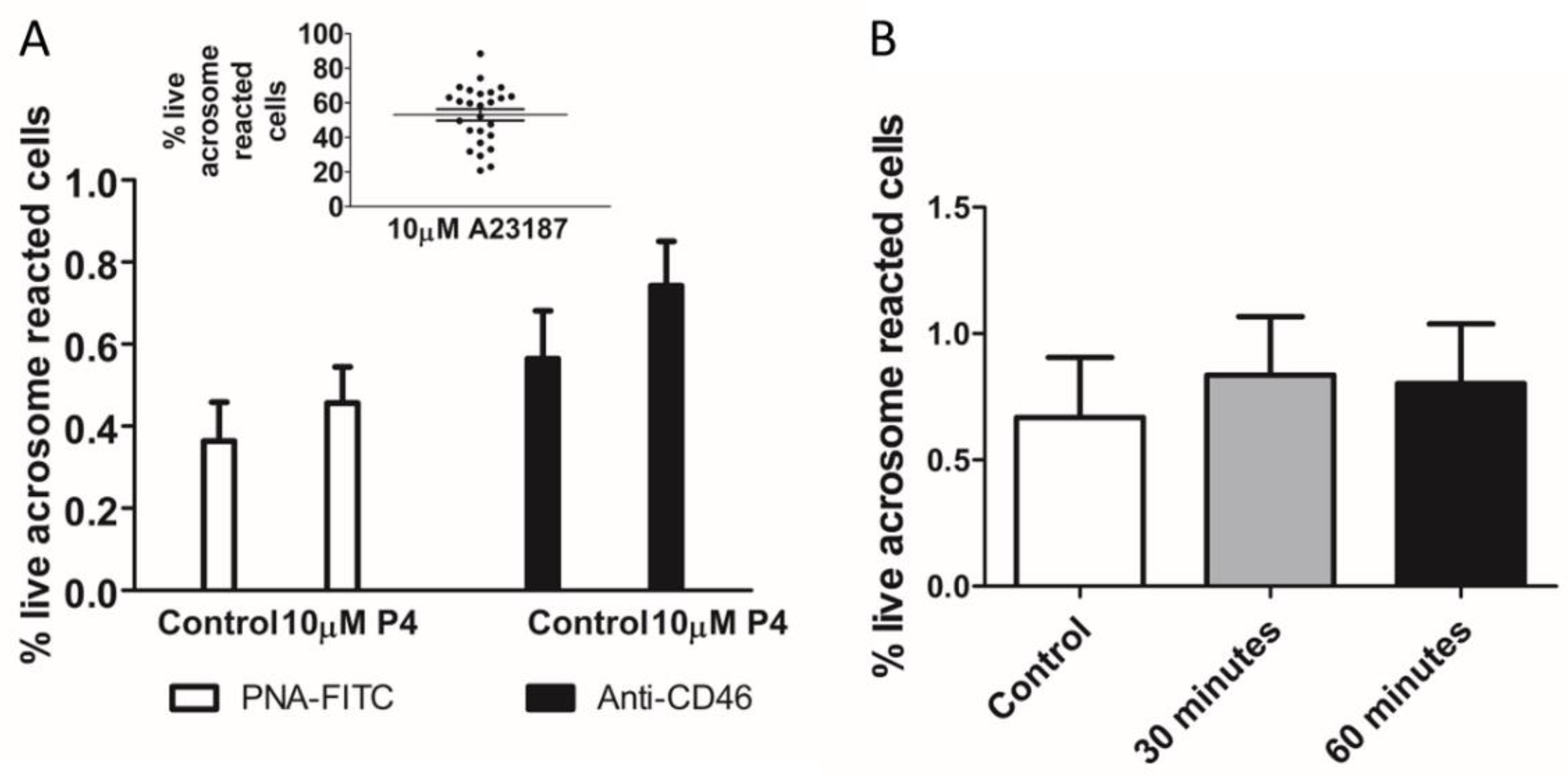
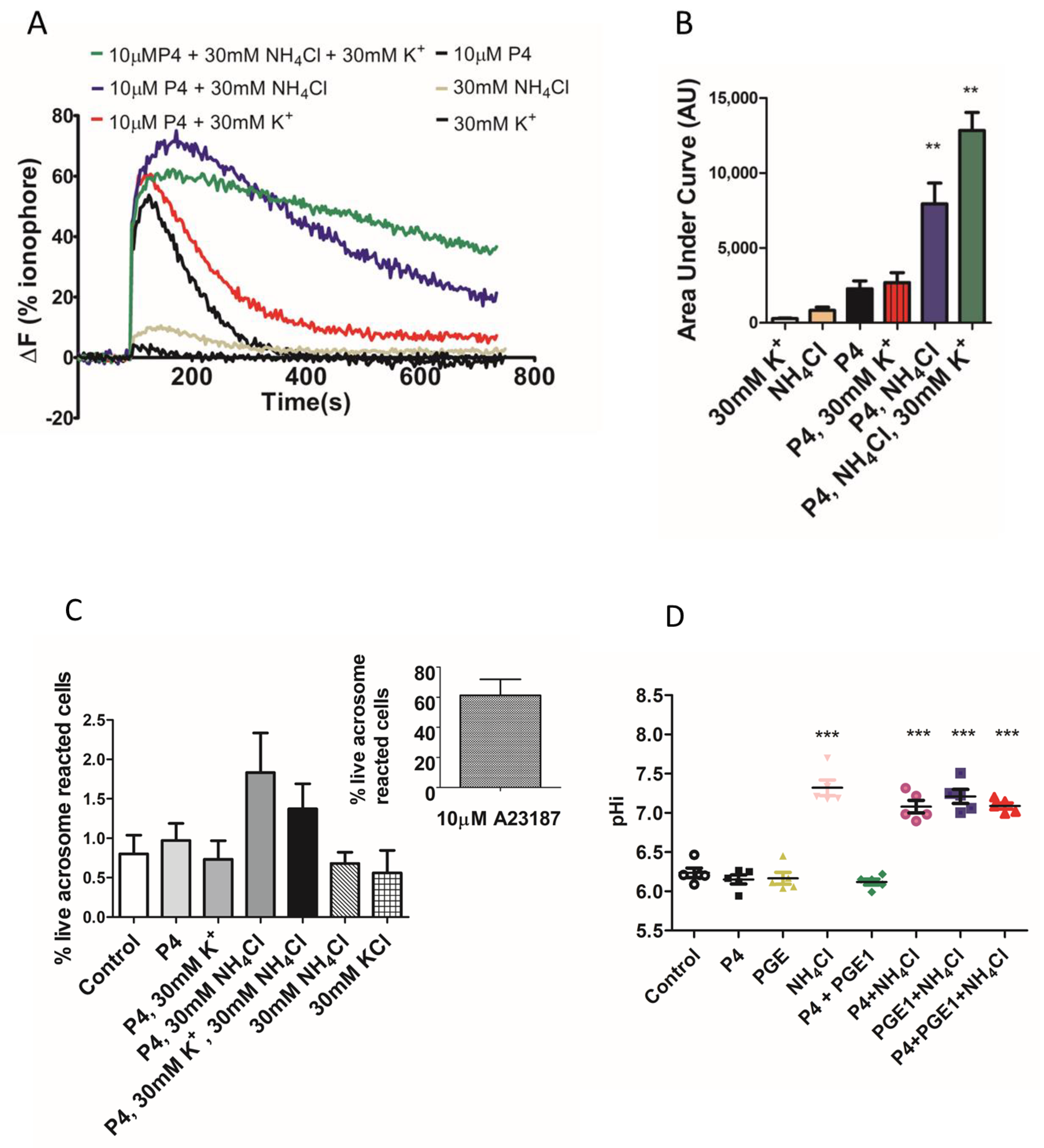
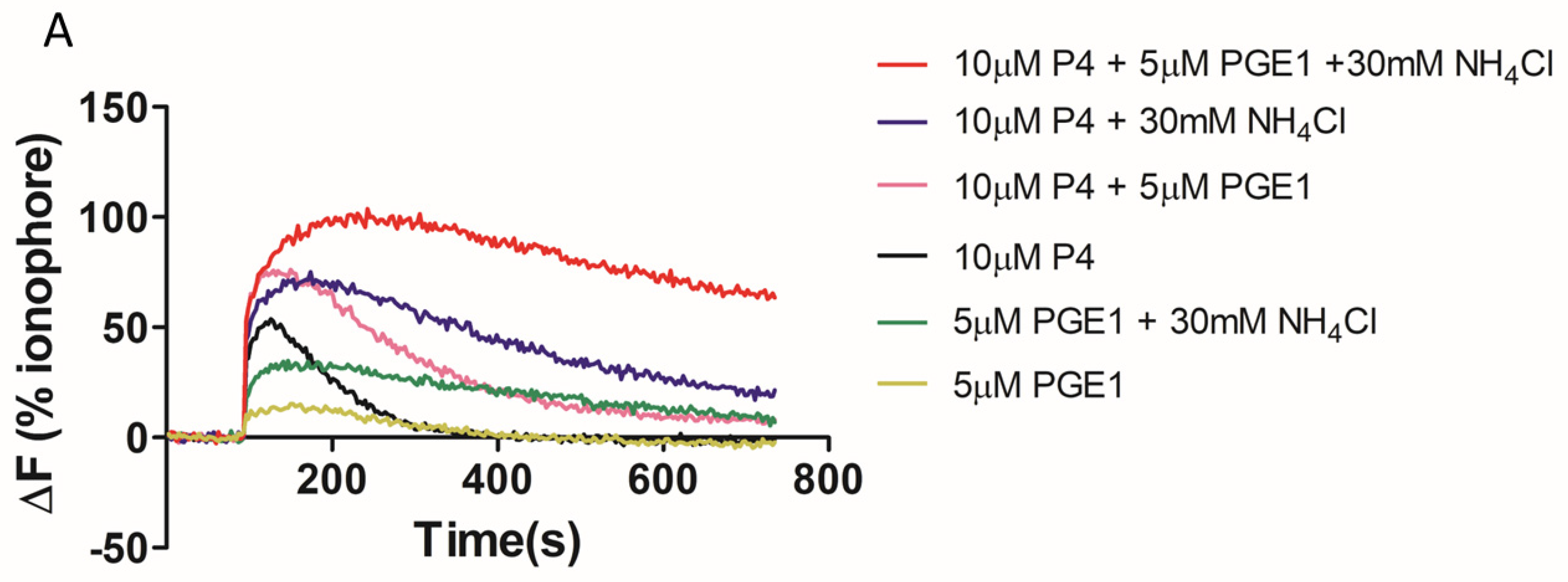
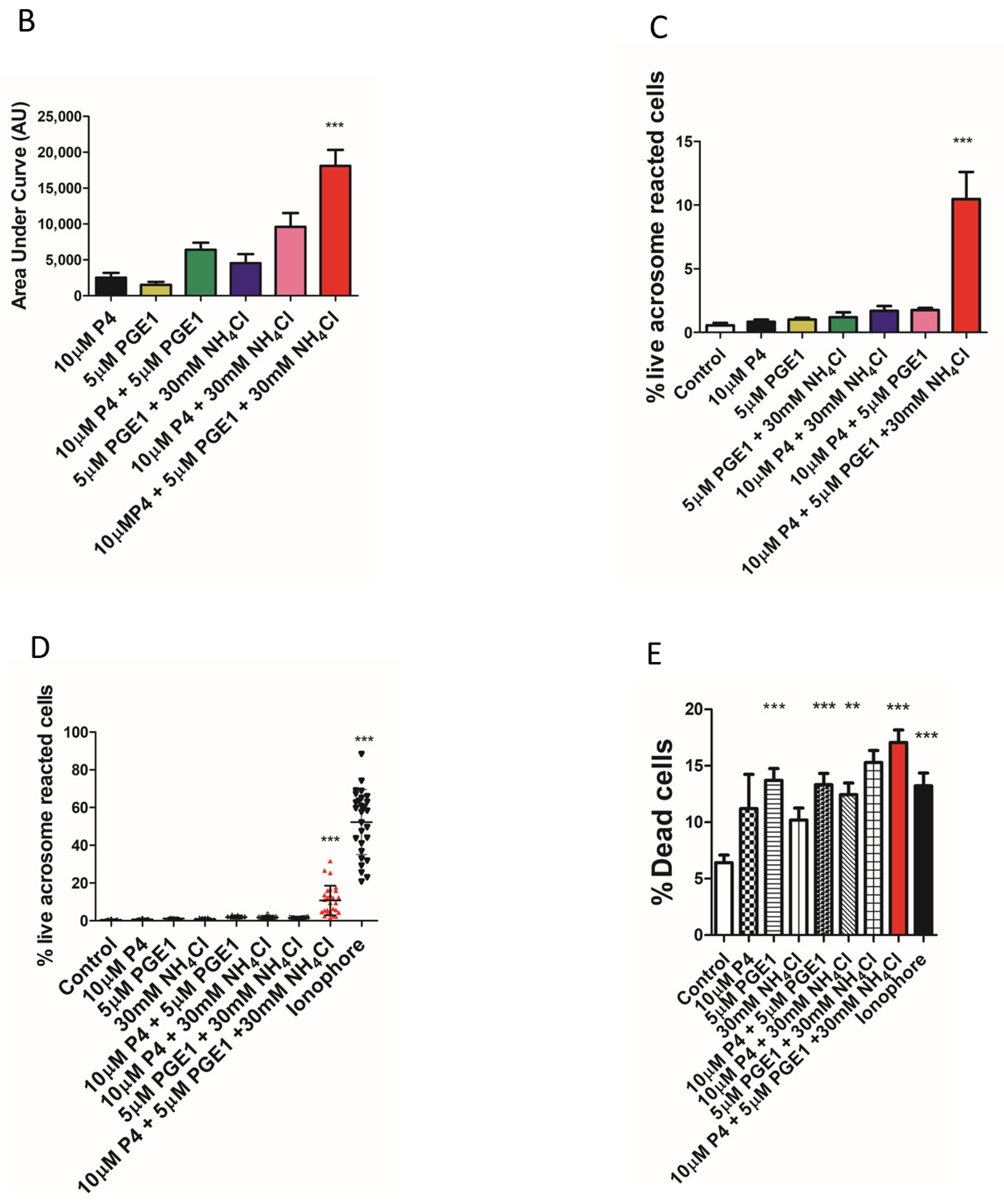
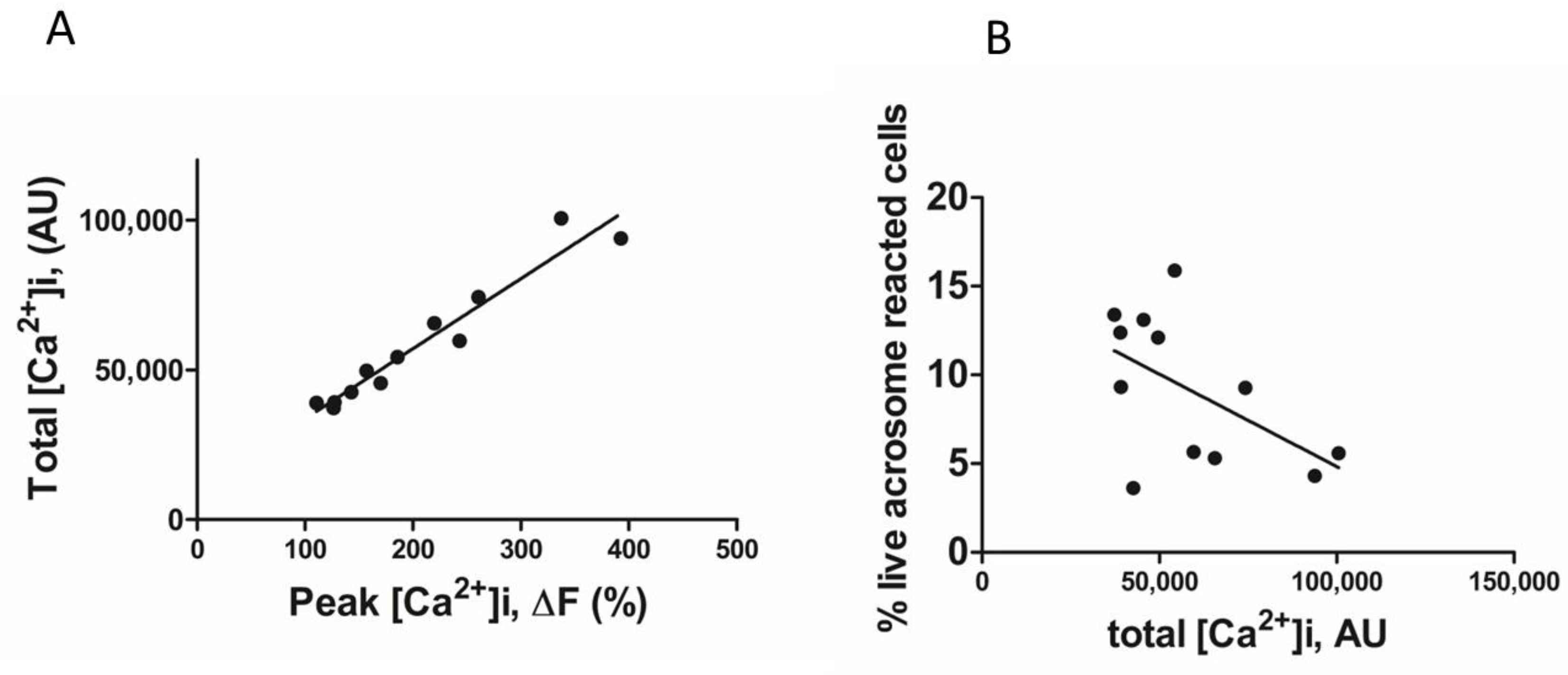
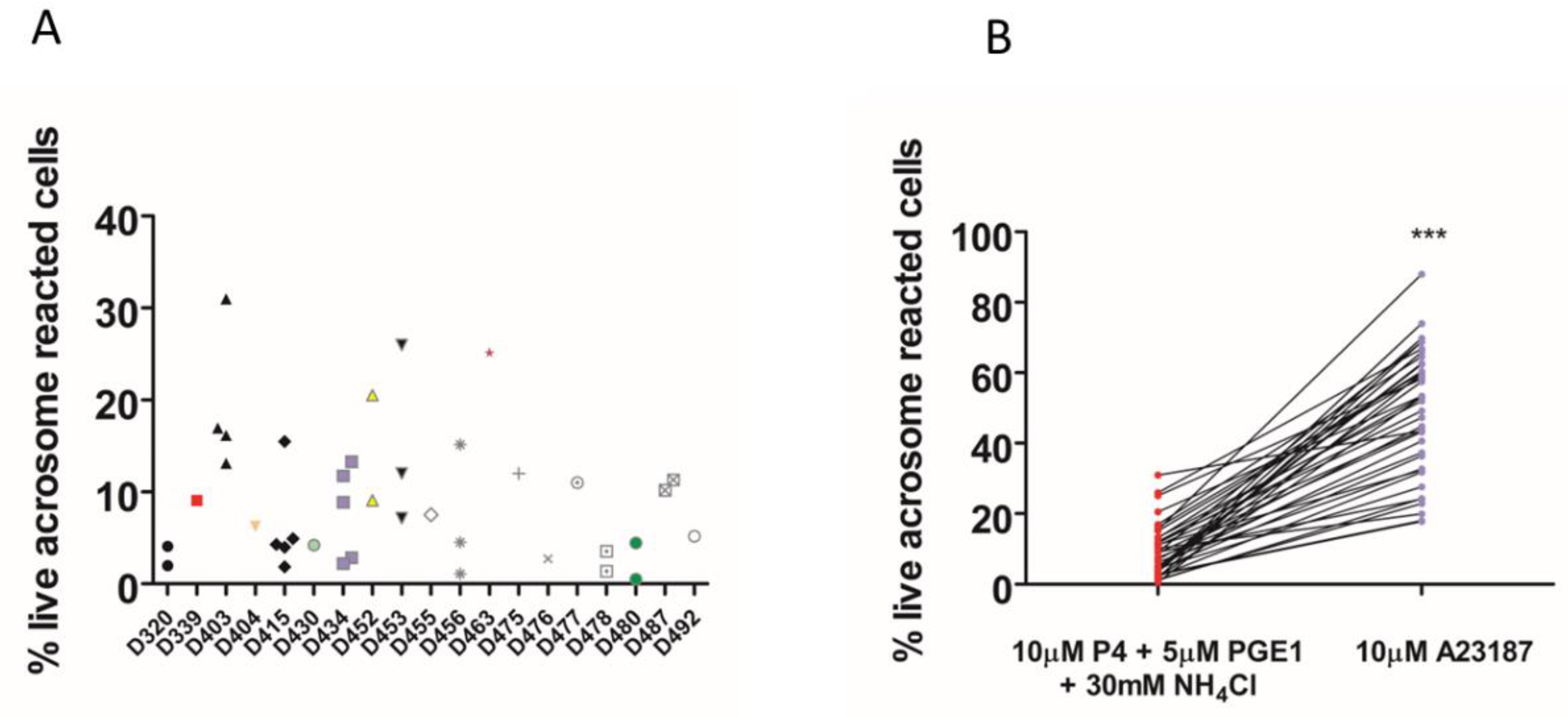
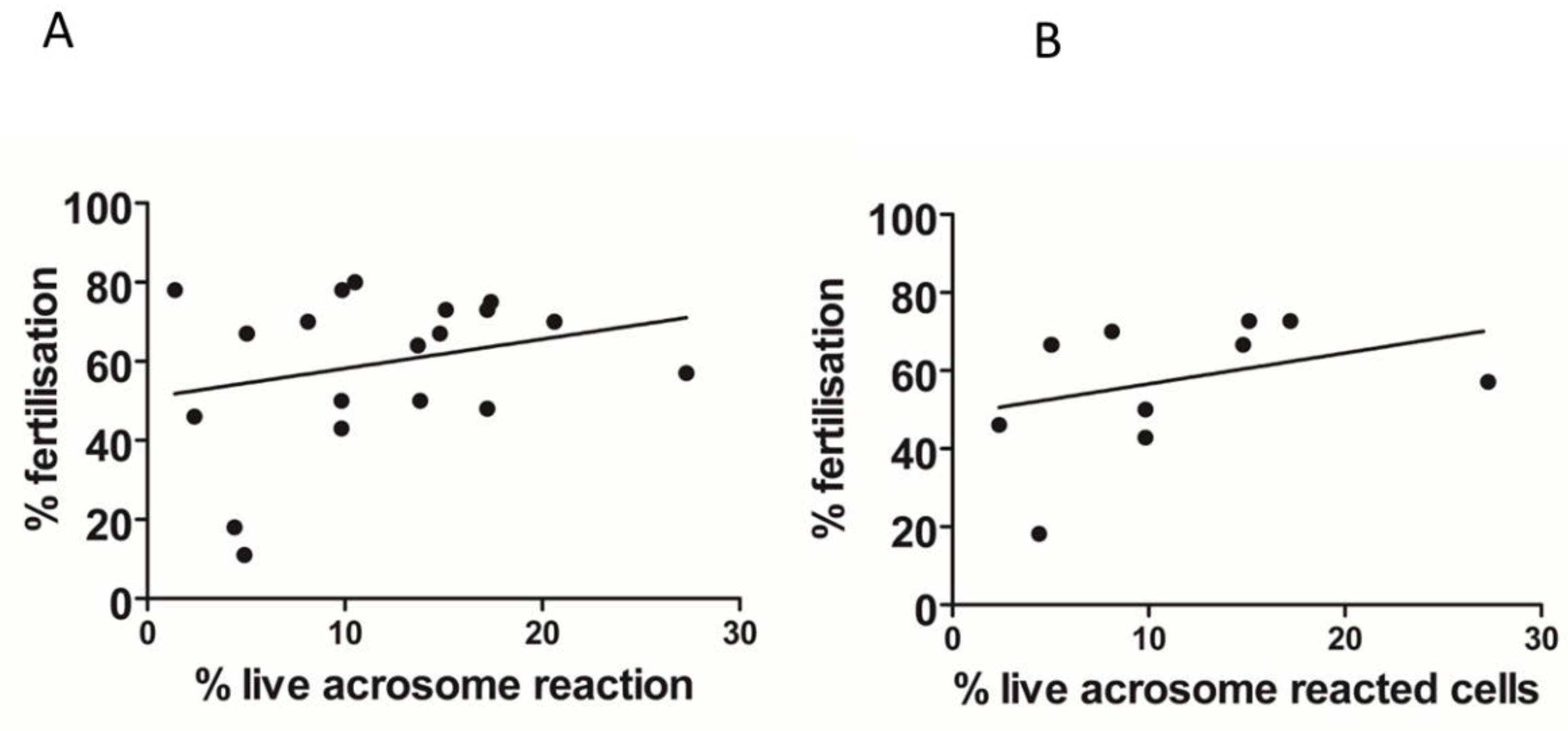
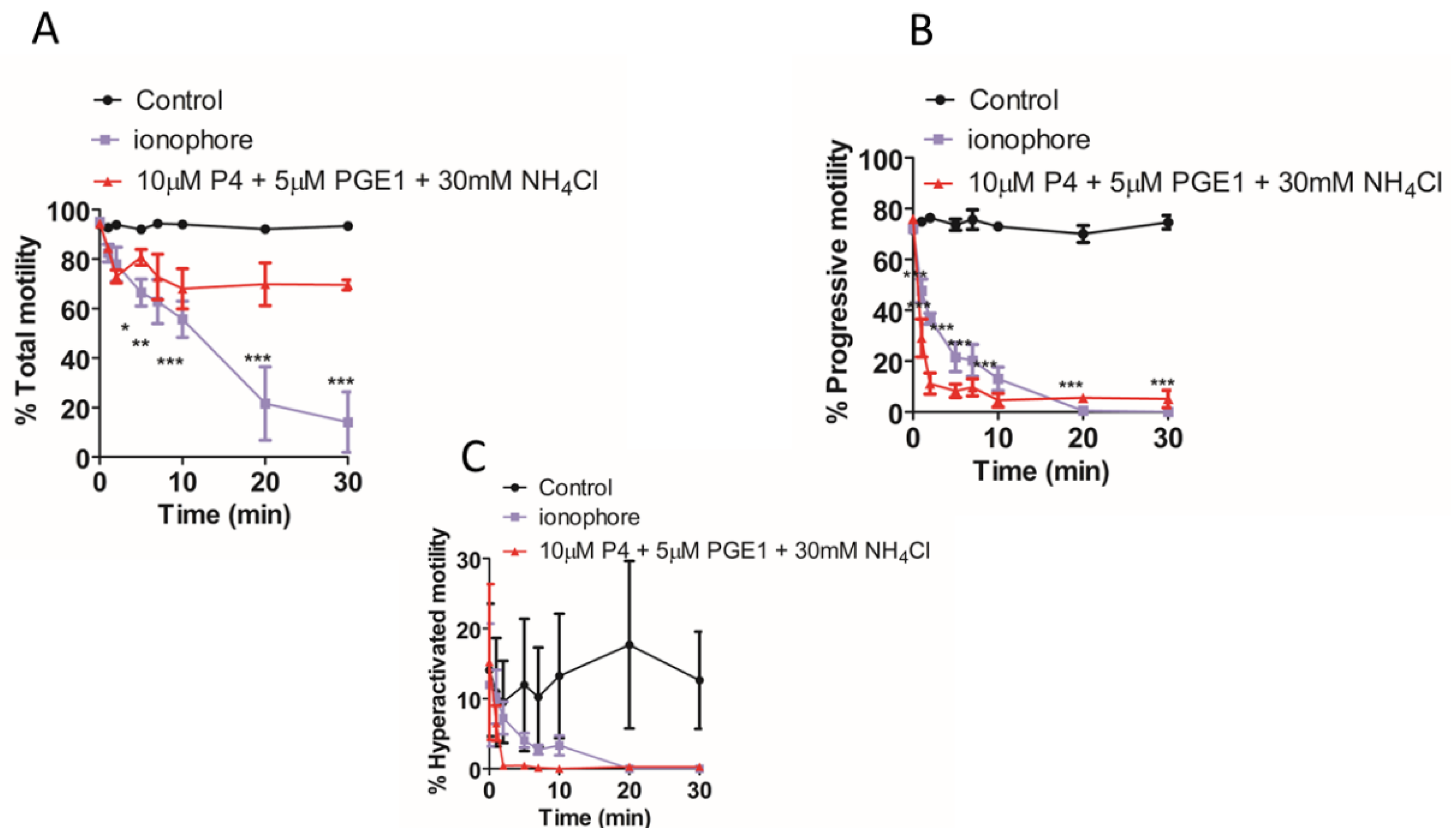
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Solutions
4.3. Sperm Samples
4.4. Preparation of Donor Samples
4.5. Preparation of Patient Sperm
4.6. Computer-Assisted Sperm Analysis
4.7. Determination of Acrosome Reaction by Flow Cytometry
4.8. Intracellular Calcium([Ca2+]i) Measurement
4.9. Intracellular pH (pHi) Measurements
4.10. Fertilization Rate at IVF
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulsiani, D.R.; Abou-Haila, A.; Loeser, C.R.; Pereira, B.M. The biological and functional significance of the sperm acrosome and acrosomal enzymes in mammalian fertilization. Exp. Cell Res. 1998, 240, 151–164. [Google Scholar] [CrossRef]
- Barbaux, S.; Ialy-Radio, C.; Chalbi, M.; Dybal, E.; Homps-Legrand, M.; Do Cruzeiro, M.; Vaiman, D.; Wolf, J.P.; Ziyyat, A. Sperm SPACA6 protein is required for mammalian Sperm-Egg Adhesion/Fusion. Sci. Rep. 2020, 10, 5335. [Google Scholar] [CrossRef] [PubMed]
- Bastiaan, H.S.; Menkveld, R.; Oehninger, S.; Franken, D.R. Zona pellucida induced acrosome reaction, sperm morphology, and sperm-zona binding assessments among subfertile men. J. Assist. Reprod. Genet. 2002, 19, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Schuffner, A.A.; Bastiaan, H.S.; Duran, H.E.; Lin, Z.Y.; Morshedi, M.; Franken, D.R.; Oehninger, S. Zona pellucida-induced acrosome reaction in human sperm: Dependency on activation of pertussis toxin-sensitive G(i) protein and extracellular calcium, and priming effect of progesterone and follicular fluid. Mol. Hum. Reprod. 2002, 8, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Franken, D.R.; Habenicht, U.F. Zona pellucida as physiological trigger for the induction of acrosome reaction. Andrologia 1998, 30, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tollner, T.L.; Yudin, A.I.; Cherr, G.N.; Overstreet, J.W. Real-time observations of individual macaque sperm undergoing tight binding and the acrosome reaction on the zona pellucida. Biol. Reprod. 2003, 68, 664–672. [Google Scholar] [CrossRef]
- Crozet, N.; Dumont, M. The site of the acrosome reaction during in vivo penetration of the sheep oocyte. Gamete Res. 1984, 10, 97–105. [Google Scholar] [CrossRef]
- Uto, N.; Yoshimatsu, N.; Lopata, A.; Yanagimachi, R. Zona-induced acrosome reaction of hamster spermatozoa. J. Exp. Zool. 1988, 248, 113–120. [Google Scholar] [CrossRef]
- Avella, M.A.; Dean, J. Fertilization with acrosome-reacted mouse sperm: Implications for the site of exocytosis. Proc. Natl. Acad. Sci. USA 2011, 108, 19843–19844. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Hino, T.; Muro, Y.; Tamura-Nakano, M.; Okabe, M.; Tateno, H.; Yanagimachi, R. The Behavior and Acrosomal Status of Mouse Spermatozoa In Vitro, and Within the Oviduct During Fertilization after Natural Mating. Biol. Reprod. 2016, 95, 50. [Google Scholar] [CrossRef] [PubMed]
- Muro, Y.; Hasuwa, H.; Isotani, A.; Miyata, H.; Yamagata, K.; Ikawa, M.; Yanagimachi, R.; Okabe, M. Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization. Biol. Reprod. 2016, 94, 80. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sathananthan, A.H. Early penetration of human sperm through the vestments of human eggs in vitro. Arch. Androl. 1986, 16, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Oren-Benaroya, R.; Orvieto, R.; Gakamsky, A.; Pinchasov, M.; Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 2008, 23, 2339–2345. [Google Scholar] [CrossRef]
- Sadat Tahajjodi, S.; Farashahi Yazd, E.; Agha-Rahimi, A.; Aflatoonian, R.; Ali Khalili, M.; Mohammadi, M.; Aflatoonian, B. Biological and physiological characteristics of human cumulus cells in adherent culture condition. Int. J. Reprod. Biomed. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Brown, S.G.; Costello, S.; Kelly, M.C.; Ramalingam, M.; Drew, E.; Publicover, S.J.; Barratt, C.L.R.; da Silva, S.M. Complex CatSper-dependent and independent [Ca2+]i signalling in human spermatozoa induced by follicular fluid. Hum. Reprod. 2017, 32, 1995–2006. [Google Scholar] [CrossRef]
- Jeschke, J.K.; Biagioni, C.; Schierling, T.; Wagner, I.V.; Borgel, F.; Schepmann, D.; Schuring, A.; Kulle, A.E.; Holterhus, P.M.; von Wolff, M.; et al. The Action of Reproductive Fluids and Contained Steroids, Prostaglandins, and Zn2+ on CatSper Ca2+ Channels in Human Sperm. Front. Cell Dev. Biol. 2021, 9, 699554. [Google Scholar] [CrossRef]
- Taiwo, B.G.; Frettsome-Hook, R.L.; Taylor, A.E.; Correia, J.N.; Lefievre, L.; Publicover, S.J.; Conner, S.J.; Kirkman-Brown, J.C. Complex combined steroid mix of the female tract modulates human sperm. Reprod. Biol. 2021, 21, 100561. [Google Scholar] [CrossRef]
- Osman, R.A.; Andria, M.L.; Jones, A.D.; Meizel, S. Steroid induced exocytosis: The human sperm acrosome reaction. Biochem. Biophys. Res. Commun. 1989, 160, 828–833. [Google Scholar] [CrossRef]
- Morales, P.; Llanos, M.; Gutierrez, G.; Kohen, P.; Vigil, P.; Vantman, D. The acrosome reaction-inducing activity of individual human follicular fluid samples is highly variable and is related to the steroid content. Hum. Reprod. 1992, 7, 646–651. [Google Scholar] [CrossRef]
- Strunker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.L.; Mansell, S.; Alasmari, W.; Brown, S.G.; Wilson, S.M.; Sutton, K.A.; Miller, M.R.; Lishko, P.V.; Barratt, C.L.; Publicover, S.J.; et al. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum. Reprod. 2015, 30, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Chen, H.Y.; Zou, Q.X.; Wang, T.; Cheng, Y.M.; Wang, H.F.; Wang, F.; Jin, Z.L.; Chen, Y.; Weng, S.Q.; et al. A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum. Reprod. 2019, 34, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Allgeyer, M.; Kirschenhofer, N.; Mann, U.; Brucker, C. Measurement of induced acrosome reactions in human sperm using physiologic stimuli-relevance for the prediction of fertilization outcome. Andrologia 2006, 38, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, C.; Baldi, E.; Krausz, C.; Casano, R.; Failli, P.; Forti, G. Decreased responsiveness to progesterone of spermatozoa in oligozoospermic patients. J. Androl. 1996, 14, 17–22. [Google Scholar]
- Tesarik, J.; Mendoza, C. Defective function of a nongenomic progesterone receptor as a sole sperm anomaly in infertile patients. Fertil. Steril. 1992, 58, 793–797. [Google Scholar] [CrossRef]
- Oehninger, S.; Blackmore, P.; Morshedi, M.; Sueldo, C.; Acosta, A.A.; Alexander, N.J. Defective calcium influx and acrosome reaction (spontaneous and progesterone-induced) in spermatozoa of infertile men with severe teratozoospermia. Fertil. Steril. 1994, 61, 349–354. [Google Scholar] [CrossRef]
- Krausz, C.; Bonaccorsi, L.; Luconi, M.; Fuzzi, B.; Criscuoli, L.; Pellegrini, S.; Forti, G.; Baldi, E. Intracellular calcium increase and acrosome reaction in response to progesterone in human spermatozoa are correlated with in-vitro fertilization. Hum. Reprod. 1995, 10, 120–124. [Google Scholar] [CrossRef][Green Version]
- Kelly, M.C.; Brown, S.G.; Costello, S.M.; Ramalingam, M.; Drew, E.; Publicover, S.J.; Barratt, C.L.R.; da Silva, S.M. Single-cell analysis of [Ca2+]i signalling in sub-fertile men: Characteristics and relation to fertilization outcome. Hum. Reprod. 2018, 33, 1023–1033. [Google Scholar] [CrossRef]
- Jiang, T.; Qin, Y.; Ye, T.; Wang, Y.; Pan, J.; Zhu, Y.; Duan, L.; Li, K.; Teng, X. Correlation analysis of the progesterone-induced sperm acrosome reaction rate and the fertilisation rate in vitro. Andrologia 2015, 47, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Bonaccorsi, L.; Maggio, P.; Luconi, M.; Criscuoli, L.; Fuzzi, B.; Pellegrini, S.; Forti, G.; Baldi, E. Two functional assays of sperm responsiveness to progesterone and their predictive values in in-vitro fertilization. Hum. Reprod. 1996, 11, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Uhler, M.L.; Leung, A.; Chan, S.Y.; Wang, C. Direct effects of progesterone and antiprogesterone on human sperm hyper-activated motility and acrosome reaction. Fertil. Steril. 1992, 58, 1191–1198. [Google Scholar] [CrossRef]
- Parinaud, J.; Vieitez, G.; Moutaffian, H.; Richoilley, G.; Labal, B. Variations in spontaneous and induced acrosome reaction: Correlations with semen parameters and in-vitro fertilization results. Hum. Reprod. 1995, 10, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Palme, D.L.E.; Rehfeld, A.; Bang, A.K.; Nikolova, K.A.; Kjaerulff, S.; Petersen, M.R.; Jeppesen, J.V.; Glensbjerg, M.; Juul, A.; Skakkebaek, N.E.; et al. Viable acrosome-intact human spermatozoa in the ejaculate as a marker of semen quality and fertility status. Hum. Reprod. 2018, 33, 361–371. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Minetti, F.; Forti, G.; Muratori, M.; Baldi, E. The CatSper calcium channel in human sperm: Relation with motility and involvement in progesterone-induced acrosome reaction. Hum. Reprod. 2014, 29, 418–428. [Google Scholar] [CrossRef]
- Parinaud, J.; Labal, B.; Vieitez, G.; Richoilley, G.; Grandjean, H. Comparison between fluorescent peanut agglutinin lectin and GB24 antibody techniques for the assessment of acrosomal status. Hum. Reprod. 1993, 8, 1685–1688. [Google Scholar] [CrossRef]
- Fisher, H.M.; Brewis, I.A.; Barratt, C.L.; Cooke, I.D.; Moore, H.D. Phosphoinositide 3-kinase is involved in the induction of the human sperm acrosome reaction downstream of tyrosine phosphorylation. Mol. Hum. Reprod. 1998, 4, 849–855. [Google Scholar] [CrossRef]
- Rehfeld, A.; Egeberg, D.L.; Almstrup, K.; Petersen, J.H.; Dissing, S.; Skakkebaek, N.E. EDC IMPACT: Chemical UV filters can affect human sperm function in a progesterone-like manner. Endocr. Connect. 2018, 7, 16–25. [Google Scholar] [CrossRef]
- Rehfeld, A.; Andersson, A.M.; Skakkebaek, N.E. Bisphenol A Diglycidyl Ether (BADGE) and Bisphenol analogs, but Not Bisphenol A (BPA), Activate the CatSper Ca(2+) Channel in Human Sperm. Front. Endocrinol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Jaiswal, B.S.; Eisenbach, M.; Tur-Kaspa, I. Detection of partial and complete acrosome reaction in human spermatozoa: Which inducers and probes to use? Mol. Hum. Reprod. 1999, 5, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Emiliozzi, C.; Cordonier, H.; Guerin, J.F.; Ciapa, B.; Benchaib, M.; Fenichel, P. Effects of progesterone on human spermatozoa prepared for in-vitro fertilization. Int. J. Androl. 1996, 19, 39–47. [Google Scholar] [CrossRef]
- Bronson, R.A.; Peresleni, T.; Golightly, M. Progesterone promotes the acrosome reaction in capacitated human spermatozoa as judged by flow cytometry and CD46 staining. Mol. Hum. Reprod. 1999, 5, 507–512. [Google Scholar] [CrossRef]
- Xu, F.; Guo, G.; Zhu, W.; Fan, L. Human sperm acrosome function assays are predictive of fertilization rate in vitro: A retrospective cohort study and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Parinaud, J.; Labal, B.; Vieitez, G. High progesterone concentrations induce acrosome reaction with a low cytotoxic effect. Fertil. Steril. 1992, 58, 599–602. [Google Scholar] [CrossRef]
- Harper, C.V.; Cummerson, J.A.; White, M.R.; Publicover, S.J.; Johnson, P.M. Dynamic resolution of acrosomal exocytosis in human sperm. J. Cell Sci. 2008, 121, 2130–2135. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Cardenas, C.; Servin-Vences, M.R.; Jose, O.; Trevino, C.L.; Hernandez-Cruz, A.; Darszon, A. Acrosome reaction and Ca(2)(+) imaging in single human spermatozoa: New regulatory roles of [Ca(2)(+)]i. Biol. Reprod. 2014, 91, 67. [Google Scholar] [CrossRef]
- Cross, N.L.; Morales, P.; Overstreet, J.W.; Frederick, W.H. Two simple methods for detecting acrosome-reacted human sperm. Gamete Res. 1986, 15, 213–226. [Google Scholar] [CrossRef]
- Mortimer, D.; Curtis, E.F.; Miller, R.G. Specific labelling by peanut agglutinin of the outer acrosomal membrane of the human spermatozoon. J. Reprod. Fertil. 1987, 81, 127–135. [Google Scholar] [CrossRef]
- Fierro, R.; Foliguet, B.; Grignon, G.; Daniel, M.; Bene, M.C.; Faure, G.C.; Barbarino-Monnier, P. Lectin-binding sites on human sperm during acrosome reaction: Modifications judged by electron microscopy/flow cytometry. Arch. Androl. 1996, 36, 187–196. [Google Scholar] [CrossRef][Green Version]
- Carver-Ward, J.A.; Jaroudi, K.A.; Hollanders, J.M.; Einspenner, M. High fertilization prediction by flow cytometric analysis of the CD46 antigen on the inner acrosomal membrane of spermatozoa. Hum. Reprod. 1996, 11, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Borland, R.M.; Biggers, J.D.; Lechene, C.P.; Taymor, M.L. Elemental composition of fluid in the human Fallopian tube. J. Reprod. Fertil. 1980, 58, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ying, Y.K.; Hong, P.; Maddaiah, V.T. Potassium increases intracellular calcium simulating progesterone action in human sperm. Arch. Androl. 2000, 44, 93–101. [Google Scholar] [CrossRef]
- Linares-Hernandez, L.; Guzman-Grenfell, A.M.; Hicks-Gomez, J.J.; Gonzalez-Martinez, M.T. Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of intracellular calcium and membrane potential. Biochim. Biophys. Acta 1998, 1372, 1–12. [Google Scholar] [CrossRef]
- Roblero, L.S.; Guadarrama, A.; Ortiz, M.E.; Fernandez, E.; Zegers-Hochschild, F. High potassium concentration and the cumulus corona oocyte complex stimulate the fertilizing capacity of human spermatozoa. Fertil. Steril. 1990, 54, 328–332. [Google Scholar] [CrossRef]
- Brenker, C.; Rehfeld, A.; Schiffer, C.; Kierzek, M.; Kaupp, U.B.; Skakkebaek, N.E.; Strunker, T. Synergistic activation of CatSper Ca2+ channels in human sperm by oviductal ligands and endocrine disrupting chemicals. Hum. Reprod. 2018, 33, 1915–1923. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.J. Dual Sensing of Physiologic pH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494.e19. [Google Scholar] [CrossRef]
- Chavez, J.C.; Darszon, A.; Trevino, C.L.; Nishigaki, T. Quantitative Intracellular pH Determinations in Single Live Mamma-lian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front. Cell Dev. Biol. 2020, 7, 366. [Google Scholar] [CrossRef]
- Molina, L.C.P.; Luque, G.M.; Balestrini, P.A.; Marin-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef]
- Garcia, M.A.; Meizel, S. Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol. Reprod. Dev. 1999, 52, 189–195. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wu, H.; Zhang, H.; Zhang, H.; Mao, J.; Liu, D.; Zhao, L.; Lin, H.; Tang, W.; et al. Sodi-um-Hydrogen-Exchanger expression in human sperm and its relationship with semen parameters. J. Assist. Reprod. Genet. 2014, 34, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Mahony, M.C.; Giuffrida, A.; Picone, R.P.; Makriyannis, A. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol. Reprod. Dev. 2002, 63, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Riffo, M.; Leiva, S.; Astudillo, J. Effect of zinc on human sperm motility and the acrosome reaction. Int. J. Androl. 1992, 15, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kennedy, K.; de Blas, G.A.; Orta, G.; Pavarotti, M.A.; Arias, R.J.; de la Vega-Beltran, J.L.; Li, Q.; Dai, H.; Perozo, E.; et al. Role of human Hv1 channels in sperm capacitation and white blood cell respiratory burst established by a designed peptide inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, E11847–E11856. [Google Scholar] [CrossRef]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2011, 108, 20008–20011. [Google Scholar] [CrossRef]
- Kaneko, T.; Iida, H.; Bedford, J.M.; Mori, T. Spermatozoa of the shrew, Suncus murinus, undergo the acrosome reaction and then selectively kill cells in penetrating the cumulus oophorus. Biol. Reprod. 2001, 65, 544–553. [Google Scholar] [CrossRef][Green Version]
- Mahi, C.A.; Yanagimachi, R. Maturation and sperm penetration of canine ovarian oocytes in vitro. J. Exp. Zool. 1976, 196, 189–196. [Google Scholar] [CrossRef]
- Balbach, M.; Hamzeh, H.; Jikeli, J.F.; Brenker, C.; Schiffer, C.; Hansen, J.N.; Neugebauer, P.; Trotschel, C.; Jovine, L.; Han, L.; et al. Molecular Mechanism Underlying the Action of Zona-pellucida Glycoproteins on Mouse Sperm. Front. Cell Dev. Biol. 2020, 8, 572735. [Google Scholar] [CrossRef]
- Plachot, M.; Mandelbaum, J.; Junca, A.M. Acrosome reaction of human sperm used for in vitro fertilization. Fertil. Steril. 1984, 42, 418–423. [Google Scholar] [CrossRef]
- Hoshi, K.; Sugano, T.; Endo, C.; Yoshimatsu, N.; Yanagida, K.; Sato, A. Induction of the acrosome reaction in human spermatozoa by human zona pellucida and effect of cervical mucus on zona-induced acrosome reaction. Fertil. Steril. 1993, 60, 149–153. [Google Scholar] [CrossRef]
- Pier, B.; Edmonds, J.W.; Wilson, L.; Arabshahi, A.; Moore, R.; Bates, G.W.; Prasain, J.K.; Miller, M.A. Comprehensive pro-filing of prostaglandins in human ovarian follicular fluid using mass spectrometry. Prostaglandins Other Lipid Mediat. 2018, 134, 7–15. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.J.; Pangas, S.A.; Carson, S.A.; Kovanci, E.; Cisneros, P.; Buster, J.E.; Amato, P.; Matzuk, M.M. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum. Reprod. 2004, 19, 2869–2874. [Google Scholar] [CrossRef]
- Viggiano, J.M.; Herrero, M.B.; Cebral, E.; Boquet, M.G.; de Gimeno, M.F. Prostaglandin synthesis by cumulus-oocyte com-plexes: Effects on in vitro fertilization in mice. Prostaglandins Leukot. Essent. Fatty Acids 1995, 53, 261–265. [Google Scholar] [CrossRef]
- Nieder, J.; Augustin, W. Prostaglandin E and F profiles in human fallopian tubes during different phases of the menstrual cycle. Gynecol. Obstet. Investig. 1986, 21, 202–207. [Google Scholar] [CrossRef]
- Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Montik, N.; Ciavattini, A.; Polidori, A.R.; Candela, F.A.; Vaccari, L.; Cignitti, M.; Carnevali, O. The Impact of Controlled Ovarian Stimulation Hormones on the Metabolic State and Endocannabinoid System of Human Cumulus Cells. Int. J. Mol. Sci. 2020, 21, 7124. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cardenas, C.; Montoya, F.; Navarrete, F.A.; Hernandez-Cruz, A.; Corkidi, G.; Visconti, P.E.; Darszon, A. Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biol. Reprod. 2018, 99, 1010–1021. [Google Scholar] [CrossRef]
- Morales, P.; Cross, N.L.; Overstreet, J.W.; Hanson, F.W. Acrosome intact and acrosome-reacted human sperm can initiate binding to the zona pellucida. Dev. Biol. 1989, 133, 385–392. [Google Scholar] [CrossRef]
- Morales, P.; Vigil, P.; Franken, D.R.; Kaskar, K.; Coetzee, K.; Kruger, T.F. Sperm-oocyte interaction: Studies on the kinetics of zona pellucida binding and acrosome reaction of human spermatozoa. Andrologia 1994, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Bansal, P.; Talwar, P.; Gupta, S.K. Delineation of downstream signalling components during acrosome reaction mediated by heat solubilized human zona pellucida. Reprod. Biol. Endocrinol. 2010, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Wong, B.S.; Lee, C.L.; Lam, K.K.; Chung, M.K.; Lee, K.F.; Koistinen, R.; Koistinen, H.; Gupta, S.K.; Seppala, M.; et al. Zona pellucida-induced acrosome reaction in human spermatozoa is potentiated by glycodelin-A via down-regulation of extracellular signal-regulated kinases and up-regulation of zona pellucida-induced calcium influx. Hum. Reprod. 2010, 25, 2721–2733. [Google Scholar] [CrossRef]
- Patrat, C.; Auer, J.; Fauque, P.; Leandri, R.L.; Jouannet, P.; Serres, C. Zona pellucida from fertilised human oocytes induces a voltage-dependent calcium influx and the acrosome reaction in spermatozoa, but cannot be penetrated by sperm. BMC Dev. Biol. 2006, 6, 59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, D.Y.; Liu, M.L.; Baker, H.W.G. Defective protein kinase A and C pathways are common causes of disordered zona pellucida (ZP)-induced acrosome reaction in normozoospermic infertile men with normal sperm-ZP binding. Fertil. Steril. 2013, 99, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Veaute, C.; Liu, D.Y.; Furlong, L.I.; Biancotti, J.C.; Baker, H.W.; Vazquez-Levin, M.H. Anti-human proacrosin antibody inhibits the zona pellucida (ZP)-induced acrosome reaction of ZP-bound spermatozoa. Fertil. Steril. 2010, 93, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.L.; Razy-Faulkner, P. Control of human sperm intracellular pH by cholesterol and its relationship to the response of the acrosome to progesterone. Biol. Reprod. 1997, 56, 1169–1174. [Google Scholar] [CrossRef]
- Naz, R.K. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol. Reprod. 1999, 60, 1402–1409. [Google Scholar] [CrossRef]
- Graf, C.B.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential As-sessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 383. [Google Scholar] [CrossRef]
- Balestrini, P.A.; Sanchez-Cardenas, C.; Luque, G.M.; Graf, C.B.; Sierra, J.M.; Hernandez-Cruz, A.; Visconti, P.E.; Krapf, D.; Darszon, A.; Buffone, M.G. Membrane hyperpolarization abolishes calcium oscillations that prevent induced acrosomal exocytosis in human sperm. FASEB J. 2021, 35, e21478. [Google Scholar] [CrossRef]
- Torrezan-Nitao, E.; Brown, S.G.; Mata-Martinez, E.; Trevino, C.L.; Barratt, C.; Publicover, S. [Ca2+]i oscillations in human sperm are triggered in the flagellum by membrane potential-sensitive activity of CatSper. Hum. Reprod. 2021, 36, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Aldana, A.; Carneiro, J.; Martinez-Mekler, G.; Darszon, A. Discrete Dynamic Model of the Mammalian Sperm Acrosome Reaction: The Influence of Acrosomal pH and Physiological Heterogeneity. Front Physiol. 2021, 12, 682790. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, I.; Torres-Rodriguez, P.; Sanchez-Carranza, O.; Solis-Lopez, A.; Santi, C.M.; Darszon, A.; Trevino, C.L. Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Gunderson, S.; Riley, J.; Lybaert, P.; Borrego-Alvarez, A.; Jungheim, E.S.; Santi, C.M. Membrane Potential Determined by Flow Cytometry Predicts Fertilizing Ability of Human Sperm. Front Cell Dev. Biol. 2020, 7, 387. [Google Scholar] [CrossRef]
- Sati, L.; Cayli, S.; Delpiano, E.; Sakkas, D.; Huszar, G. The pattern of tyrosine phosphorylation in human sperm in response to binding to zona pellucida or hyaluronic acid. Reprod. Sci. 2014, 21, 573–581. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Moreno-Irusta, A.; Torres-Rodriguez, P.; Giojalas, L.; Gervasi, M.G.; Visconti, P.E.; Trevino, C.L. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: The case of capacitation-induced tyrosine phosphorylation. Mol. Hum. Reprod. 2018, 24, 64–73. [Google Scholar] [CrossRef]
- Chung, J.J.; Shim, S.H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Ded, L.; Hwang, J.Y.; Miki, K.; Shi, H.F.; Chung, J.J. 3D in situ imaging of the female reproductive tract reveals molecular signatures of fertilizing spermatozoa in mice. Elife 2020, 9, 62043. [Google Scholar] [CrossRef]
- Escoffier, J.; Navarrete, F.; Haddad, D.; Santi, C.M.; Darszon, A.; Visconti, P.E. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 2015, 92, 121. [Google Scholar] [CrossRef]
- Simons, J.; Fauci, L. A Model for the Acrosome Reaction in Mammalian Sperm. Bull. Math. Biol. 2018, 80, 2481–2501. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; da Silva, S.J.M. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.S.; Johnston, Z.C.; Barratt, C.L.; Andrews, P.D. A phenotypic screening platform utilising human spermatozoa identifies compounds with contraceptive activity. Elife 2020, 9, e51739. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Bjorndahl, L.; Barratt, C.L.; Mortimer, D.; Jouannet, P. ‘How to count sperm properly’: Checklist for acceptability of studies based on human semen analysis. Hum. Reprod. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- Brenker, C.; Zhou, Y.; Muller, A.; Echeverry, F.A.; Trotschel, C.; Poetsch, A.; Xia, X.M.; Bonigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife 2014, 3, e01438. [Google Scholar] [CrossRef]
- Mata-Martinez, E.; Darszon, A.; Trevino, C.L. pH-dependent Ca(+2) oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef]
- Achikanu, C.; Pendekanti, V.; Teague, R.; Publicover, S. Effects of pH manipulation, CatSper stimulation and Ca2+-store mobilization on [Ca2+]i and behaviour of human sperm. Hum. Reprod. 2018, 33, 1802–1811. [Google Scholar] [CrossRef]
- McBrinn, R.C.; Fraser, J.; Hope, A.G.; Gray, D.W.; Barratt, C.L.R.; da Silva, S.J.M.; Brown, S.G. Novel pharmacological actions of trequinsin hydrochloride improve human sperm cell motility and function. Br. J. Pharmacol. 2019, 176, 4521–4536. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, P.; Kane, S.; McBrinn, R.C.; Dean, M.S.; Martins da Silva, S.J.; Brown, S.G. Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction. Int. J. Mol. Sci. 2022, 23, 11253. https://doi.org/10.3390/ijms231911253
Prajapati P, Kane S, McBrinn RC, Dean MS, Martins da Silva SJ, Brown SG. Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction. International Journal of Molecular Sciences. 2022; 23(19):11253. https://doi.org/10.3390/ijms231911253
Chicago/Turabian StylePrajapati, Priyanka, Shruti Kane, Rachel C. McBrinn, Morven S. Dean, Sarah J. Martins da Silva, and Sean G. Brown. 2022. "Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction" International Journal of Molecular Sciences 23, no. 19: 11253. https://doi.org/10.3390/ijms231911253
APA StylePrajapati, P., Kane, S., McBrinn, R. C., Dean, M. S., Martins da Silva, S. J., & Brown, S. G. (2022). Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction. International Journal of Molecular Sciences, 23(19), 11253. https://doi.org/10.3390/ijms231911253






