Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy
Abstract
1. Introduction
2. Mechanism of Action of Platinum Drugs
3. Mechanism of Resistance and Limitations of Platinum Drugs
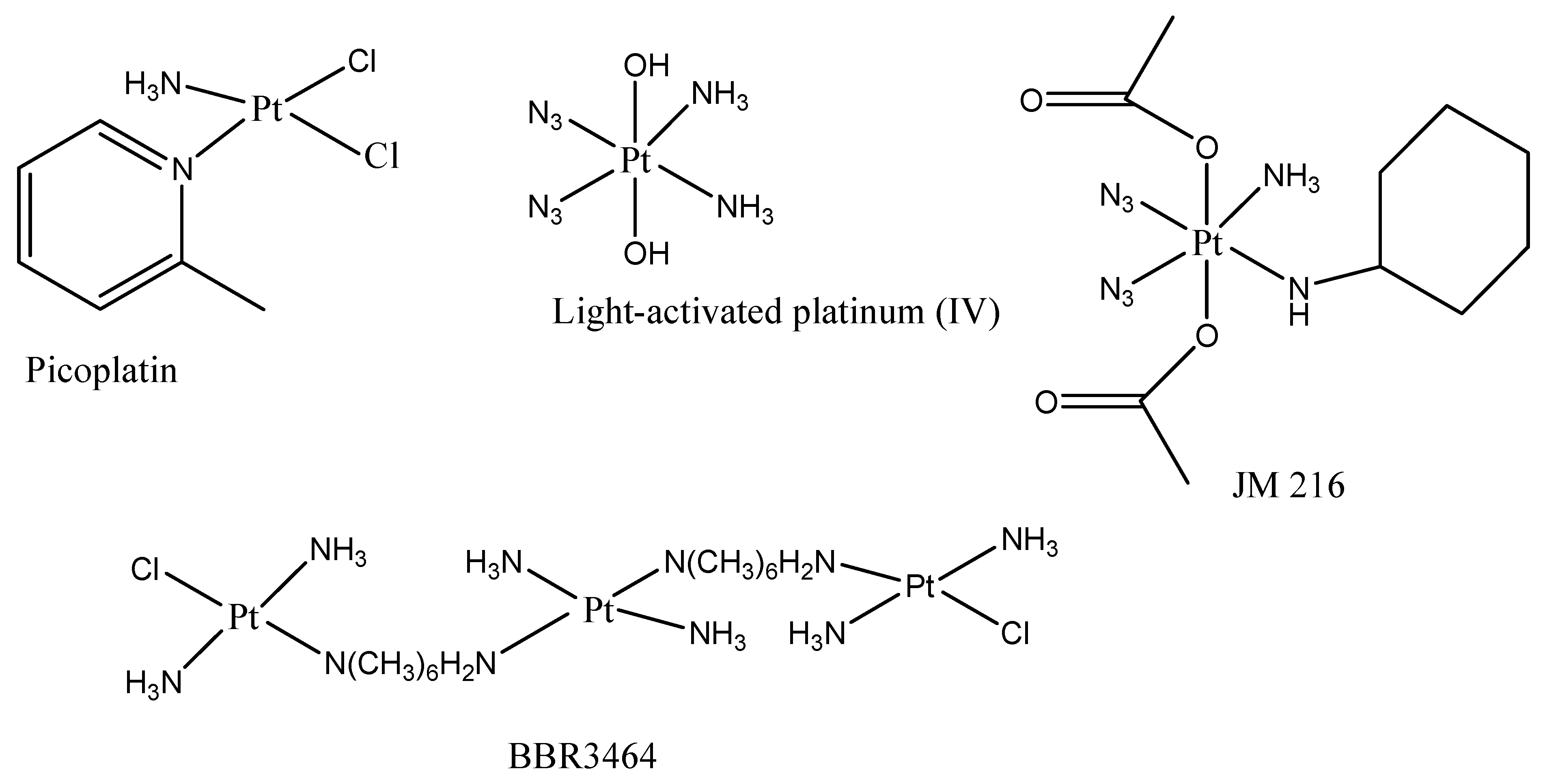
Clinical Trials of Platinum-Based Drugs in CRC
4. Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Treatment
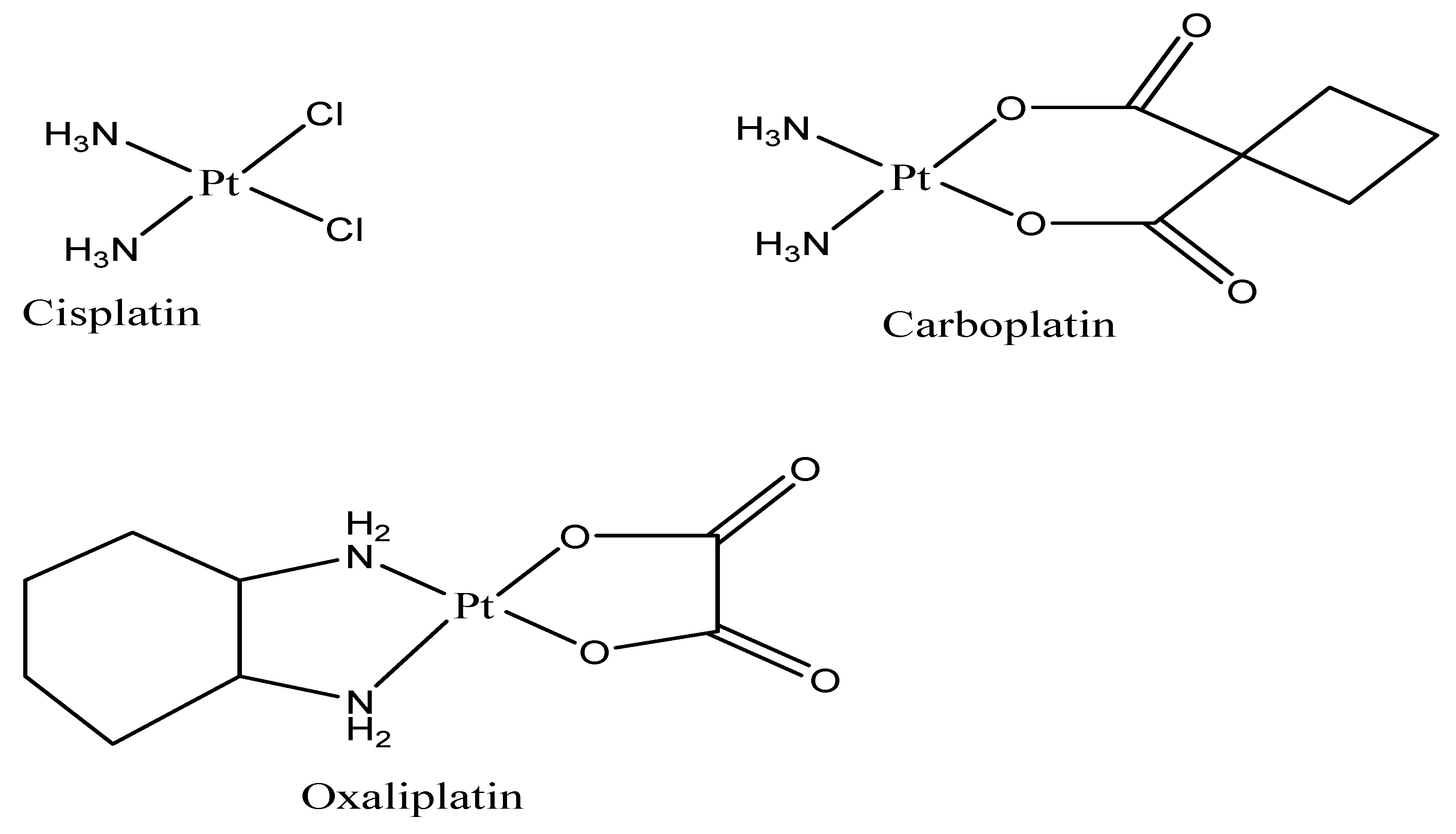
4.1. Nanoparticles Loaded with Cisplatin
4.2. Nanoparticles Loaded with Carboplatin
4.3. Nanoparticles Loaded with Oxaliplatin
4.3.1. Nanoparticles
4.3.2. Lipid-Based Nanoparticles Loaded with Oxaliplatin
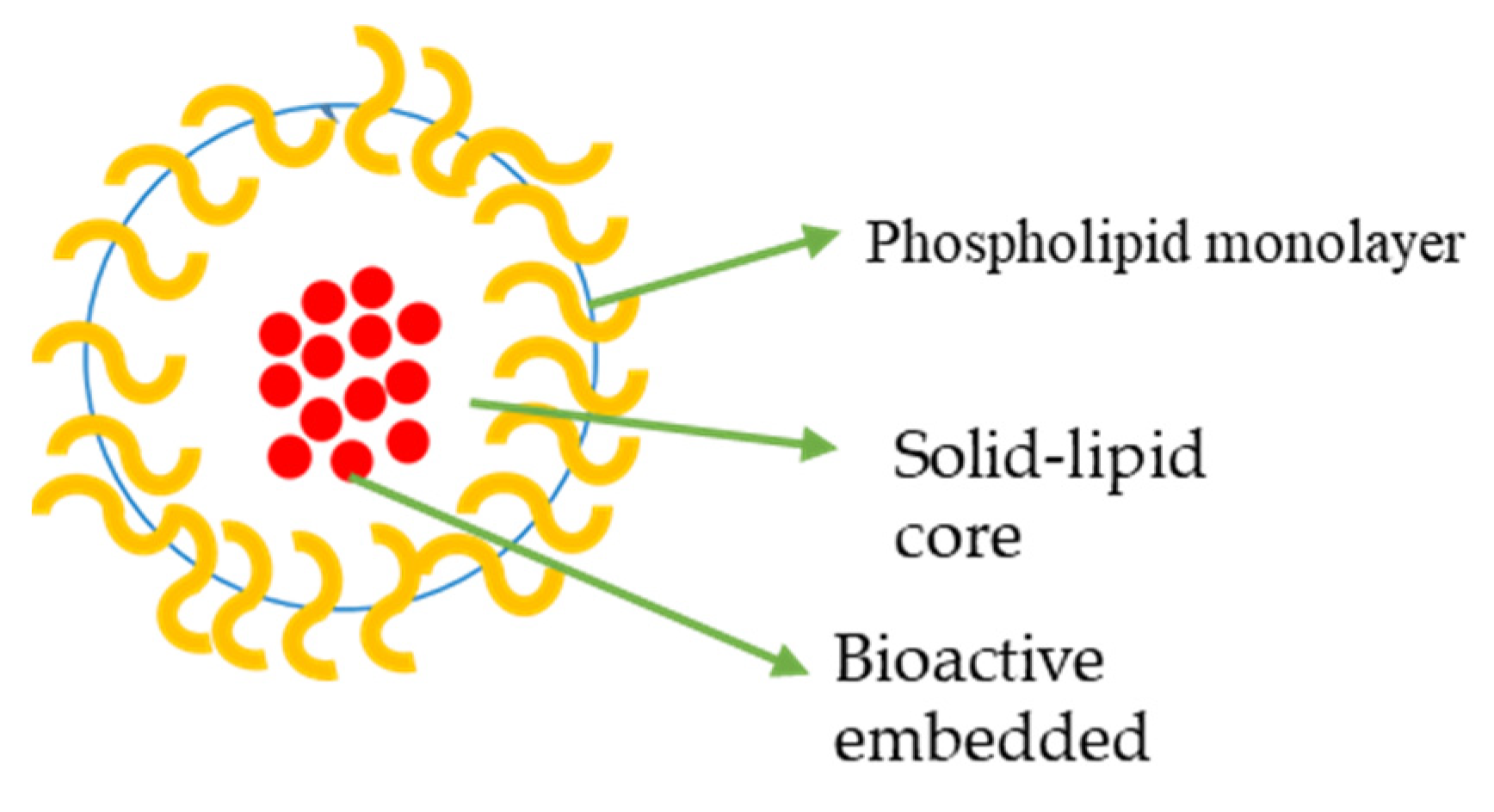
4.3.3. Solid Lipid Nanoparticles (SLNs) Loaded with Oxaliplatin
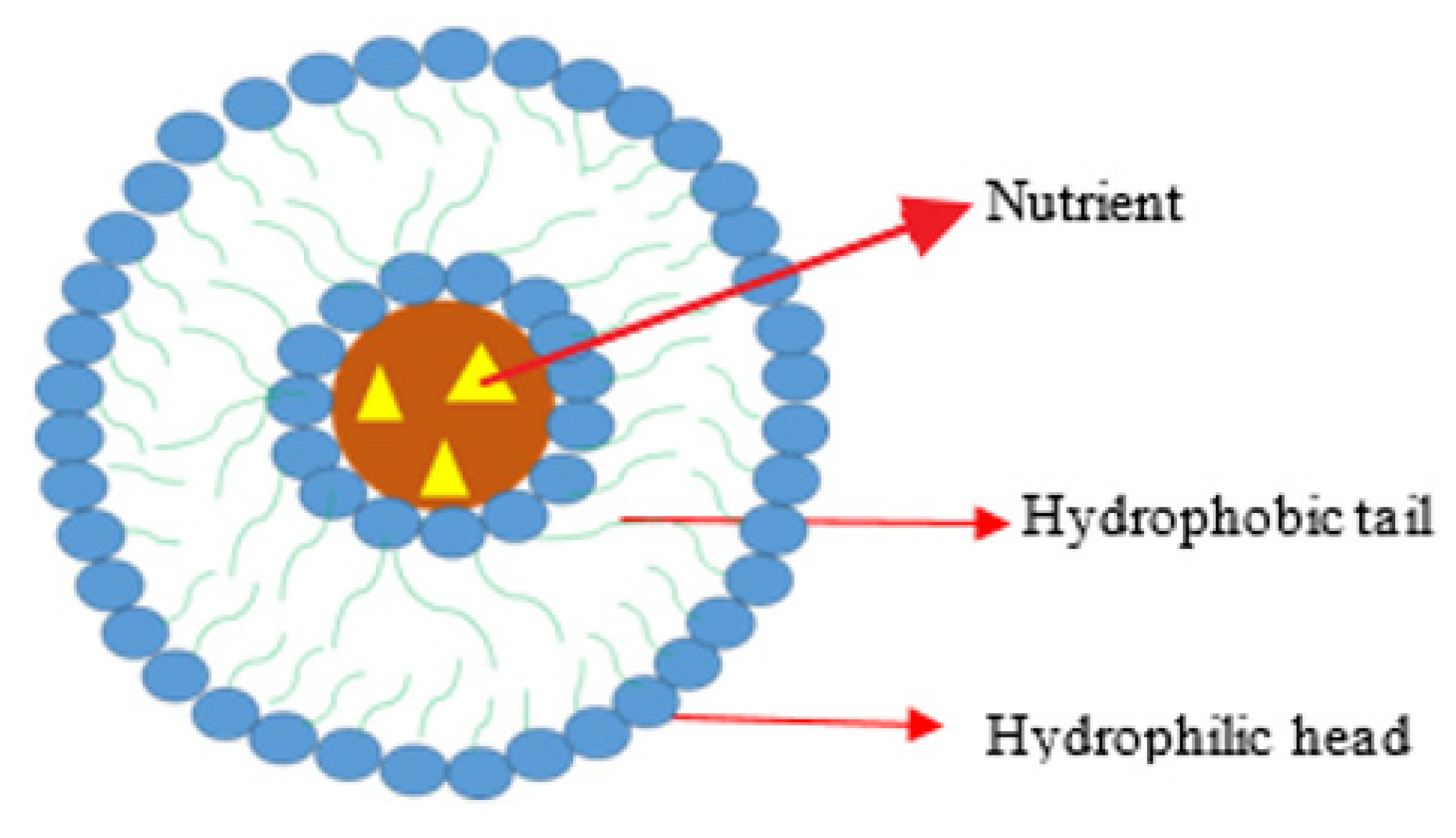
4.3.4. Liposome Loaded with Oxaliplatin
4.3.5. Polymeric Nanoparticles (PNPs) Loaded with Oxaliplatin
4.3.6. Carbon Nanotubes (CNTs) Loaded with Oxaliplatin
4.3.7. Multiwalled Carbon Nanotube Loaded with Oxaliplatin
4.3.8. Metal Nanoparticles Loaded with Oxaliplatin
5. Commercially Available Nanomaterials for Colorectal Cancer Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| CTR1 | Copper transport protein |
| MSN | Medium spiny neurons |
| PLGA | Poly D,L-lactic-co-glycolic acid |
| LNPs | Lipid nanoparticles |
| PAMAM | Polyamidoamines |
| PEG | Polyethylene glycol |
| PNP | Psychoneuroplasticity |
| SWCNTs | Single-walled carbon nanotubes |
| FU | Fluorouracil |
| HACTNPs | Hyaluronic acid Coupled Chitosan Nanoparticles |
| CPX- | Ciprofloxacin |
| SLNPs | Solid lipid nanoparticles |
| TME | Tumour microenvironment |
| NSCLC | Non-small cancer lung cell |
| SWCNTs | Single-walled carbon nanotubes |
References
- Bray, A.; Ferlay, F.; Soerjomataram, J.; Siegel, I.; Torre, R.L.; Jemal, L.A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Liu, Y.; Sethi, N.S.; Hinoue, T.; Schneider, B.G.; Cheriack, A.D.; Vega, F.S.; Seoane, J.A.; Farshidfar, F.; Bowlby, R.; Islam, M.; et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018, 33, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.P.; Kloor, M.; Pox, H. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Marmol, M.J.R.; Sanchez-De-Diego, C.; Dieste, A.P.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.; Kasi, P.M.; Wallace, B. Colorectal cancer. Lancet. 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Franke, A.J.; Skelton, W.P.; Starr, J.S.; Parekh, H.; Lee, J.J.; Overman, M.J.; Allegra, C.; George, T.J. Immunotherapy for colorectal cancer: A review of current and novel therapeutic approaches. J. Natl. Cancer Inst. 2019, 111, 1131–1141. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Yoldi, M.J.R. Modifiable and non-modifiable risk factors of colorectal cancer. In Advances in Health and Disease, 1st ed.; Duncan, L.T., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2018; Volume 7, pp. 67–116. [Google Scholar]
- Lee, M.M.; MacKinlay, A.; Semira, C.; Schieber, C.; Jimeno Yepes, A.J.; Lee, B.; Wong, R.; Hettiarachchige, C.K.H.; Gunn, N.; Tie, J.; et al. Stage-based Variation in the E ect of Primary Tumor Side on All Stages of Colorectal Cancer Recurrence and Survival. Clin. Color. Cancer. 2018, 17, e569–e577. [Google Scholar] [CrossRef]
- Pavitra, E.; Dariya, B.; Srivani, G.; Kang, S.M.; Alam, A.; Sudhir, P.R.; Kamal, M.A.; Raju, G.S.R.; Han, Y.K.; Lakkakula, B.V.K.S.; et al. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Semin. Cancer Biol. 2021, 69, 293–306. [Google Scholar] [CrossRef]
- Pinto, J.F. Site-specific drug delivery systems within the gastro-intestinal tract: From the mouth to the colon. Int. J. Pharm. 2010, 395, 44–52. [Google Scholar] [CrossRef]
- Omar, S.; Aldosari, B.; Refai, H.; Gohary, O.A. Colon-specific drug delivery for mebeverine hydrochloride. J. Drug Target. 2007, 15, 691–700. [Google Scholar] [CrossRef]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin nanoformulations for colorectal cancer: A review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell. 2015, 6, 480–503. [Google Scholar] [CrossRef]
- Griffin, B.T.; Guo, J.; Presas, E.; Donovan, M.D.; Alonso, M.J.; O’Drisscol, C.M. Pharmacokinetic, pharmacodynamic and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv. Drug. Deliv. Rev. 2016, 106, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Jain, S. Advances in oral delivery of anti-cancer prodrugs. Expert Opin. Drug Deliv. 2016, 13, 1759–1775. [Google Scholar] [CrossRef] [PubMed]
- Alavian, F.; Shams, N. Oral and intra-nasal administration of nanoparticles in the cerebral ischemia treatment in animal experiments: Considering its advantages and disadvantages. Curr. Clin. Pharmacol. 2018, 15, 20–29. [Google Scholar]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–510. [Google Scholar] [CrossRef]
- Hall, M.D.; Okabe, M.; Shen, D.W.; Liang, X.J.; Gottesman, M.M. The Role of Cellular Accumulation in Determining Sensitivity to Platinum-Based Chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Knipp, M. Metallothioneins and Platinum(II) Anti-Tumor Compounds. Curr. Med. Chem. 2009, 16, 522–530. [Google Scholar] [CrossRef]
- Martin, L.P.; Schilder, R.J.; Hamilton, T.C. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008, 14, 1291–1300. [Google Scholar] [CrossRef]
- Benedetti, V.; Perego, P.; Beretta, G.L.; Corna, E.; Tinelli, S.; Righetti, S.C.; Leone, R.; Apostoli, P.; Lanzi, C.; Zunino, F. Modulation of survival pathways in ovarian carcinoma cell lines resistant to platinum compounds. Mol. Cancer Ther. 2008, 7, 679–687. [Google Scholar] [CrossRef]
- Fricker, S.P. Metal based drugs: From serendipity to design. Dalt. Trans. 2007, 4903–4910. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Cai, J. Research on application of computer database technology in information management. Drug Target. 2014, 22, 648–650. [Google Scholar] [CrossRef]
- Varghese, S.S.; Ram, T.S.; Pavamani, S.P.; Thomas, E.M.; Jeyasseelan, V.; Viswanathan, N. Concurrent chemo-irradiation with weekly cisplatin and paclitaxel in the treatment of locally advanced squamous cell carcinoma of cervix: A phase II study. J. Cancer Res. Ther. 2014, 10, 330–340. [Google Scholar] [CrossRef]
- Hussian, M.; Daignault, S.; Agarwal, N.; Petros, D.; Grivas, A.O.; Radke, S.; Puzanov, I.; McVicar, G.R.; Levine, E.L.; Srinivas, S.; et al. A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 2014, 120, 2684–2690. [Google Scholar] [CrossRef]
- Setua, S.; Ouberai, M.; Piccirillo, S.G.; Watts, C.; Welland, M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale 2014, 6, 10865–10870. [Google Scholar] [CrossRef]
- Hannon, M.J. Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 2007, 79, 2243–2261. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalt. Trans. 2010, 39, 8113–8120. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Batalla, C. The Rational Design of Anticancer Platinum Complexes: The Importance of the Structure-Activity Relationship. Curr. Med. Chem. 2009, 16, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Dasari, P.B.; Tchounwou, S. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cardús, A.; Martinez-Balibrea, E.; Bandrés, E.; Malumbres, R.; Ginés, A.; Manzano, J.L.; Taron, M.; Garcia-Foncillas, J.; Abad, A. Pharmacogenomic approach for the identification of novel determinants of acquired resistance to oxaliplatin in colorectal cancer. Mol. Cancer Ther. 2009, 8, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.M.; Chan, S.; Salinas, D.; Woynarowska, R.; Woynarowski, B. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Bergamo, G.; Dyson, A.; Sava, P.J. The mechanism of tumour cell death by metal-based anticancer drugs is not only a matter of DNA interactions. Coord. Chem. Rev. 2018, 360, 17–33. [Google Scholar] [CrossRef]
- Palermo, U.; Magistrato, G.; Riedel, A.; von Erlach, T.; Davey, T.; Dyson, C.A.; Rothlisberger, P.J. Fighting Cancer with Transition Metal Complexes: From Naked DNA to Protein and Chromatin Targeting Strategies. Chem. Med. Chem. 2016, 11, 1199–1210. [Google Scholar] [CrossRef]
- Gibson, D. The mechanism of action of platinum anticancer agents-what do we really know about it? Dalt. Trans. 2009, 48, 10681–10689. [Google Scholar] [CrossRef]
- Khoury, A.; Deo, K.M.; Aldrich-wright, J.R. Recent advances in platinum-based chemotherapeutics that exhibit inhibitory and targeted mechanisms of action. J. Inorg. Biochem. 2020, 207, 111070. [Google Scholar] [CrossRef]
- Schoch, S.; Gajewski, S.; Rothfuß, J.; Hartwig, A.; Köberle, B. Comparative Study of the Mode of Action of Clinically Approved Platinum-Based Chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6928. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef]
- De Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Brazilian J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, A.J.; Goodisman, J.; Dabrowiak, J.C. Inorganica Chimica Acta Understanding how the platinum anticancer drug carboplatin works: From the bottle to the cell. Inorganica Chim. Acta. 2012, 389, 29–35. [Google Scholar] [CrossRef]
- Misset, J.L.; Bleiberg, H.; Sutherland, W.; Bekradda, M.; Cvitkovic, E. Oxaliplatin clinical activity A review. Crit. Rev. Oncol. 2000, 35, 75–93. [Google Scholar] [CrossRef]
- Raez, L.E.; Kobina, S.; Santos, E.S. Review Oxaliplatin in First-line Therapy for Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer. 2010, 11, 18–24. [Google Scholar] [CrossRef]
- Stordal, B.; Pavlakis, N.; Davey, R. Oxaliplatin for the treatment of cisplatin-resistant cancer: A systematic review. Cancer Treat. Rev. 2007, 33, 347–357. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Yamasaki, M.; Makino, T.; Masuzawa, T.; Kurokawa, Y.; Miyata, H.; Takiguchi, S.; Nakajima, K.; Fujiwara, Y.; Matsuura, T.; Mori, M.; et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br. J. Cancer. 2011, 104, 707–713. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalt. Trans. 2018, 47, 6645. [Google Scholar] [CrossRef]
- Boztepe, T.; Castro, G.R.; Leon, I.E. Lipid, polymeric, inorganic-based drug delivery applications for platinum-based anticancer drugs. Int. J. Pharm. 2021, 605, 120788. [Google Scholar] [CrossRef]
- Holzer, A.K.; Manorek, G.H.; Howell, S.B. Contribution of the Major Copper Influx Transporter CTR1 to the Cellular Accumulation of Cisplatin, Carboplatin, and Oxaliplatin. Mol. Pharmacol. 2006, 70, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhan, L.; Zhang, L.; Wang, Q.; Juan, L.; Jiang, Z.; Hu, X.; Yuan, X. Stanniocalcin 2 induces oxaliplatin resistance in colorectal cancer cells by upregulating P-glycoprotein. Can. J. Physiol. Pharmacol. 2016, 94, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Covaletti, G.; Antonacopoulou, A.; Genazzani, A.; Briani, C.; Bruna, J.; Terrazzino, S.; Velasco, R.; Alberti, P.; Campagnolo, M.; et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: Results from a prospective multicenter study. Cancer 2013, 119, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Machover, D.; Diaz-Rubio, E.; de Gramont, A.; Schilf, A.; Gastiaburu, J.J.; Brienza, S.; Itzhaki, M.; Metzger, G.; N’Daw, D.; Vignoud, J.; et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients wit advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann. Oncol. 1996, 7, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Misset, J.L.; Brienza, S.; Adam, R.; Metzger, G.; Itzakhi, M.; Caussanel, J.P.; Kunstlinger, F.; Lecouturier, S.; Descorps-Declère, A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumour effectiveness against metastatic colorectal cancer. Cancer 1992, 69, 893–900. [Google Scholar] [CrossRef]
- Giacchetti, S.; Perpoint, B.; Zidani, R.; Le Bail, N.; Faggiuolo, R.; Focan, C.; Chollet, P.; Llory, J.F.; Letourneau, Y.; Coudert, B.; et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2000, 18, 136–147. [Google Scholar] [CrossRef]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Rothenberg, M.L.; Oza, A.M.; Bigelow, R.H.; Berlin, J.D.; Marshall, J.L.; Ramanathan, R.K.; Hart, L.L.; Gupta, S.; Garay, C.A.; Burger, B.G.; et al. Superiority of oxaliplatin and fluorouracil–leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil–leucovorin: Interim results of a Phase III trial. J. Clin. Oncol. 2003, 21, 2059–2069. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Sargent, D.J.; Morton, R.F.; Fuchs, C.S.; Ramanathan, R.K.; Williamson, S.K.; Findlay, B.P.; Pitot, H.C.; Alberts, S.R. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 2003, 22, 23–29. [Google Scholar] [CrossRef]
- Fu, D.; Wu, J.; Lai, J.; Liu, Y.; Zhou, L.; Chen, L.; Zhang, Q. T cell recruitment triggered by optimal dose platinum compounds contributes to the therapeutic efficacy of sequential PD-1 blockade in a mouse model of colon cancer. Am. J. Cancer Res. 2020, 10, 473–490. [Google Scholar]
- Petrelli, N.J.; Creaven, P.J.; Herrera, L.; Mittelman, A. Phase II trial of continuous-infusion iproplatin (CHIP) and 5-fluorouracil (5-FU) in advanced colorectal carcinoma. Cancer Chemother. Pharm. 1989, 23, 61–62. [Google Scholar] [CrossRef]
- Asbury, R.F.; Kramer, A.; Green, M.; Qazi, R.; Skeel, R.T.; Haller, D.G. A phase II study of carboplatin and CHIP in patients with metastatic colon carcinoma. Am. J. Clin. Oncol. 1989, 12, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Nolè, F.; Biganzoli., L.; Buzzoni., R.; Bajetta., E. Carboplatin in patients with advanced colorectal cancer pretreated with fluoropyrimidines. Eur. J. Cancer 1993, 29, 1330–1331. [Google Scholar] [CrossRef]
- Blitzer, J.B.; Newman, N.; Ginsberg, S.J.; Louie, A.; Scalzo, A.; Poiesz, B. Phase II trial of iproplatin (CHIP) in previously untreated patients with colorectal cancer. Am. J. Clin. Oncol. 1988, 11, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Nguyen, T.; Yang, L.Y.; Khokhar, A.R.; Perez-Soler, R. Cellular accumulation and DNA damage induced by liposomal cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexaneplatinum(II) in LoVo and LoVo/PDD cells. Anti-Cancer Drugs 1994, 5, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Rademaker-Lakhai, J.M.; Terret, C.; Howell, S.B.; Baud, C.M.; De Boer, R.F.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M.; Droz, J.P. A phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin. Cancer Res. 2004, 10, 3386–3390. [Google Scholar] [CrossRef]
- Rice, J.R.; Gerberich, J.L.; Nowtnik, D.P.; Howell, S.B. Preclinical efficacy and pharmacokinetics of AP5346, a novel diaminocyclohexane-platinum tumor-targeting drug delivery system. Clin. Cancer Res. 2006, 12, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Sood, P.; Thurmond, K.B.; Jacob, J.E.; Waller, L.K.; Silva, G.O.; Steward, D.R.; Nowotnik, D.P. Synthesis and Characterization of AP5346, a Novel Polymer-Linked Diaminocyclohexyl Platinum Chemotherapeutic Agent. Bioconjug. Chem. 2006, 17, 1270–1280. [Google Scholar] [CrossRef]
- Stewart, D.; Rice, J.; Sood, P.; John, J.S.; Sheychuk, S.; Thurmond, K.B.; Nguyen, D.; Russel-Jones, D.; Nowotnik, D. Preclinical development of AP5346: A dachplatinum polymer conjugate. J. Contol. Release. 2003, 91, 255–260. [Google Scholar]
- Howell, B.A.; Fan, D. Poly(amidoamine) dendrimer-supported organoplatinum antitumour agents. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 466, 1515–1520. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Kumae, A.; Maher, D.M.; CHauhan, S.C.; Vangara, K.K.; Palakurthi, S. Biotinylated PAMAM dendrimers for intracellular delivery of cisplatin to ovarian cancer. Anticancer Res. 2011, 31, 897–900. [Google Scholar]
- Haririan, I.; Alavidjeh, M.S.; Khorramizadeh, M.R.; Ardestani, M.S.; Ghane, Z.Z.; Namazi, H. Anionic linear-globular dendrimer-cis-platinum (II) conjugates promote cytotoxicity in vitro against different cancer cell lines. Int. J. Nanomed. 2010, 5, 63–75. [Google Scholar] [CrossRef]
- Kapp, T.; Dullin, A.; Gust, R. Platinum(II)−Dendrimer Conjugates: Synthesis and Investigations on Cytotoxicity, Cellular Distribution, Platinum Release, DNA, and Protein Binding. Bioconj. Chem. 2010, 21, 328–330. [Google Scholar] [CrossRef]
- Boulikas, T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol. Rep. 2004, 12, 3–12. [Google Scholar] [CrossRef]
- Oberoi, H.S. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, Y.; Li, R. Influence of corn straw Fibers and polymer latexes on vibration damping property of cement-based materials. Macromol. Biosci. 2012, 12, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Qi, R.; Liu, S.; Hu, X.; Zheng, Y.; Huang, Y.; Jing, X. Biodegradable polymer -cisplatin(IV) conjugate as a pro-drug of cisplatin(II). Biomaterials 2011, 32, 7732–7740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Noble, G.T.; Stefanick, G.T.; Qi, R.; Kiziltepe, T.; Jing, X.; Bilgicer, B. Photosensitive Pt(IV)-azide prodrug-loaded nanoparticles exhibit controlled drug release and enhanced efficacy in vivo. J. Control. Release 2014, 173, 11–17. [Google Scholar] [CrossRef]
- Xiao, H.; Qi, R.; Wang, R.; Liu, S.; Zheng, Y.; Xie, Z.; Huang, Y.; Jing, X. The use of polymeric platinum (IV) prodrugs to deliver multinuclear platinum (II) drugs with reduced systemic toxicity and enhanced antitumor efficacy. Biomaterials 2012, 33, 8657–8660. [Google Scholar] [CrossRef]
- Wheate, N.J.; Wagstaff, A.J.; Brown, S.D.; Holdem, M.R.; Craig, G.E.; Plumb, J.A.; Brown, R.E.; Schreiter, N. Chrzanowski, Cisplatin drug delivery using gold-coated iron oxide nanoparticles for enhanced tumour targeting with external magnetic field. Inorg. Chim. Acta. 2012, 393, 328–330. [Google Scholar]
- Craig, G.E.; Brown, S.D.; Lamprou, D.A.; Graham, D.; Wheaate, N.J. Cisplatin-Tethered Gold Nanoparticles That Exhibit Enhanced Reproducibility, Drug Loading, and Stability: A Step Closer to Pharmaceutical Approval? Inorg. Chem. 2012, 51, 3490–3500. [Google Scholar] [CrossRef]
- Xing, R.; Wang, X.; Zhang, C.; Wang, J.; Zhang, Y.; Song, Y.; Guo, Z. Superparamagnetic magnetite nanocrystal clusters as potential magnetic carriers for the delivery of platinum anticancer drugs. J. Mater. Chem. 2011, 21, 11142–11150. [Google Scholar] [CrossRef]
- Tao, Z.; Xie, Y.; Goodisman, J.; Asefa, T. Isomer-Dependent Adsorption and Release of Cis- and Trans-platin Anticancer Drugs by Mesoporous Silica Nanoparticles. Langmuir 2010, 26, 8914–8920. [Google Scholar] [CrossRef]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano 2010, 4, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Rieter, W.J.; Pott, K.M.; Taylor, K.M.L.; Lin, W. Nanoscale Coordination Polymers for Platinum-Based Anticancer Drug Delivery. J. Am. Chem. Soc. 2008, 130, 11584–11590. [Google Scholar] [CrossRef]
- Lin, W.; Taylor-Pashow, K.M.L.; Rocca, J.D.; Xie, Z.; Tran, S. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2008, 131, 14261–14270. [Google Scholar]
- Liu, D.; Poon, C.; Lu, K.; He, C.; Lin, W. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 2014, 5, 4182–4190. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, Y.; Qiu, L. Amino-functionalized nano-vesicles for enhanced anticancer efficacy and reduced myelotoxicity of carboplatin. Colloids Surf. B Biointerfaces 2017, 157, 56–64. [Google Scholar] [CrossRef]
- Profirio, D.D.; Pessine, F.B.T. Formulation of functionalized PLGA nanoparticles with folic acid- conjugated chitosan for carboplatin encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Li, M.; Mao, L.; Chen, M.; Li, K.; Wang, J.M. Characterization of an Amphiphilic Phosphonated Calixarene Carrier Loaded With Carboplatin and Paclitaxel: A Preliminary Study to Treat Colon Cancer in vitro and in vivo. Front. Bioeng. Biotechnol. 2019, 7, 328. [Google Scholar] [CrossRef]
- Abdelwahab, H.A.; Asker, M.E.; Kotb, N.S.; Mohamed, H.E. Cytotoxic evaluation of anticancer folate decorated carboplatin loaded albumin nanoparticles using folate receptor. Az. J. Pharm Sci. 2021, 64, 109–121. [Google Scholar]
- Pairoj, S.; Damrongsak, P.; Damrongsak, B.; Jinawath, N.; Kaewkhaw, K.; Ruttanasirawit, C.; Leelawattananon, T.; Locharoenrat, K. Antitumor activities of carboplatin–doxorubicin–ZnO complexes in different human cancer cell lines (breast, cervix uteri, colon, liver and oral ) under UV exposition. Artif. Cells Nanomed. Biotechnol. 2021, 49, 120–135. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ray, U. What Are the Different Types of Nanoparticles? Azonano. 2018. Available online: www.azonano.com/article.aspx?ArticleID=4938 (accessed on 20 September 2021).
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Guven, E. Lipid-based nanoparticles in the treatment of erectile dysfunction. IJIR 2020, 32, 578–586. [Google Scholar] [CrossRef]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef]
- Xiaowei, D. Lipid-Based Paclitaxel and Doxorubicin Nanoparticles to Overcome P-Gpmediated Drug Resistance in Solid Tumors. Ph.D. Thesis, University of Kentucky, Lexington, Kentucky, 2009. Available online: https://uknowledge.uky.edu/gradschool_diss/724 (accessed on 30 March 2022).
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, X.; Zhai, Y.; Li, G.L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar]
- Rajpoot, K.; Jain, S.K. Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: Preparation, optimization, and in vitro evaluation. Artif. Cells Nanomed. Biotec. 2018, 46, 1236–1247. [Google Scholar] [CrossRef]
- Al-Najjar, B.Y.; Hussain, S.A. Solid Lipid Nanoparticles Delivery Systems for Colon Cancer Chemotherapy: A Critical Review. Sys. Rev. Pharm. 2020, 11, 1152–1161. [Google Scholar]
- Kim, H.; Jo, A.; Baek, S.; Lim, D.; Park, S.Y.; Cho, S.K.; Chung, J.W.; Yoon, J. Synergistically enhanced selective intracellular uptake of anticancer drug carrier comprising folic acid conjugated hydrogels containing magnetite nanoparticles. Sci. Rep. 2017, 7, 41090. [Google Scholar] [CrossRef]
- Tummala, S.; Kumar, M.N.S.; Gowthamarajan, K.; Prakash, A.; Raju, K.R.S.; Mulukutla, S. Preparation, physicochemical characterization and in vitro evaluation of oxaliplatin solid lipid nanoparticles for the treatment of colorectal cancer. Indo Am. J. Pharm. Res. 2014, 4, 2231–6876. [Google Scholar]
- Nobili, S.; Checcacci, D.; Filippelli, F.; Del Buono, S.; Mazzocchi, V.; Mazzei, T. Mini E: Bimonthly chemotherapy with oxaliplatin, irinotecan, infusional 5-fluorouracil/folinic acid in patients with metastatic colorectal cancer pretreated with irinotecan-or oxaliplatin-based chemotherapy. J Chemother. 2008, 20, 622–631. [Google Scholar] [CrossRef]
- Dragovich, T.; Mendelson, D.; Richardson, S.K.K.; Hoff, D.V.; Hoos, A.A. Phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother. Pharmacol. 2006, 58, 759–764. [Google Scholar] [CrossRef]
- Ying, K.; Bai, B.; Gao, X.; Xu, Y.; Wang, H.; Xie, B. Orally Administrable Therapeutic Nanoparticles for the Treatment of Colorectal Cancer. Front. Bioeng. Biotechnol. 2021, 9, 670124. [Google Scholar] [CrossRef]
- Shaji, J.; Menona, I. Recent advances in nanocarrier based therapeutic and diagnostic tools for colorectal cancer. Int. J. Curr. Pharm. Res. 2015, 7, 9–16. [Google Scholar]
- Garrido, M.J.; Zalba, S.; Contreras, A.M.; Haeri, A.; Hagen, T.L.M.; Navarro, I.; Koning, G. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J. Control. Release 2015, 210, 26–38. [Google Scholar]
- You, X.; Kang, J.; Hollett, G.; Chen, X.; Zhao, W.; Gu, Z.; Wu, J. Polymeric nanoparticles for colon cancer therapy: Overview and perspectives. J. Mater. Chem. B 2016, 4, 7779. [Google Scholar] [CrossRef]
- Tummala, S.; Gowthamarajan, K.; Kumar, M.N.S.; Wadhwani, A. Oxaliplatin immuno hybrid nanoparticles for active targeting: An approach for enhanced apoptotic activity and drug delivery to colorectal tumors. Drug Deliv. 2016, 23, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Du, L.; Wang, Y.; Liu, X.; Tian, H.; Wang, L.; Li, P.; Zhao, Y.; Duan, W.; et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol. Cancer 2019, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, H.Z.; Fu, Z.X.; Lu, W.D. Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol. 2011, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, C.; Wang, A.Z.; Lin, W. Application of liposomal technologies for delivery of platinum analogs in oncology. Int. J. Nanomed. 2013, 8, 3309–3319. [Google Scholar]
- Yang, C.; Liu, H.Z.; Lu, W.D.; Fu, Z.X. PEG-liposomal oxaliplatin potentialization of antitumor efficiency in a nude mouse tumor-xenograft model of colorectal carcinoma. Oncol. Rep. 2011, 25, 1621–1628. [Google Scholar]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym. Plast. Technol. Mater 2021, 60, 1–29. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, W.; Dong, H.; Zhang, W.; Mao, J.; Dai, Y. Combination of Oxaliplatin and Vit.E-TPGS in Lipid Nanosystem For Enhanced Therapeutic Efficacy in Colon Cancers. Pharm. Res. 2018, 35, 27. [Google Scholar] [CrossRef]
- Luiza, A.C.; Oliveira, D.S.L.; Fernandes, R.; Júnior, D.A.; Carvalho, T.G.D.; Chan, A.B.; Timo, S.; Tamburini, F.; Geus-Oei, L.F.D.; Cruz, L.J. Effect of Oxaliplatin-Loaded Poly(d,l-Lactide-co-Glycolic Acid) (PLGA) Nanoparticles Combined with Retinoic Acid and Cholesterol on Apoptosis, Drug Resistance, and Metastasis Factors of Colorectal Cancer. Pharmaceutics 2020, 12, 193. [Google Scholar]
- Narmani, A.; Kamali, M.; Amini, B.; Salimi, A.; Panahi, Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies, Process. Biochem 2018, 69, 178–187. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reisabc, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B. 2020, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Shankar, S.J.; Munisamy, M.; Akshatha, R.S.; Sagar, V.S. Development of pH-dependent chronomodulated delivery systems of 5-fluorouracil and oxaliplatin to treat colon cancer. Int. J. App. Pharm. 2020, 12, 118–130. [Google Scholar]
- Maspes, A.; Pizzetti, F.; Rossetti, A.; Makvandi, F.; Sitia, G.; Rossi, F. Advances in Bio-Based Polymers for Colorectal Cancer Treatment: Hydrogels and Nanoplatforms. Ges 2021, 7, 6. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine 2010, 6, 179–190. [Google Scholar] [CrossRef]
- Selim, A.; Lila, A.; Kiwada, H.; Ishida, T. Selective Delivery of Oxaliplatin to Tumor Tissue by Nanocarrier System Enhances Overall Therapeutic Efficacy of the Encapsulated Oxaliplatin. Biol. Pharm. Bull. 2014, 37, 206–211. [Google Scholar]
- Hassanzadeganroudsari, M.; Apostolopoulos, V.; Nurgali, K. Development and characterization of targeted nanoparticles loaded with Oxaliplatin for colorectal cancer treatment. J. Nanomed. Nanotechnol. 2018, 9, 41. [Google Scholar]
- Urbanska, A.M.; Karagiannis, E.D.; Guajardo, G.; Langer, R.S.; Anderson, D.G. Therapeutic effect of orally administered microencapsulated oxaliplatin for colorectal cancer. Biomaterials 2012, 33, 4752–4761. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Lipid-Based Drug Delivery Nanoplatforms for Colorectal Cancer Therapy. Nanomaterials 2020, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G. Introduction to Carbon Materials Research. Topics Appl. Phys. 2001, 80, 1–9. [Google Scholar]
- Debnath, K.S.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 644564. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef] [PubMed]
- Kukovecz, A.; Kozma, G.; Konya, Z. Multi-Walled Carbon Nanotubes. In Springer Handbook of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–188. [Google Scholar]
- Lee, P.C.; Lin, C.Y.; Peng, C.L.; Shieh, M.J. Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater. Sci. 2016, 4, 1742–1753. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated multi-walled carbon nanotubes for encapsulation and sustained release of oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef]
- Mordorski, B.; Friedman, A. Chapter 4—Metal Nanoparticles for Microbial Infection. A Strategy to Address Microbial Drug Resistance Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 77–109. [Google Scholar]
- Mody, V.V.; Siwale, R.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Wheate, N.J.; Collins, J.G. Multi-Nuclear Platinum Drugs: A New Paradigm in Chemotherapy. Curr. Med. Chem Anti-Cancer Agents 2005, 5, 267–279. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling§, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4680. [Google Scholar] [CrossRef]
- Gholami, M.; Hekmat, A.; Khazaei, M.; Darroudi, M. OXA-CuS@ UiO-66-NH2 as a drug delivery system for Oxaliplatin to colorectal cancer cells. J. Mater. Sci.: Mater. Med. 2022, 33, 26. [Google Scholar] [CrossRef]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–Biomimetic Magnetic Nanoparticle Assemblies for Colon Cancer-Targeted Chemotherapy: An In Vitro Study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Prados, P.J.; Melguizo, J.; Ortiz, C.; Perazzoli, R.; Cabeza, G.; Alvarez, L. Colon cancer therapy: Recent developments in nanomedicine to improve the efficacy of conventional chemotherapeutic drugs. Anticancer Agents Med. Chem. 2013, 13, 1204–1216. [Google Scholar] [CrossRef]
- Hu, L.; Aryal, C.M.; Zhang, S. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Cavalieri, F. Engineering nanomedicines to overcome multidrug resistance in cancer therapy. Curr. Med. Chem. 2016, 23, 3–22. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Vahidnezhad, H.; Youssefian, L.; Mosafer, J.; Baradaran, B.; Uitto, A.J. Applications of spherical nucleic acid nanoparticles as delivery systems. Trends Mol. Med. 2019, 25, 1066–1079. [Google Scholar] [CrossRef]
- Blanco, E.; Hsiao, A.; Mann, A.P.; Landry, M.G.; Meric-Bernstam, F. Nanomedicine in cancer therapy: Innovative trends and prospects. Cancer Sci. 2011, 102, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Sengupta, S. Nanoparticles in cancer chemotherapy. Prog. Mol. Biol. Transl. Sci. 2011, 104, 489–507. [Google Scholar]
- Li, S.; Wang, A.; Jiang, W.; Guan, Z. Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer. 2008, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, B.; Deeba, F.; Bano, S.; Kulshreshtha, A. Lipophilic 5-fluorouracil prodrug encapsulated xylan-stearic acid conjugates nanoparticles for colon cancer therapy. Int. J. Biol. Macromol. 2019, 128, 204–213. [Google Scholar]
- Batist, G.; Gelmon, K.A.; Chi, K.N.; Miller, W.J.; Chia, S.K.; Mayer, L.D. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 692–700. [Google Scholar] [CrossRef]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: Extract standardization, nanoparticle optimization, and cytotoxicity evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef] [PubMed]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent advances in designing 5-fluorouracil delivery systems: A stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016, 513, 648–658. [Google Scholar] [CrossRef]
- Cevenini, A.; Celia, C.; Orrù, S.; Sarnataro, D.; Raia, M.; Mollo, V.; Locatelli, M.; Imperlini, E.; Peluso, N.; Peltrini, R.; et al. Liposome-embedding silicon microparticle for oxaliplatin delivery in tumor chemotherapy. Pharmaceutics 2020, 12, 559. [Google Scholar] [CrossRef]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. Ph Responsive PEG-shedding and targeting peptide-modified nanoparticles for dual-delivery of irinotecan and microRNA to enhance tumor-specific therapy. Small 2019, 15, e1903296. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Chan, R.; Ji, Y.; Lu, J.; Liao, Y.P. Improved efficacy and reduced toxicity using a custom-designed irinotecan-delivering silicasome for orthotopic colon cancer. ACS Nano 2019, 13, 38–53. [Google Scholar] [CrossRef]
- Huang, J.R.; Lee, M.H.; Li, W.S.; Wu, H.C. Liposomal irinotecan for treatment of colorectal cancer in a preclinical model. Cancers 2019, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, X.; Chen, Y.; Lee, R.J.; Wang, J.; Yao, J. Anticancer activity of polymeric nanoparticles containing linoleic acid-SN38 (LA-SN38) conjugate in a murine model of colorectal cancer. Colloids Surf. B Biointerfaces 2019, 181, 822–829. [Google Scholar] [CrossRef]
- Ning, S.T.; Lee, S.Y.; Wei, M.F.; Peng, C.L.; Lin, S.Y.; Tsai, M.H. Targeting Colorectal cancer stem-like cells with Anti-CD133 antibody conjugated SN-38 nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17793–17804. [Google Scholar] [CrossRef]
- Han, W.; Xie, B.; Li, Y.; Shi, L.; Wan, J.; Chen, X. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q. Co-Administration Of iRGD enhances tumor-targeted delivery and antitumor effects of paclitaxel-loaded PLGA nanoparticles for colorectal cancer treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef]
- Dehvari, K.; Chen, Y.; Tsai, Y.H.; Tseng, S.H.; Lin, K.S. Superparamagnetic iron oxide nanorod carriers for paclitaxel delivery in the treatment and imaging of colon cancer in mice. J. Biomed. Nanotechnol. 2016, 12, 1734–1745. [Google Scholar] [CrossRef]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth, K.B.; Sarkar, D.A. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Dinarvand, R.; Ostad, S.N. Chitosan- Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar]
- Chuah, L.H.; Billa, N.; Roberts, C.J.; Burley, J.C.; Manickam, S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm. Dev. Technol. 2013, 18, 591–599. [Google Scholar] [CrossRef]
- Wang, C.; Ho, P.C.; Lim, L.Y. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int. J. Pharm. 2010, 400, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Wang, T.; Yin, W.; Tran, T.; Nguyen, T.; Lee, B.J. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, G.; Song, D.; Gao, R.; Teng, P.; Zhou, L. Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater. Sci. 2019, 7, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J.; Le, V.M.; Gong, Q.; Li, S.; Gao, F. EpCAM Aptamer functionalized cationic liposome-based nanoparticles loaded with miR-139-5p for targeted therapy in colorectal cancer. Mol. Pharm. 2019, 16, 4696–4710. [Google Scholar] [CrossRef] [PubMed]
- Minelli, R.; Serpe, L.; Pettazzoni, P.; Minero, V.; Barrera, G.; Gigliotti, C. Cholesteryl butyrate solid lipid nanoparticles inhibit the adhesion and migration of colon cancer cells. Br. J. Pharmacol. 2012, 166, 587–601. [Google Scholar] [CrossRef]
- Chen, W.; Hu, S. Suitable carriers for encapsulation and distribution of endostar: Comparison of endostar-loaded particulate carriers. Int. J. Nanomed. 2011, 6, 1535–1541. [Google Scholar]
- Marill, J.; Mohamed, A.N.; Paris, S. DNA damage enhancement by radiotherapy-activated hafnium oxide nanoparticles improves cGAS-STING pathway activation in human colorectal cancer cells. Radiother. Oncol. 2019, 141, 262–266. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Park, C.; Koo, H.; Kim, J.H.; Hong, K. Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef]
- Kumagai, M.; Sarma, T.K.; Cabral, H.; Kaida, S.; Sekino, M. Enhanced in vivo magnetic resonance imaging of tumors by PEGylated iron-oxide-gold core-shell nanoparticles with prolonged blood circulation properties. Macromol. Rapid Commun. 2010, 31, 1521–1528. [Google Scholar] [CrossRef]
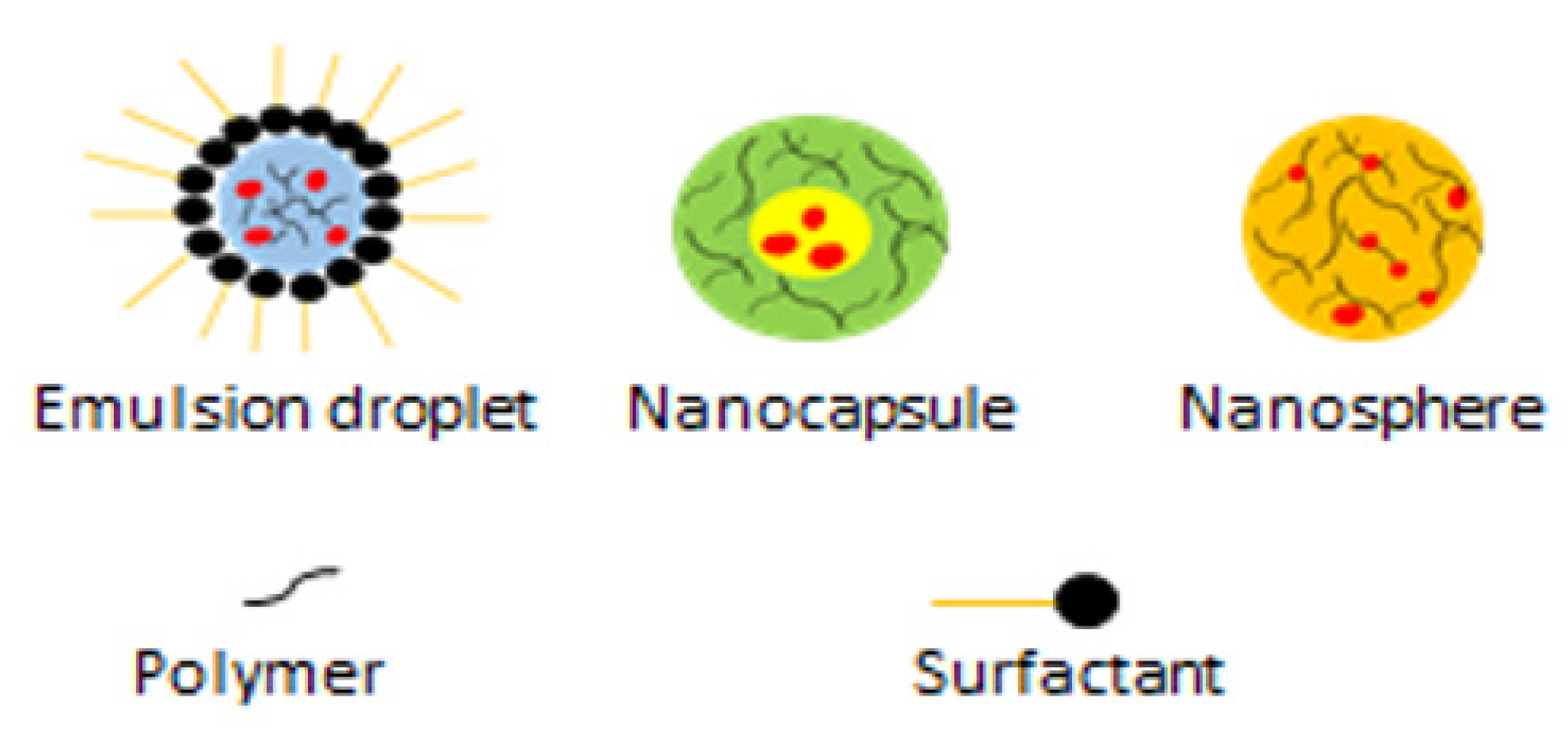
| Nanoparticle Formulations | Drugs Used | Type of Nanoparticles | References |
|---|---|---|---|
| FOLFOX | 5-FU, Oxaliplatin | Lipid-based NPs | [121] |
| 5-FU/PEG-PBLG | 5-FU | Polymeric NPs | [155] |
| HACTNP | 5-FU | Polymeric NPs | [156] |
| Xyl-SA/5-FUSA | 5-FU | Conjugate NPs | [157] |
| CPX-1 | Irinotecan HCl | Liposomes | [158] |
| SLNPs containing 5-FU | 5-FU | Solid NPs | [159,160] |
| 5-FU/GSH-GNPs | 5-FU | Conjugate NPs | [161] |
| Chitosan-HA-Oxa NPs | Oxaliplatin | Polymeric NPs | [153] |
| Oxaliplatin encapsulated in chitosan-coated alginate microspheres | Oxaliplatin | Polymeric NPs | [133] |
| PEG-liposomal L-oHP | Oxaliplatin | Polymeric NPs | [116] |
| Liposome-embedding silicon microparticle | Oxaliplatin | Liposomes | [162] |
| Nanoscale coordination polymer (NCP) core–shell particles | Oxaliplatin, DHA | Liposomes | [128] |
| pH-responsive PEG-shedding and targeting Peptide-modified nanoparticles | Irinotecan, miR-200 | Polymeric NPs | [163] |
| Lipid bilayer-coated MSNP carrier | Irinotecan | Liposomes and Polymeric NPs | [164] |
| Liposomal irinotecan (Lipo-IRI) | Irinotecan | Liposomes | [165] |
| SN38 (LA-SN38)-loaded NPs | SN38 | Lipid-based NPs | [166] |
| CD133Ab-NPs-SN-38 | SN38 | Polymeric NPs | [167] |
| nSN38 | nCUR SN38, curcumin | Conjugated NPs | [168] |
| PLGA-PTX | Paclitaxel | Polymeric NPs | [169] |
| Paclitaxel-loaded magnetic nanocarriers | Paclitaxel | Polymeric NPs | [170] |
| Celecoxib-containing Hap-Cht NPs | Celecoxib | Conjugated NPs | [171] |
| Chitosan NPs | Gemcitabine, curcumin | Polymeric NPs | [172,173] |
| WGA-conjugated PLGA NPs loaded with Pac | Paclitaxel | Conjugated NPs | [174] |
| Aspirin-loaded nanoexosomes | Aspirin | Conjugated NPs | [175] |
| A33Ab-US-Exo/Dox | Doxorubicin | Conjugated NPs | [176] |
| EGFR-targeted evodiamine NPs | Evodiamine | Polymeric NPs | [177] |
| miR-139-5p-EpCAM Apt-HSPC/DOTAP/Chol/DSPE-PEG2000-COOH nanoparticles | MANPs miR-139-5p | Liposomes | [178] |
| Chol-butyrate SLNP formulation | Butyric acid | Lipid-based NPs | [179] |
| PEG-PLGA-endostar | Endostatin | Polymeric NPs | [180] |
| Hafnium oxide nanoparticles (NBTXR3) | - | NBTX 3 | [181] |
| Silver nanoparticles (AgNPs) | - | Metallic NPs | [182] |
| PEG-AuIONs | - | Polymeric NPs | [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyana, B.; Naki, T.; Alven, S.; Aderibigbe, B.A. Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 11261. https://doi.org/10.3390/ijms231911261
Buyana B, Naki T, Alven S, Aderibigbe BA. Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy. International Journal of Molecular Sciences. 2022; 23(19):11261. https://doi.org/10.3390/ijms231911261
Chicago/Turabian StyleBuyana, Buhle, Tobeka Naki, Sibusiso Alven, and Blessing Atim Aderibigbe. 2022. "Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy" International Journal of Molecular Sciences 23, no. 19: 11261. https://doi.org/10.3390/ijms231911261
APA StyleBuyana, B., Naki, T., Alven, S., & Aderibigbe, B. A. (2022). Nanoparticles Loaded with Platinum Drugs for Colorectal Cancer Therapy. International Journal of Molecular Sciences, 23(19), 11261. https://doi.org/10.3390/ijms231911261








