Rhamnolipid Micellization and Adsorption Properties
Abstract
1. Introduction
2. Results and Discussion
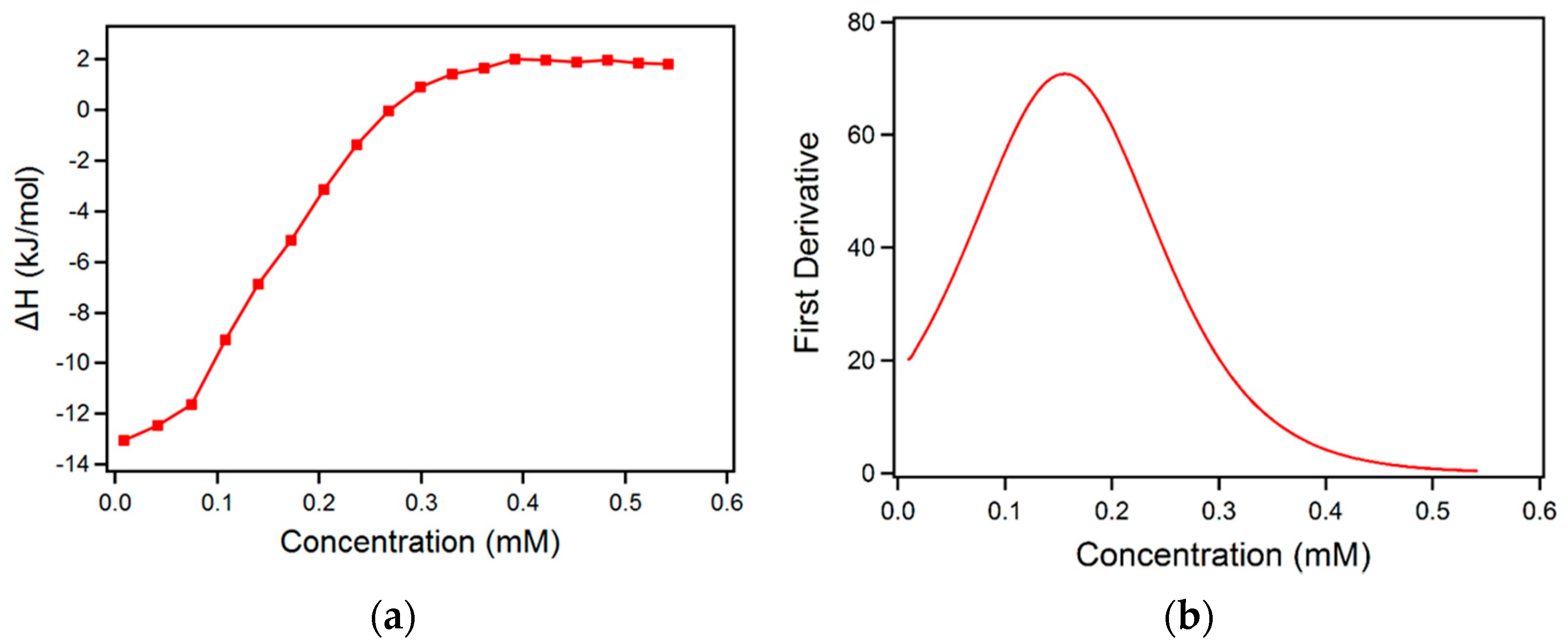
2.1. Micellization in Aqueous Solution
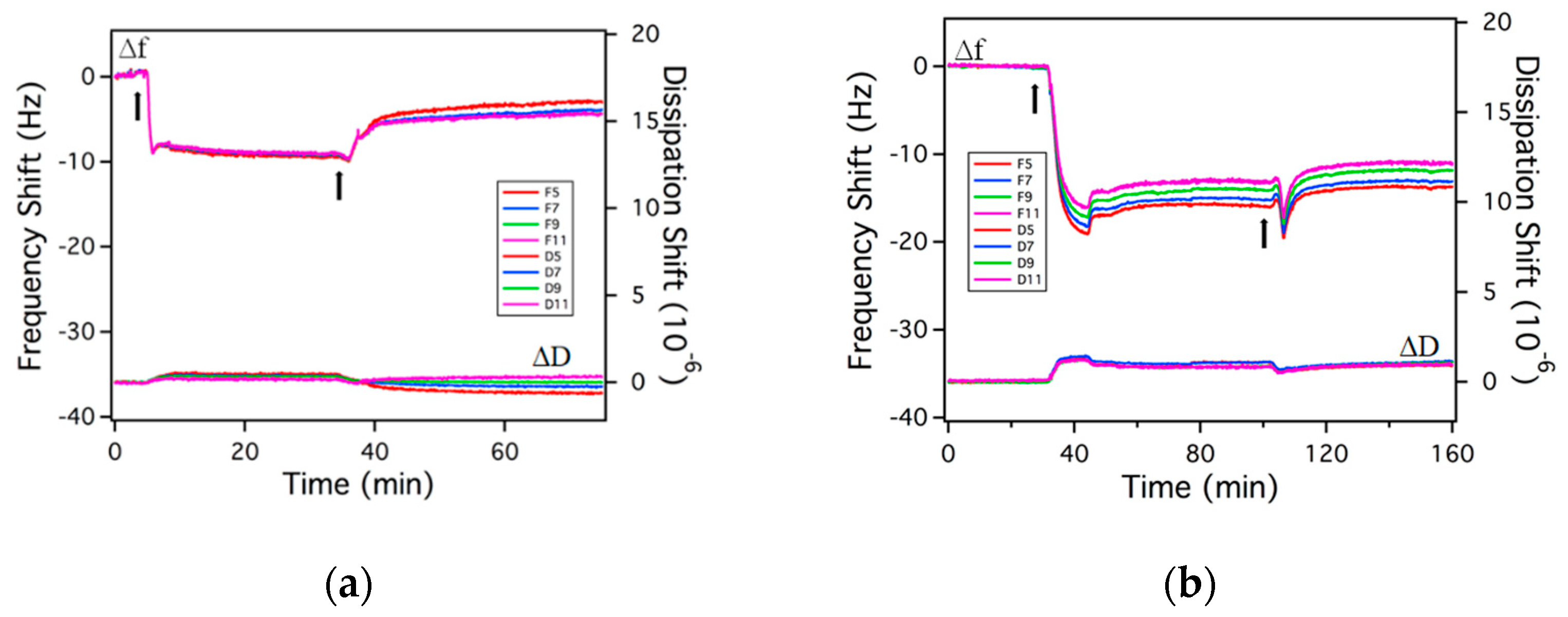
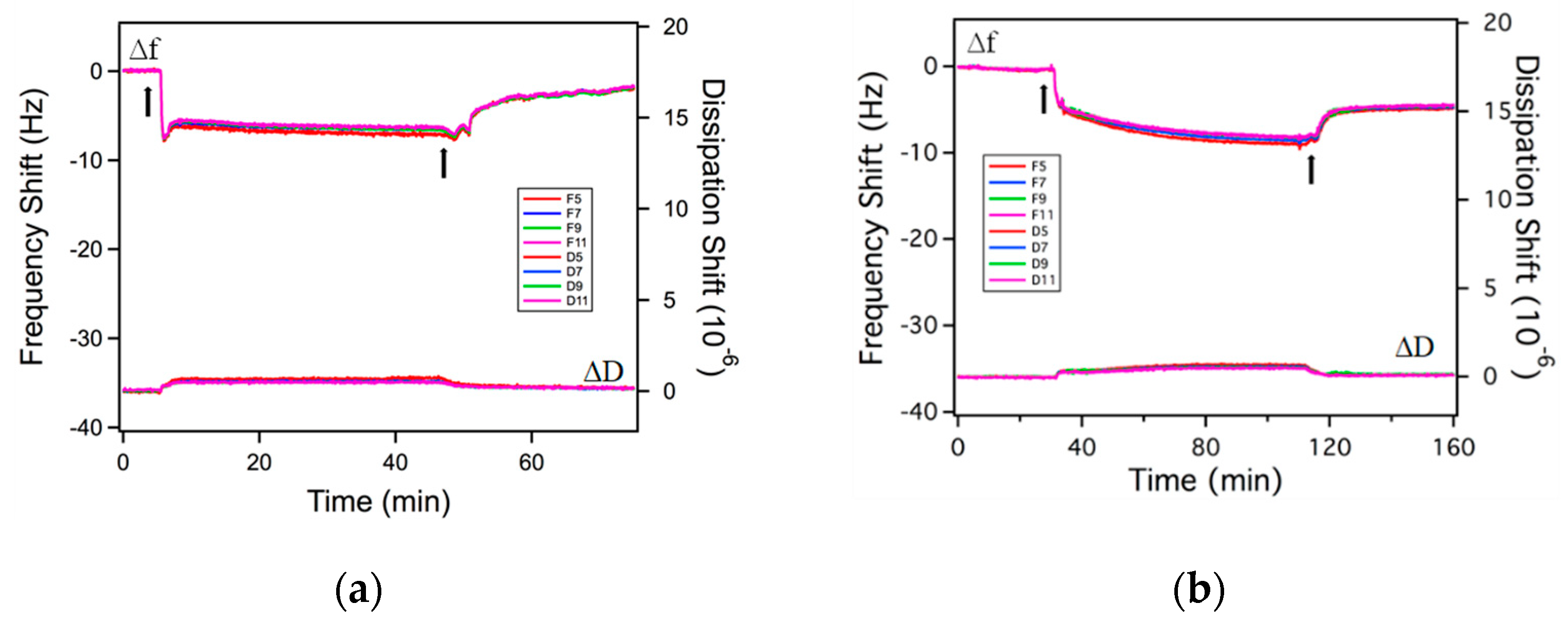
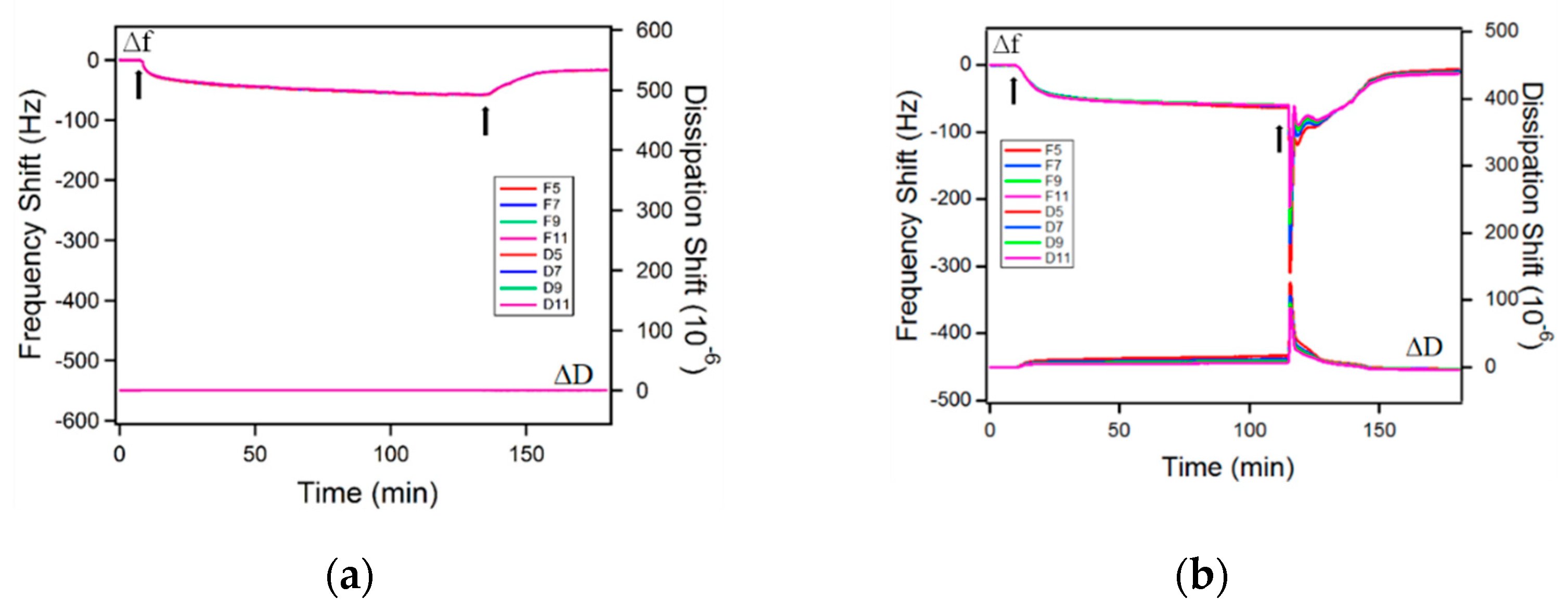
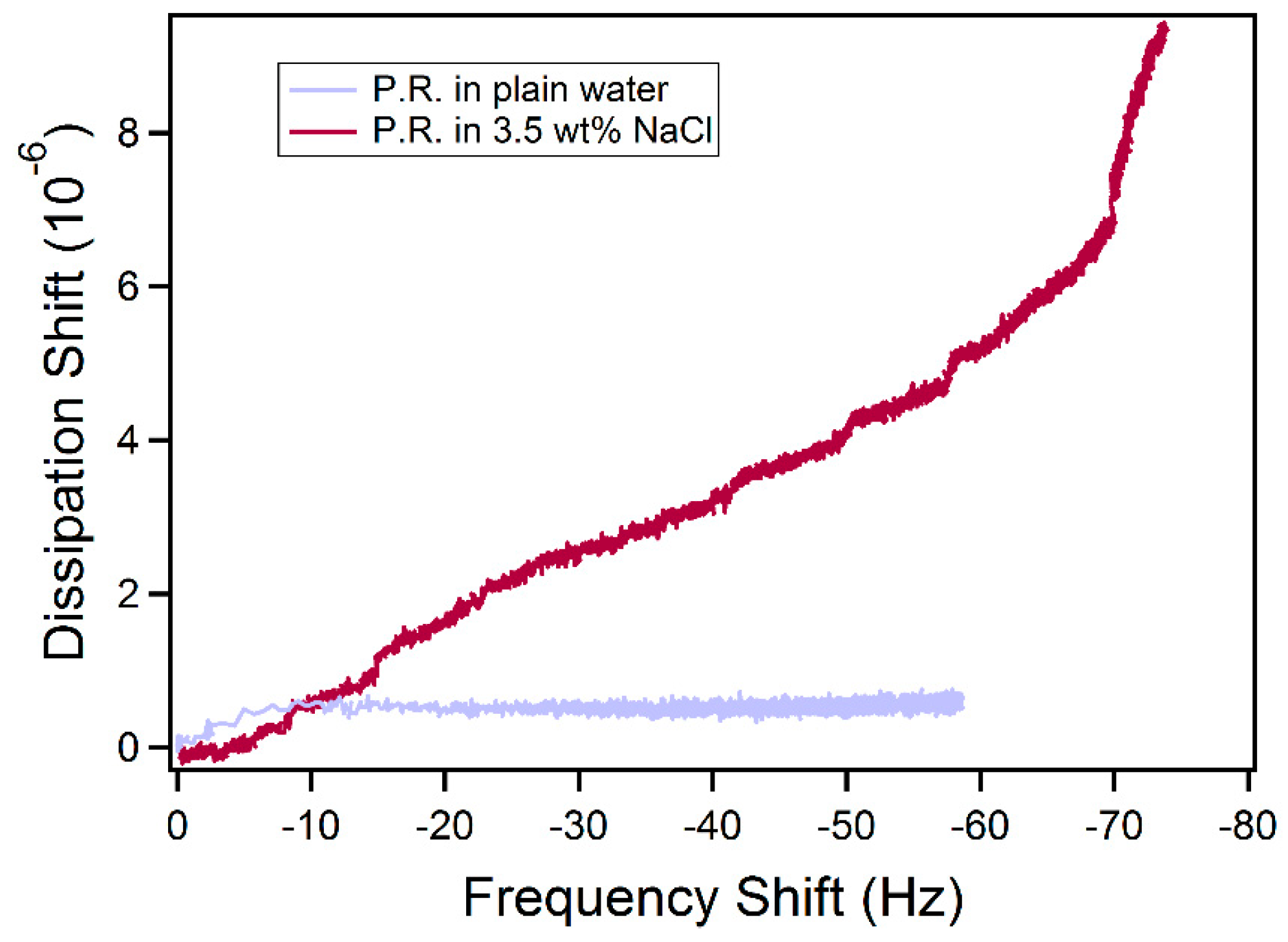
2.2. Adsorption on Solid Surfaces
3. Materials and Methods
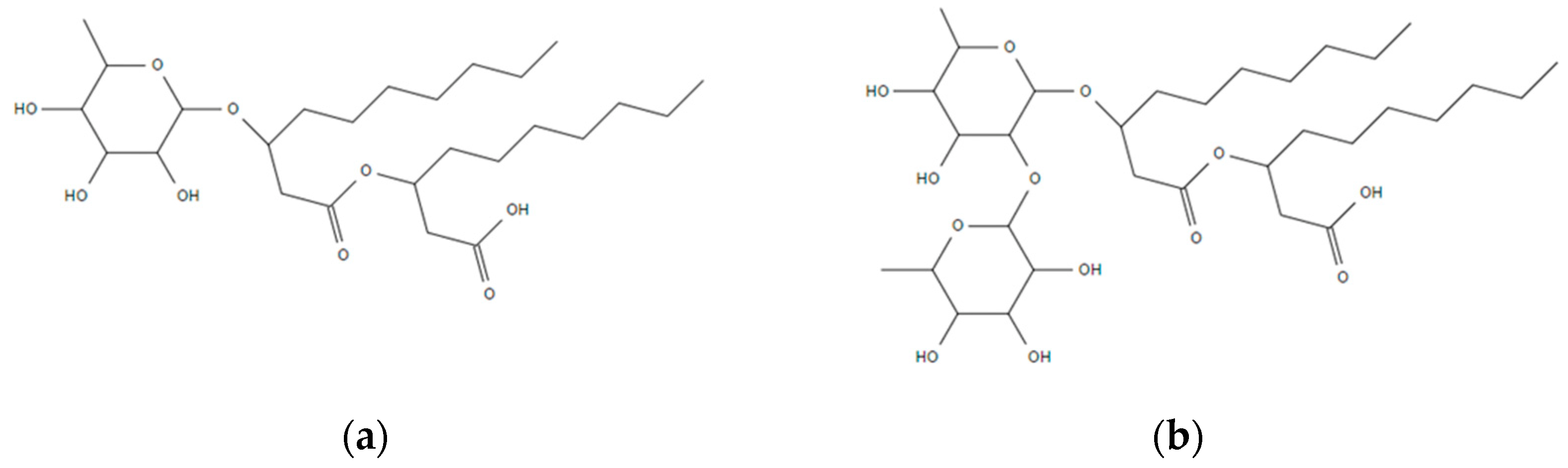
3.1. Rhamnolipids
3.2. Other Chemicals
3.3. Sample Preparation
3.4. Fluorescence Spectroscopy
3.5. Isothermal Titration Calorimetry
3.6. Quartz Crystal Microbalance with Dissipation (QCM-D)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. [Google Scholar] [CrossRef]
- Souza, E.C.; Vessoni-Penna, T.C.; de Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Alexandridis, P. Rheological properties of oppositely charged polyelectrolyte−surfactant mixtures: Effect of polymer molecular weight and surfactant architecture. Macromolecules 2001, 34, 5005–5018. [Google Scholar] [CrossRef]
- Kancharla, S.; Bedrov, D.; Tsianou, M.; Alexandridis, P. Structure and composition of mixed micelles formed by nonionic block copolymers and ionic surfactants in water determined by small-angle neutron scattering with contrast variation. J. Colloid Interface Sci. 2022, 609, 456–468. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Sarkar, B.; Alexandridis, P. Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Adv. Colloid Interface Sci. 2017, 244, 132–163. [Google Scholar] [CrossRef]
- Chandaroy, P.; Sen, A.; Alexandridis, P.; Hui, S.W. Utilizing temperature-sensitive association of Pluronic F-127 with lipid bilayers to control liposome-cell adhesion. Biochim. Biophys. Acta-Biomembr. 2002, 1559, 32–42. [Google Scholar] [CrossRef]
- Kaizu, K.; Alexandridis, P. Effect of surfactant phase behavior on emulsification. J. Colloid Interface Sci. 2016, 466, 138–149. [Google Scholar] [CrossRef]
- Save, M.; Hellaye, M.L.; de Villedon, V.; Adoumaz, I.; Pillet, M.; Atanase, L.; Lahcini, M.; Deniau, E.; Khoukh, A.; Pellerin, V.; et al. Biosourced Polymeric Emulsifiers for Miniemulsion Copolymerization of Myrcene and Styrene: Toward Biobased Waterborne Latex as Pickering Emulsion Stabilizer. Biomacromolecules 2022, 23, 2536–2551. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Wu, J.; Jahan, R.; Sarkar, B.; Tsianou, M.; Alexandridis, P. Mono- and di-valent salts as modifiers of PEO-PPO-PEO block copolymer interactions with silica nanoparticles in aqueous dispersions. J. Dispers. Sci. Technol. 2015, 36, 1806–1815. [Google Scholar] [CrossRef]
- Lin, Y.; Smith, T.W.; Alexandridis, P. Adsorption of a polymeric siloxane surfactant on carbon black particles dispersed in mixtures of water with polar organic solvents. J. Colloid Interface Sci. 2002, 255, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Tsianou, M. Block copolymer-directed metal nanoparticle morphogenesis and organization. Eur. Polym. J. 2011, 47, 569–583. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Alexandridis, P.; Mallory, R.; Petrou, A.; Mountziaris, T.J. Templated synthesis of ZnSe nanostructures using lyotropic liquid crystals. Nanotechnology 2005, 16, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Benhur, A.M.; Pingali, S.; Amin, S. Application of Biosurfactants and Biopolymers in Sustainable Cosmetic Formulation Design. J. Cosmet. Sci. 2020, 71, 455–480. [Google Scholar]
- John, V.; Arnosti, C.; Field, J.; Kujawinski, E.; McCormick, A. The role of dispersants in oil spill remediation: Fundamental concepts, rationale for use, fate, and transport issues. Oceanography 2016, 29, 108–117. [Google Scholar] [CrossRef]
- Atta, D.Y.; Negash, B.M.; Yekeen, N.; Habte, A.D. A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J. Mol. Liq. 2021, 321, 114888. [Google Scholar] [CrossRef]
- Fragkou, E.; Antoniou, E.; Daliakopoulos, I.; Manios, T.; Theodorakopoulou, M.; Kalogerakis, N. In Situ Aerobic Bioremediation of Sediments Polluted with Petroleum Hydrocarbons: A Critical Review. J. Mar. Sci. Eng. 2021, 9, 1003. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Priya, N.S.; Doble, M.; Sangwai, J.S. Bioremediation of costal and marine pollution due to crude oil using a microorganism Bacillus subtilis. Procedia Eng. 2015, 116, 213–220. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 1998, 20, 48–52. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Macroscopic and microscopic properties of some surfactants and biosurfactants. Int. J. Mol. Sci. 2018, 19, 1934. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z.; Rosenberg, E. Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Yang, X.; Liu, Y.; Shao, B.; Liu, Z. Advances in applications of rhamnolipids biosurfactant in environmental remediation: A review. Biotechnol. Bioeng. 2018, 115, 796–814. [Google Scholar] [CrossRef]
- Sekhon Randhawa, K.K.; Rahman, P.K.S.M. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Jin, L.; Black, W.; Sawyer, T. Application of Environment-Friendly Rhamnolipids against Transmission of Enveloped Viruses Like SARS-CoV2. Viruses 2021, 13, 322. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactants: Production and application prospects in the food industry. Biotechnol. Prog. 2020, 36, e3030. [Google Scholar] [CrossRef]
- Available online: https://mayluclean.com/ (accessed on 1 July 2022).
- Available online: https://www.jeneilbiotech.com/ (accessed on 1 July 2022).
- Costa, S.G.V.A.O.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 2010, 45, 1511–1516. [Google Scholar] [CrossRef]
- Lan, G.; Fan, Q.; Liu, Y.; Chen, C.; Li, G.; Liu, Y.; Yin, X. Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem. Eng. J. 2015, 101, 44–54. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl. Biochem. Biotechnol. 2010, 160, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; Taherzadeh, M.J.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; You, S.M.; Teixeira, J.A.; Bui, X.T. Bio-based rhamnolipids production and recovery from waste streams: Status and perspectives. Bioresour. Technol. 2021, 319, 124213. [Google Scholar] [CrossRef] [PubMed]
- Pornsunthorntawee, O.; Chavadej, S.; Rujiravanit, R. Solution properties and vesicle formation of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa SP4. Colloids Surf. B Biointerfaces 2009, 72, 6–15. [Google Scholar] [CrossRef]
- Jiang, J.J.; Jin, M.J.; Li, X.Y.; Meng, Q.; Niu, J.; Long, X.W. Recent progress and trends in the analysis and identification of rhamnolipids. Appl. Microbiol. Biotechnol. 2020, 104, 8171–8186. [Google Scholar] [CrossRef]
- Penfold, J.; Thomas, R.K.; Shen, H.-H. Adsorption and self-assembly of biosurfactants studied by neutron reflectivity and small angle neutron scattering: Glycolipids, lipopeptides and proteins. Soft Matter 2012, 8, 578–591. [Google Scholar] [CrossRef]
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Thermodynamic properties of rhamnolipid micellization and adsorption. Colloids Surf. B Biointerfaces 2014, 119, 22–29. [Google Scholar] [CrossRef]
- Monteiro, S.A.; Sassaki, G.L.; de Souza, L.M.; Meira, J.A.; de Araújo, J.M.; Mitchell, D.A.; Ramos, L.P.; Krieger, N. Molecular and structural characterization of the biosurfactant produced by Pseudomonas aeruginosa DAUPE 614. Chem. Phys. Lipids 2007, 147, 1–13. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Kłosowska-Chomiczewska, I.E.; Mędrzycka, K.; Hallmann, E.; Karpenko, E.; Pokynbroda, T.; Macierzanka, A.; Jungnickel, C. Rhamnolipid CMC prediction. J. Colloid Interface Sci. 2017, 488, 10–19. [Google Scholar] [CrossRef]
- Caruso, F.; Serizawa, T.; Furlong, D.N.; Okahata, Y. Quartz crystal microbalance and surface plasmon resonance study of surfactant adsorption onto gold and chromium oxide surfaces. Langmuir 1995, 11, 1546–1552. [Google Scholar] [CrossRef]
- Noordman, W.H.; Brusseau, M.L.; Janssen, D.B. Adsorption of a multicomponent rhamnolipid surfactant to soil. Environ. Sci. Technol. 2000, 34, 832–838. [Google Scholar] [CrossRef]
- Özdemir, G.; Peker, S.; Helvaci, S.S. Effect of pH on the surface and interfacial behavior of rhamnolipids R1 and R2. Colloids Surf. A Physicochem. Eng. Asp. 2004, 234, 135–143. [Google Scholar] [CrossRef]
- Singh, A.K.; Cameotra, S.S. Rhamnolipids production by multi-metal-resistant and plant-growth-promoting rhizobacteria. Appl. Biochem. Biotechnol. 2013, 170, 1038–1056. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Hu, Y.-Y. Solubilization of moderately hydrophobic 17α-ethinylestradiol by mono- and di-rhamnolipid solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 12–20. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Chou, C.-L.; Chang, J.-S. Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem. Eng. J. 2005, 27, 146–154. [Google Scholar] [CrossRef]
- Rodrigues, A.I.; Gudina, E.J.; Teixeira, J.A.; Rodrigues, L.R. Sodium chloride effect on the aggregation behaviour of rhamnolipids and their antifungal activity. Sci. Rep. 2017, 7, 12907. [Google Scholar] [CrossRef]
- Lebrón-Paler, A.; Pemberton, J.E.; Becker, B.A.; Otto, W.H.; Larive, C.K.; Maier, R.M. Determination of the acid dissociation constant of the biosurfactant monorhamnolipid in aqueous solution by potentiometric and spectroscopic methods. Anal. Chem. 2006, 78, 7649–7658. [Google Scholar] [CrossRef]
- Abbasi, H.; Noghabi, K.A.; Hamedi, M.M.; Zahiri, H.S.; Moosavi-Movahedi, A.A.; Amanlou, M.; Teruel, J.A.; Ortiz, A. Physicochemical characterization of a monorhamnolipid secreted by Pseudomonas aeruginosa MA01 in aqueous media. An experimental and molecular dynamics study. Colloids Surf. B Biointerfaces 2013, 101, 256–265. [Google Scholar] [CrossRef]
- Yutaka, I.; Yasuo, G.; Hitoshi, N.; Muneo, Y.; Hisae, N.; Toshio, K. The pH-sensitive conversion of molecular aggregates of rhamnolipid biosurfactant. Chem. Lett. 1987, 16, 763–766. [Google Scholar] [CrossRef]
- Ikizler, B.; Arslan, G.; Kipcak, E.; Dirik, C.; Celenk, D.; Aktuglu, T.; Helvaci, S.S.; Peker, S. Surface adsorption and spontaneous aggregation of rhamnolipid mixtures in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 125–136. [Google Scholar] [CrossRef]
- Helvacı, Ş.Ş.; Peker, S.; Özdemir, G. Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloids Surf. B Biointerfaces 2004, 35, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Fajalia, A.I.; Tsianou, M. Charging and uncharging a neutral polymer in solution: A small-angle neutron scattering investigation. J. Phys. Chem. B 2014, 118, 10725–10739. [Google Scholar] [CrossRef]
- Benrraou, M.; Bales, B.L.; Zana, R. Effect of the nature of the counterion on the properties of anionic surfactants. 1. Cmc, ionization degree at the cmc and aggregation number of micelles of sodium, cesium, tetramethylammonium, tetraethylammonium, tetrapropylammonium, and tetrabutylammonium dodecyl sulfates. J. Phys. Chem. B 2003, 107, 13432–13440. [Google Scholar] [CrossRef]
- Chatterjee, A.; Moulik, S.P.; Sanyal, S.K.; Mishra, B.K.; Puri, P.M. Thermodynamics of micelle formation of ionic surfactants: A critical assessment for sodium dodecyl sulfate, cetyl pyridinium chloride and dioctyl sulfosuccinate (Na salt) by microcalorimetric, conductometric, and tensiometric measurements. J. Phys. Chem. B 2001, 105, 12823–12831. [Google Scholar] [CrossRef]
- Marcolongo, J.P.; Mirenda, M. Thermodynamics of Sodium Dodecyl Sulfate (SDS) Micellization: An Undergraduate Laboratory Experiment. J. Chem. Educ. 2011, 88, 629–633. [Google Scholar] [CrossRef]
- Fajalia, A.I.; Antoniou, E.; Alexandridis, P.; Tsianou, M. Self-assembly of sodium bis(2-ethylhexyl) sulfosuccinate in aqueous solutions: Modulation of micelle structure and interactions by cyclodextrins investigated by small-angle neutron scattering. J. Mol. Liq. 2015, 210, 125–135. [Google Scholar] [CrossRef]
- Patel, S.G.; Bummer, P.M. Thermodynamics of aggregate formation between a non-ionic polymer and ionic surfactants: An isothermal titration calorimetric study. Int. J. Pharm. 2017, 516, 131–143. [Google Scholar] [CrossRef]
- Dutkiewicz, E.; Jakubowska, A. Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles. Colloid Polym. Sci. 2002, 280, 1009–1014. [Google Scholar] [CrossRef]
- Umlong, I.M.; Ismail, K. Micellization of AOT in aqueous sodium chloride, sodium acetate, sodium propionate, and sodium butyrate media: A case of two different concentration regions of counterion binding. J. Colloid Interface Sci. 2005, 291, 529–536. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Alexandridis, P. Micellization thermodynamics of Pluronic P123 (EO20PO70EO20) amphiphilic block copolymer in aqueous ethylammonium nitrate (EAN) solutions. Polymers 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.J.; Espuny, M.J.; Marqués, A.; Teruel, J.A.; Manresa, Á.; Ortiz, A. Thermodynamics of the interaction of a dirhamnolipid biosurfactant secreted by pseudomonas aeruginosa with phospholipid membranes. Langmuir 2007, 23, 2700–2705. [Google Scholar] [CrossRef] [PubMed]
- Zana, R. Critical micellization concentration of surfactants in aqueous solution and free energy of micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X. Special oleophobic and hydrophilic surfaces: Approaches, mechanisms, and applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Superhydrophilic (superwetting) surfaces: A review on fabrication and application. J. Ind. Eng. Chem. 2017, 47, 19–40. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B. Correlation between surface free energy of quartz and its wettability by aqueous solutions of nonionic, anionic and cationic surfactants. J. Colloid Interface Sci. 2009, 340, 243–248. [Google Scholar] [CrossRef]
- Zhang, H.; Khatibi, M.; Zheng, Y.; Lee, K.; Li, Z.; Mullin, J.V. Investigation of OMA formation and the effect of minerals. Mar. Pollut. Bull. 2010, 60, 1433–1441. [Google Scholar] [CrossRef]
- Ozcan, O. Classification of minerals according to their critical surface tension of wetting values. Int. J. Miner. Processing 1992, 34, 191–204. [Google Scholar] [CrossRef]
- Knock, M.M.; Sanii, L.S. Effect of hydrophobization of gold QCM-D crystals on surfactant adsorption at the solid-liquid interface. ACS Symp. Ser. 2010, 1070, 175–192. [Google Scholar]
- Weckman, N.E.; Olsson, A.L.J.; Tufenkji, N. Evaluating the binding of selected biomolecules to cranberry derived proanthocyanidins using the Quartz Crystal Microbalance. Biomacromolecules 2014, 15, 1375–1381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thavorn, J.; Hamon, J.J.; Kitiyanan, B.; Striolo, A.; Grady, B.P. Competitive surfactant adsorption of AOT and Tween 20 on gold measured using a quartz crystal microbalance with dissipation. Langmuir 2014, 30, 11031–11039. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet; Wiley-VCH: New York, NY, USA, 1999. [Google Scholar]
- Alves, A.V.; Tsianou, M.; Alexandridis, P. Fluorinated Surfactant Adsorption on Mineral Surfaces: Implications for PFAS Fate and Transport in the Environment. Surfaces 2020, 3, 516–566. [Google Scholar] [CrossRef]
- Kancharla, S.; Alexandridis, P.; Tsianou, M. Sequestration of per- and polyfluoroalkyl substances (PFAS) by adsorption: Surfactant and surface aspects. Curr. Opin. Colloid Interface Sci. 2022, 58, 17. [Google Scholar] [CrossRef]
- Bleninger, T.; Niepelt, A.; Jirka, G. Desalination plant discharge calculator. Desalin. Water Treat. 2010, 13, 156–173. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Liu, X.; Vesterinen, A.-H.; Genzer, J.; Seppälä, J.V.; Rojas, O.J. Adsorption of PEO–PPO–PEO triblock copolymers with end-capped cationic chains of poly(2-dimethylaminoethyl methacrylate). Langmuir 2011, 27, 9769–9780. [Google Scholar] [CrossRef]
- Soltani, D.S.; Kohl, J.; Ju, L.-K. Rhamnolipid adsorption in soil: Factors, unique features, and considerations for use as green antizoosporic agents. J. Agric. Food Chem. 2016, 64, 3330–3337. [Google Scholar] [CrossRef]
- Renfro, T.D.; Xie, W.; Yang, G.; Chen, G. Rhamnolipid surface thermodynamic properties and transport in agricultural soil. Colloids Surf. B Biointerfaces 2014, 115, 317–322. [Google Scholar] [CrossRef]
- Nivaggioli, T.; Alexandridis, P.; Hatton, T.A.; Yekta, A.; Winnik, M.A. Fluorescence probe studies of Pluronic copolymer solutions as a function of temperature. Langmuir 1995, 11, 730–737. [Google Scholar] [CrossRef]
- Sarkar, B.; Ravi, V.; Alexandridis, P. Micellization of amphiphilic block copolymers in binary and ternary solvent mixtures. J. Colloid Interface Sci. 2013, 390, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Nivaggioli, T.; Hatton, T.A. Temperature effects on structural properties of Pluronic P104 and F108 PEO-PPO-PEO block copolymer solutions. Langmuir 1995, 11, 1468–1476. [Google Scholar] [CrossRef]
- Regev, O.; Zana, R. Aggregation behavior of Tyloxapol, a nonionic surfactant oligomer, in aqueous solution. J. Colloid Interface Sci. 1999, 210, 8–17. [Google Scholar] [CrossRef]
- He, Z.; Ma, Y.; Alexandridis, P. Comparison of ionic liquid and salt effects on the thermodynamics of amphiphile micellization in water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 159–168. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Panuganti, S.R.; Vargas, F.M.; Taghikhani, V.; Pishvaie, M.R.; Chapman, W.G. Asphaltene deposition in different depositing environments: Part 1. Model oil. Energy Fuels 2013, 28, 1617–1628. [Google Scholar] [CrossRef]
- Ekholm, P.; Blomberg, E.; Claesson, P.; Auflem, I.H.; Sjoblom, J.; Kornfeldt, A. A quartz crystal microbalance study of the adsorption of asphaltenes and resins onto a hydrophilic surface. J. Colloid Interface Sci. 2002, 247, 342–350. [Google Scholar] [CrossRef]
- Goual, L.; Horvath-Szabo, G.; Masliyah, J.H.; Xu, Z.H. Adsorption of bituminous components at oil/water interfaces investigated by quartz crystal microbalance: Implications to the stability of water-in-oil emulsions. Langmuir 2005, 21, 8278–8289. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Dudášová, D.; Silset, A.; Sjöblom, J. Quartz crystal microbalance monitoring of asphaltene adsorption/deposition. J. Dispers. Sci. Technol. 2008, 29, 139–146. [Google Scholar] [CrossRef]
- Plunkett, M.A.; Claesson, P.M.; Ernstsson, M.; Rutland, M.W. Comparison of the adsorption of different charge density polyelectrolytes: A quartz crystal microbalance and X-ray photoelectron spectroscopy study. Langmuir 2003, 19, 4673–4681. [Google Scholar] [CrossRef]
- Abudu, A.; Goual, L. Adsorption of crude oil on surfaces using quartz crystal microbalance with dissipation (QCM-D) under flow conditions. Energy Fuels 2009, 23, 1237–1248. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.-T. Application of Kevin-Voigt model in quantifying whey protein adsorption on polyethersulfone using QCM-D. J. Assoc. Lab. Autom. 2009, 14, 213–220. [Google Scholar] [CrossRef]
- Slavin, S.; Soeriyadi, A.H.; Voorhaar, L.; Whittaker, M.R.; Becer, C.R.; Boyer, C.; Davis, T.P.; Haddleton, D.M. Adsorption behaviour of sulfur containing polymers to gold surfaces using QCM-D. Soft Matter 2012, 8, 118–128. [Google Scholar] [CrossRef]
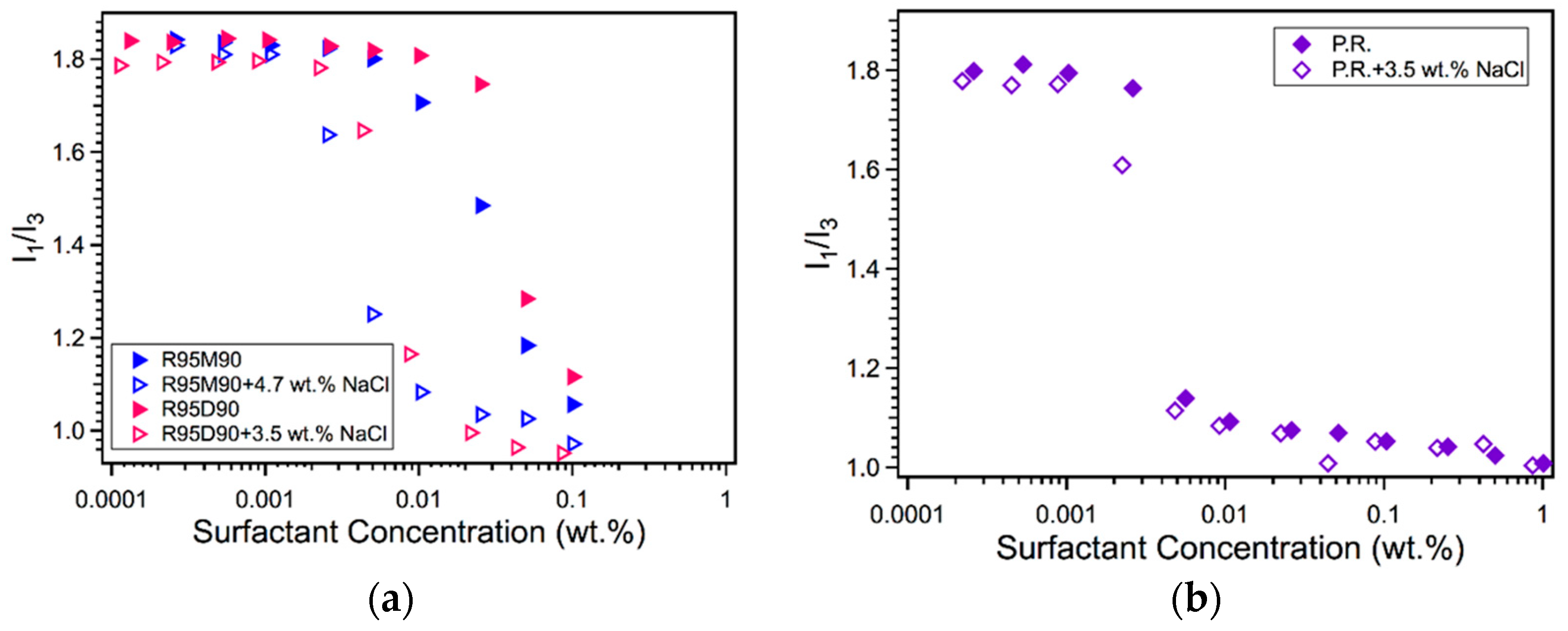

| Surfactant | NaCl (wt.%) | CMC (wt.%) | CMC (mM) |
|---|---|---|---|
| R95M90 | 0 | 0.059 | 1.17 |
| 4.7 | 0.011 | 0.22 | |
| R95D90 | 0 | 0.063 | 0.97 |
| 3.5 | 0.011 | 0.17 | |
| P.R. | 0 | 0.006 | 0.10 |
| 3.5 | 0.005 | 0.09 |
| Surfactant | NaCl (wt.%) | Model | Areal Mass Adsorption (ng/cm2) | Area Per Molecule before Rinsing (nm2/molecule) | Average Area per Molecule after Rinsing a (nm2/molecule) | Irreversibly Adsorbed Mass b (ng/cm2) |
|---|---|---|---|---|---|---|
| 0.15 wt.% R95M90 | 0 | Sauerbrey | 178 ± 24 | 0.47 | 1.07 | 79 ± 8 [44%] |
| 3.5 | Sauerbrey | 263 ± 28 | 0.32 | 0.45 | 185 ± 54 [70%] | |
| 0.15 wt.% R95D90 | 0 | Sauerbrey | 127 ± 18 | 0.85 | 2.99 | 36 ± 16 [28%] |
| 3.5 | Sauerbrey | 148 ± 14 | 0.73 | 1.34 | 81 ± 15 [55%] | |
| 0.1 wt.% P.R. | 0 | Sauerbrey | 1002 ± 49 | 0.10 | 0.32 | 297 ± 40 [30%] |
| 3.5 | Viscoelastic | 1915 ± 398 | 0.05 | 0.27 | 352 ± 19 [18%] |
| 0.15 wt.% R95D90 in Water on Gold (Our Data) | 0.10 wt.% P.R. in Water on Gold (Our Data) | Rhamnolipid in PBS on CPAC-Coated Surface [74] | |
|---|---|---|---|
| Δf after adsorption | −7 Hz (126.8 ng/cm2) | −58 Hz (1018 ng/cm2) | ~−60 Hz (~1062 ng/cm2) |
| Δf after desorption | −2 Hz (36.1 ng/cm2) [28%] | −17 Hz (303 ng/cm2) [30%] | ~−30 Hz (~531 ng/cm2) [50%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Placek, T.L.; Jahan, R.; Alexandridis, P.; Tsianou, M. Rhamnolipid Micellization and Adsorption Properties. Int. J. Mol. Sci. 2022, 23, 11090. https://doi.org/10.3390/ijms231911090
Zhang Y, Placek TL, Jahan R, Alexandridis P, Tsianou M. Rhamnolipid Micellization and Adsorption Properties. International Journal of Molecular Sciences. 2022; 23(19):11090. https://doi.org/10.3390/ijms231911090
Chicago/Turabian StyleZhang, Yi, Tess L. Placek, Ruksana Jahan, Paschalis Alexandridis, and Marina Tsianou. 2022. "Rhamnolipid Micellization and Adsorption Properties" International Journal of Molecular Sciences 23, no. 19: 11090. https://doi.org/10.3390/ijms231911090
APA StyleZhang, Y., Placek, T. L., Jahan, R., Alexandridis, P., & Tsianou, M. (2022). Rhamnolipid Micellization and Adsorption Properties. International Journal of Molecular Sciences, 23(19), 11090. https://doi.org/10.3390/ijms231911090






