Green Synthesis of Endolichenic Fungi Functionalized Silver Nanoparticles: The Role in Antimicrobial, Anti-Cancer, and Mosquitocidal Activities
Abstract
:1. Introduction
2. Results and Discussion
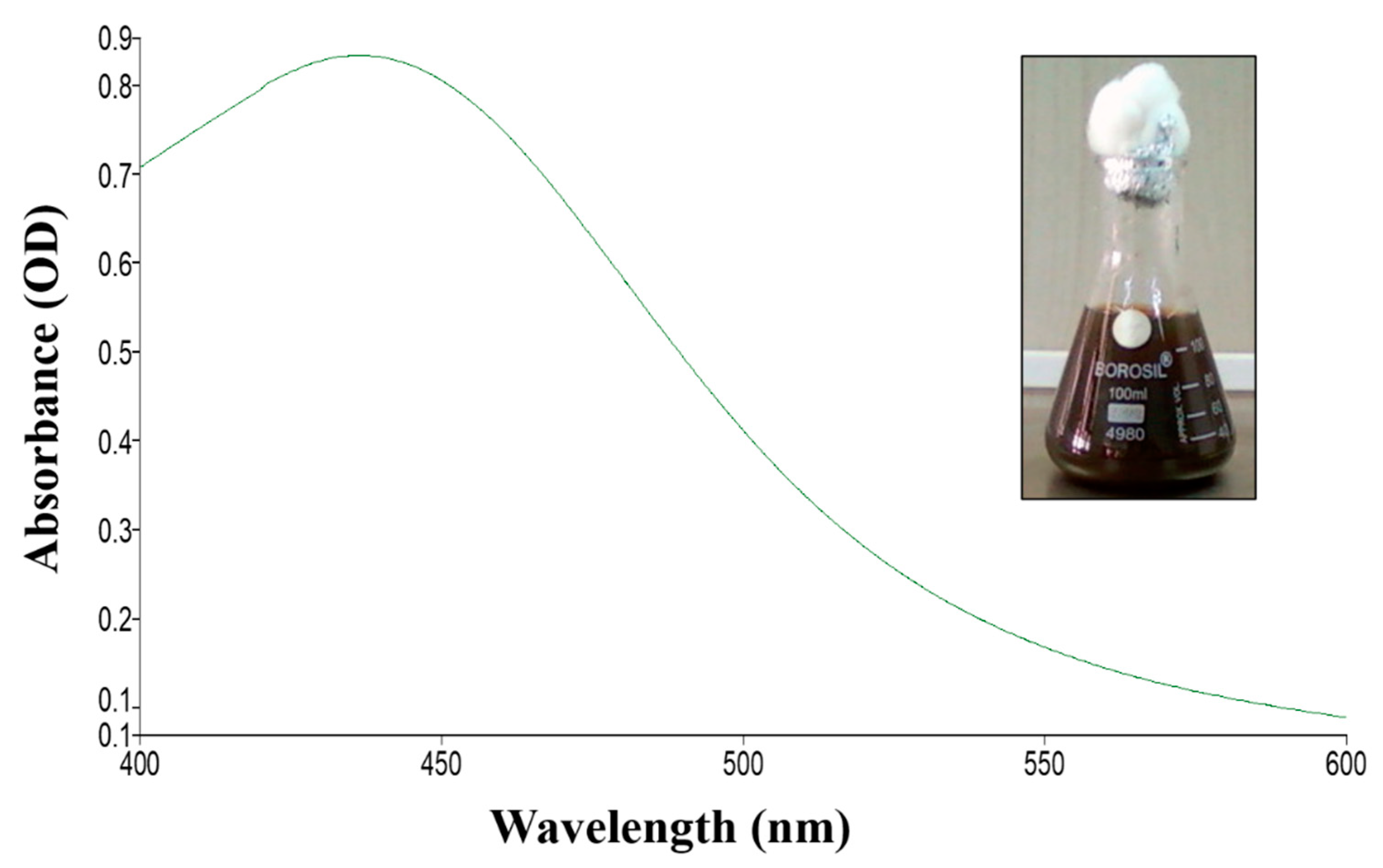
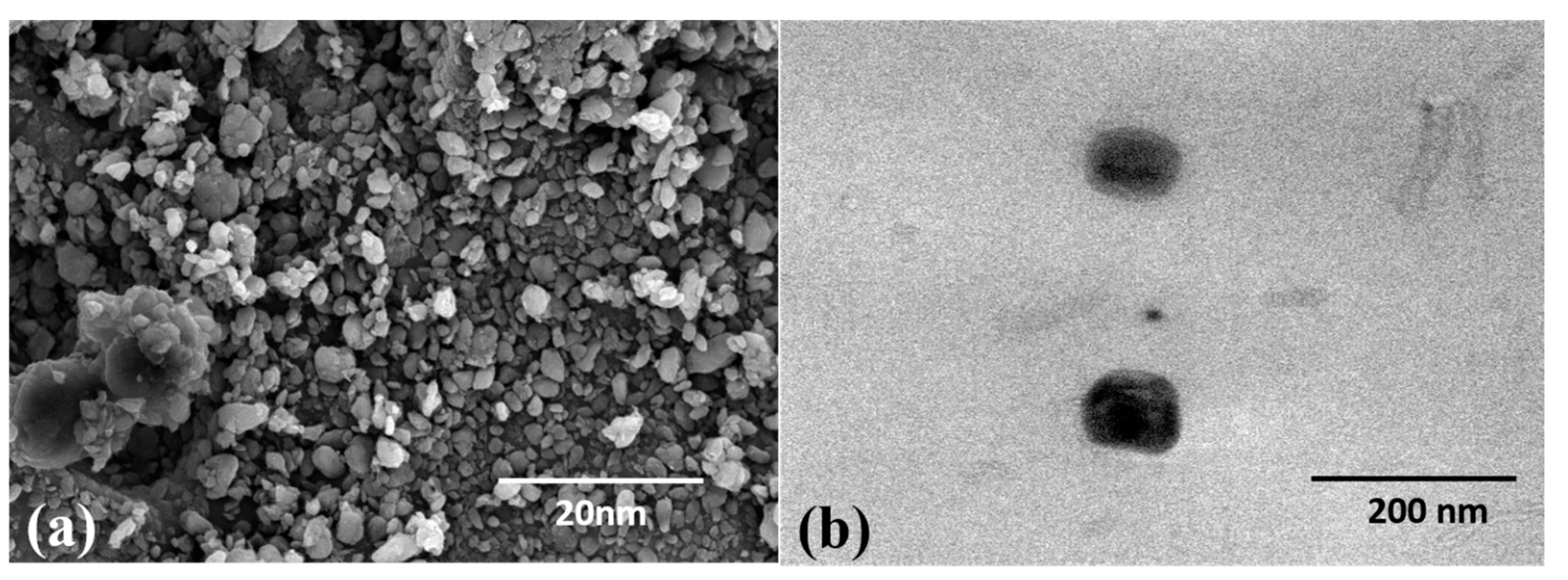
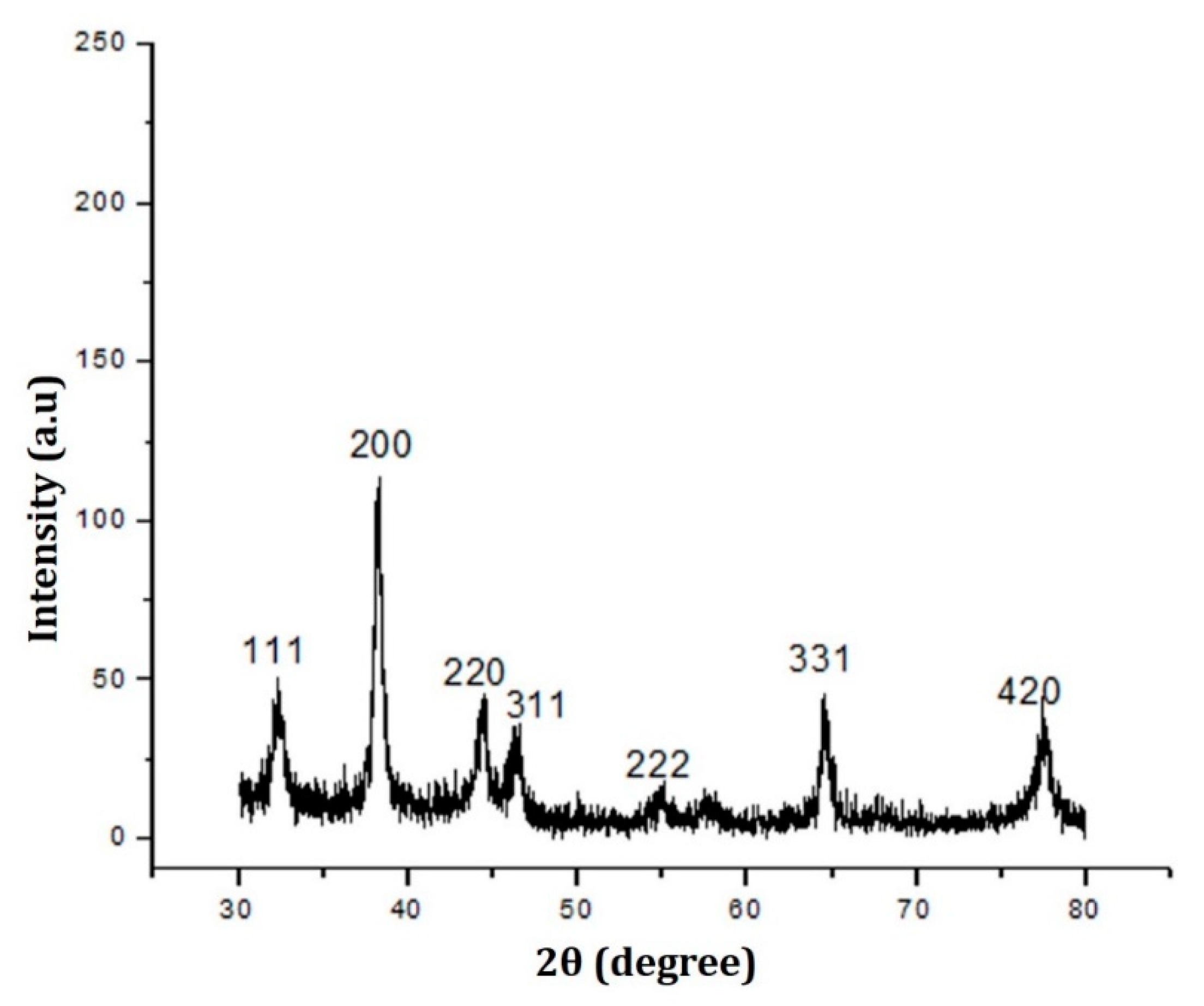
2.1. Synthesis and Characterization of TF-AgNPs
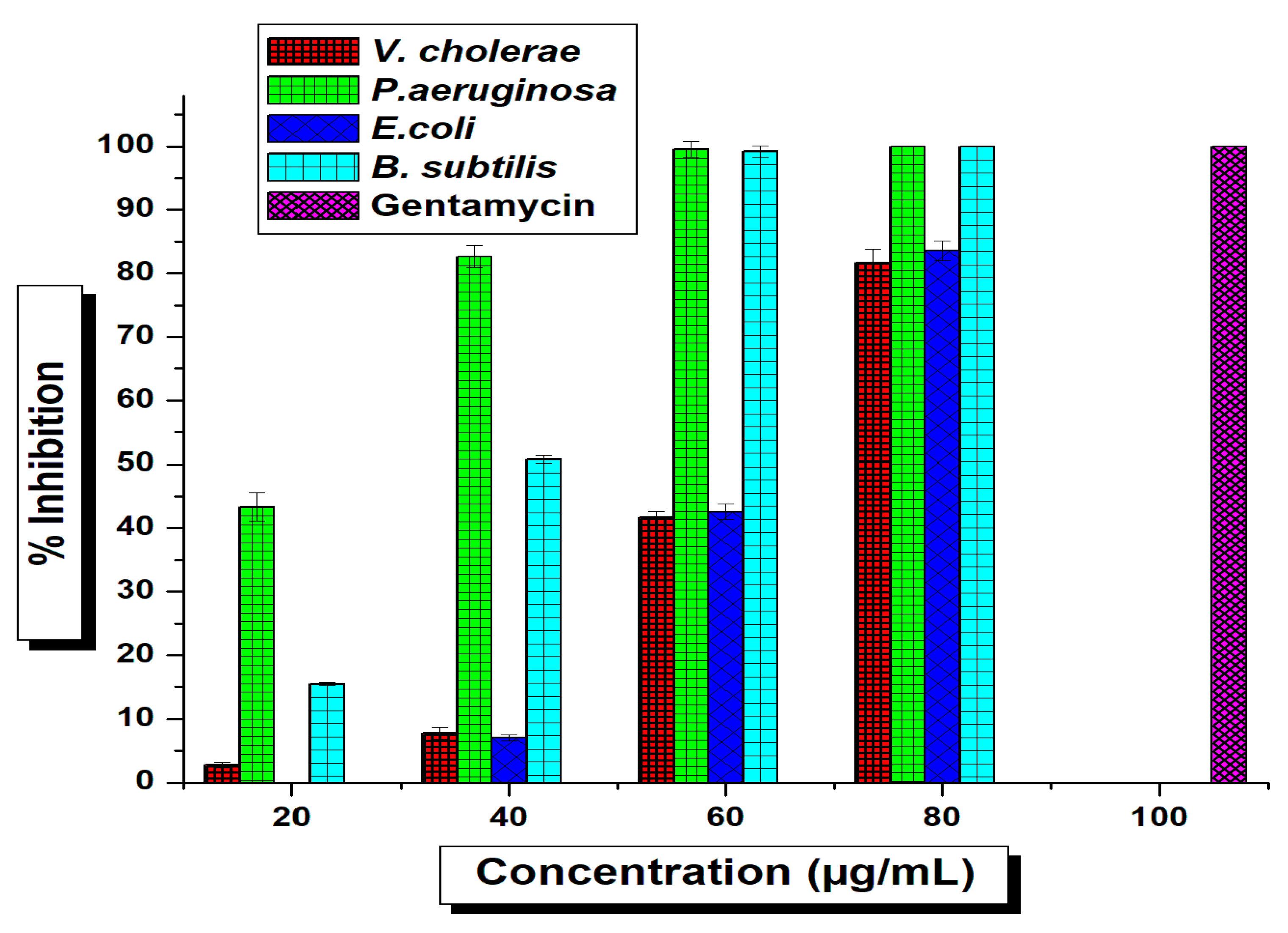
2.2. Antimicrobial Activity
2.3. Antioxidant Activity
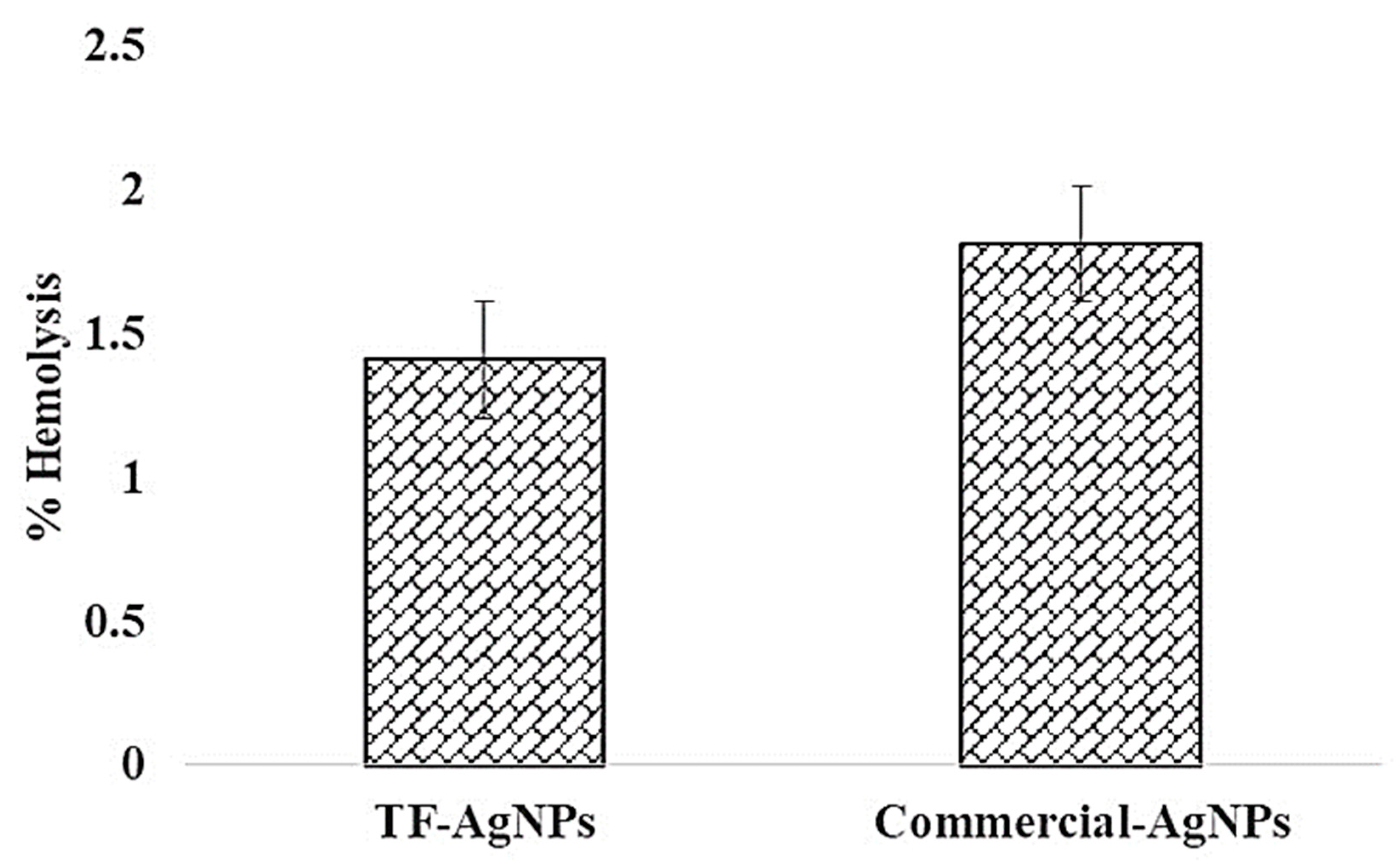
2.4. Hemo-Compatibility Assay
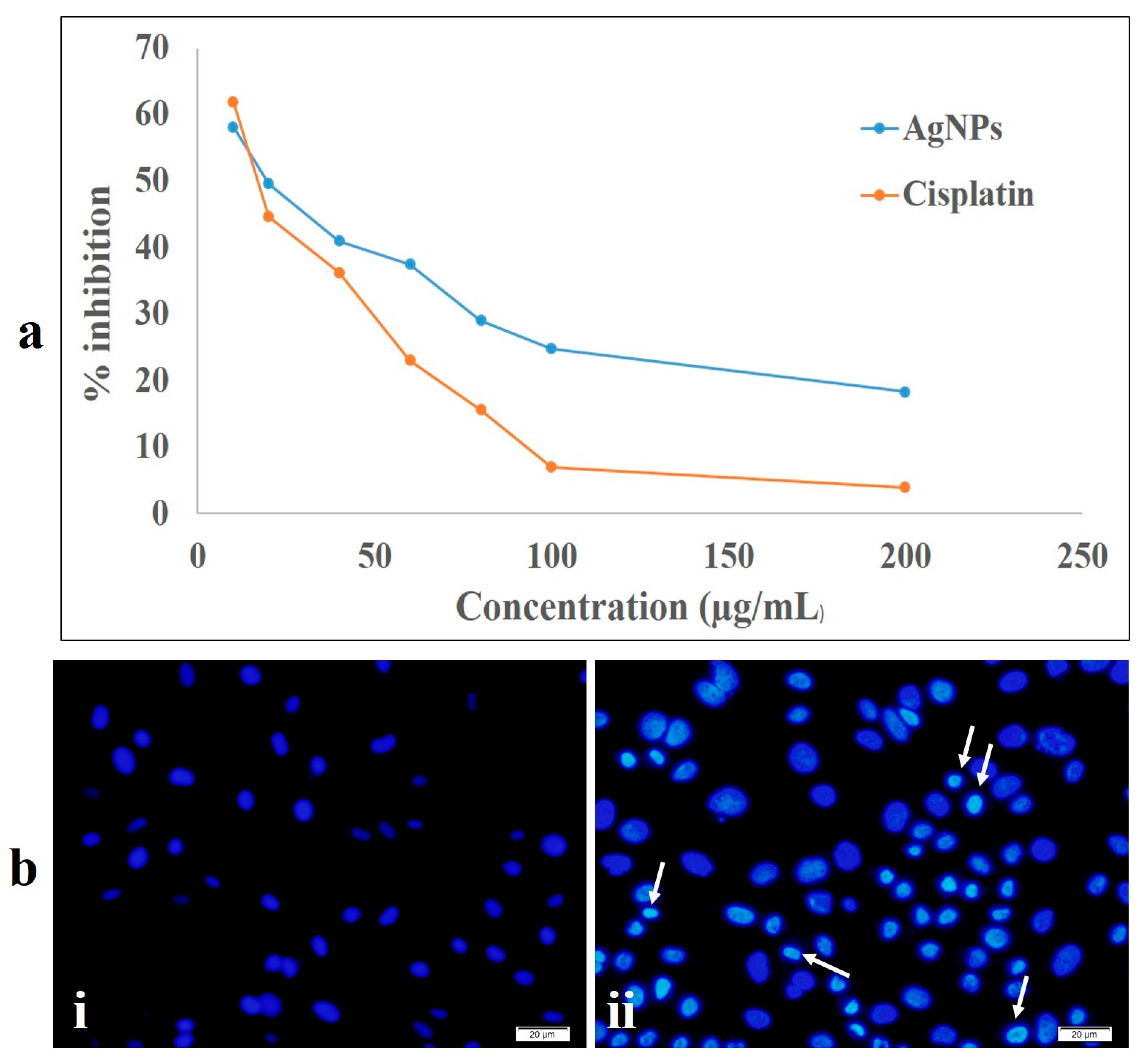
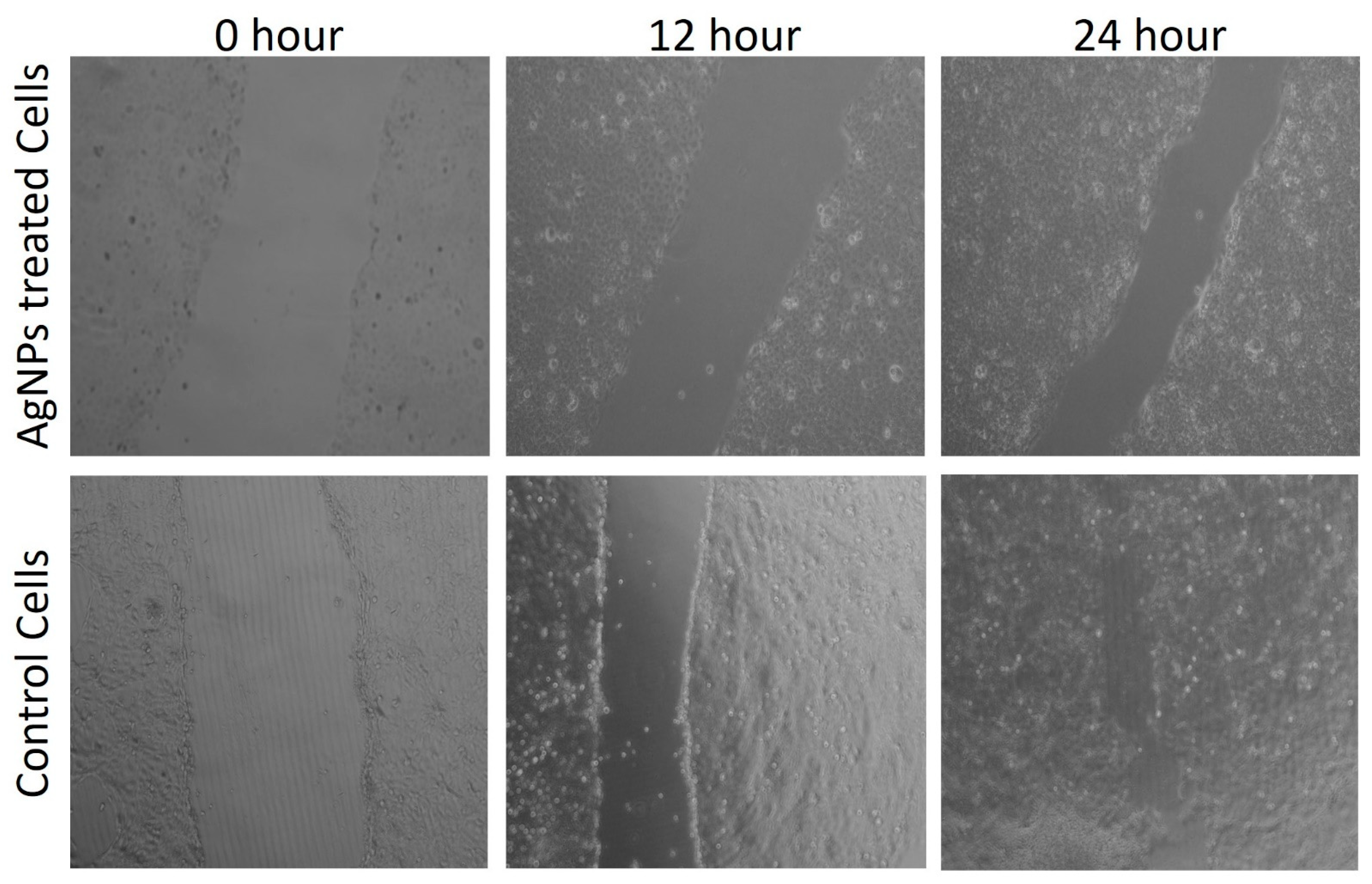
2.5. Cytotoxic Activity Study
2.6. In Vitro ROS Activity
2.7. Predation of Mesocyclops aspericornis against A. stephensi and Aedes aegypti
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell-Free Biomass Extract Preparation
3.3. Synthesis of AgNPs
3.4. Characterization Studies
3.5. Antimicrobial Activity
3.6. Antioxidant Activity
3.6.1. DPPH Scavenging Assay
3.6.2. Nitric Oxide (NO) Scavenging Activity
3.7. Hemolytic Assay
3.8. Cytotoxic Activity
3.8.1. MTT Assay
3.8.2. Chromatin Condensation Assay with Hoechst 33342 Staining
3.8.3. Scratch Wound Healing Assay
3.8.4. In Vitro ROS Activity
3.9. Predation of M. aspericornis against Malaria and Dengue Mosquitoes
3.10. Predation of M. aspericornis against Malaria and Dengue Mosquitoes Post Treatment with AgNPs
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Khatua, A.; Mohanta, Y.K.; Saravanan, M.; Meena, R.; Ghosh, I. Low-Dose Exposure of Phytosynthesized Gold Nanoparticle Combined with Glutamine Deprivation Enhances Cell Death in Cancer Cell Line HeLa via Oxidative Stress-Mediated Mitochondrial Dysfunction and G0/G1 Cell Cycle Arrest. Nanoscale 2022, 14, 10399–10417. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, A.S.; Cammarata, R.C. Nanomaterials: Synthesis, Properties and Applications; IOP Publishing: Bristol, UK, 1996. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M. Nanostructured Thin Films by Self-Assembly of Surface Modified Colloidal Particles. Curr. Sci. 2000, 78, 1089–1097. [Google Scholar]
- Philp, D.; Fraser Stoddart, J. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. (Int. Ed. Engl.) 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Dhanker, R.; Hussain, T.; Tyagi, P.; Singh, K.J.; Kamble, S.S. The Emerging Trend of Bio-Engineering Approaches for Microbial Nanomaterial Synthesis and Its Applications. Front. Microbiol. 2021, 12, 638003. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garnæs, J.; Mijakovic, I. A Sustainable Approach for the Green Synthesis of Silver Nanoparticles from Solibacillus Isronensis sp. and Their Application in Biofilm Inhibition. Molecules 2020, 25, 2783. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Spring, S.; Schleifer, K.-H. Diversity of Magnetotactic Bacteria. Syst. Appl. Microbiol. 1995, 18, 147–153. [Google Scholar] [CrossRef]
- Oliver, S.; Kuperman, A.; Coombs, N.; Lough, A.; Ozin, G.A. Lamellar Aluminophosphates with Surface Patterns That Mimic Diatom and Radiolarian Microskeletons. Nature 1995, 378, 47–50. [Google Scholar] [CrossRef]
- Pum, D.; Sleytr, U.B. The Application of Bacterial S-Layers in Molecular Nanotechnology. Trends Biotechnol. 1999, 17, 8–12. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Messner, P.; Pum, D.; Sara, M. Crystalline Bacterial Cell Surface Layers (s Layers): From Supramolecular Cell Structure to Biomimetics and Nanotechnology. Angew. Chem. Int. Ed. Engl. 1999, 38, 1034–1054. [Google Scholar] [CrossRef]
- Stark, J.F.; Stones, C. Constructing Representations of Germs in the Twentieth Century. Cult. Soc. Hist. 2019, 16, 287–314. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial Resistance and Virulence: A Successful or Deleterious Association in the Bacterial World? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T.; Mondal, A.H.; Haq, Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef] [PubMed]
- Le, O.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Biswas, K.; Mishra, A.K.; Rauta, P.R.; Al-sehemi, A.G.; Pannipara, M.; Sett, A.; Bratovcic, A.; Avula, S.K.; Mohanta, T.K. Exploring the Bioactive Potentials of C 60 -AgNPs Nano-Composites against Malignancies and Microbial Infections. Int. J. Mol. Sci. 2022, 23, 714. [Google Scholar] [CrossRef]
- Khan, F.; Mishra, A.; Mishra, S.K. Etiopathogenesis of Breast Cancer and Prevention. In Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2022; pp. 53–69. [Google Scholar]
- Pugazhendhi, A.; Edison, T.; Karuppusamy, I.; Kathirvel, B. Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Coccia, M.; Wang, L. Path-Breaking Directions of Nanotechnology-Based Chemotherapy and Molecular Cancer Therapy. Technol. Forecast. Soc. Chang. 2015, 94, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Estanqueiro, M.; Amaral, M.; Conceicao, J.; Sousa, L. Nanotechnological Carriers for Cancer Chemotherapy, The State of the Art. Colloids Surf B. 2015, 126, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Massari, F.; Del Re, M. Investigational Therapies Targeting Signal Transducer and Activator of Transcription 3 for the Treatment of Cancer. Expert Opin. Investig. Drugs 2015, 24, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Makila, E.M.; Kaasalainen, M.; Hagström, M.V.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Dual-Drug Delivery by Porous Silicon Nanoparticles for Improved Cellular Uptake., Sustained Release., and Combination Therapy. Acta Biomater. 2015, 16, 206–214. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.; Vishnubalaji, R. Rapid Biological Synthesis of Silver Nanoparticles Using Plant Seed Extracts and Their Cytotoxicity on Colorectal Cancer Cell Lines. J. Clust Sci. 2017, 28, 595–605. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Sadras, S.R. Green Synthesized Nano Silver: Synthesis, Physicochemical Profiling, Antibacterial, Anticancer Activities and Biological in Vivo Toxicity. J. Colloid Interface Sci. 2017, 499, 33–45. [Google Scholar] [CrossRef]
- Preetha, D.; Prachi, K.; Chirom, A. Synthesis and Characterization of Silver Nanoparticles Using Cannonball Leaves and Their Cytotoxic Activity against MCF-7 Cell Line. J. Nanotechnol. 2013, 2, 421–428. [Google Scholar]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically Synthesised Silver Nanoparticles from Three Diverse Family of Plant Extracts and Their Anticancer Activity against Epidermoid A431 Carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green Synthesis of Silver Nanoparticles by Trichoderma Harzianum and Their Bioefficacy Evaluation against Staphylococcus Aureus and Klebsiella Pneumonia. Ind. Crop. Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of Silver Nanoparticles Bearing Antibacterial Activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma Reesei. Insci. J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-, E.; Abd-elsalam, K.A.; Alghuthaymi, M.A.; Almoammar, H.; Rai, M. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.A.; Umer, A.; Awan, G.H.; Hassan, M.F.; Hasnain, A. Selection of Suitable Biological Method for the Synthesis of Silver Nanoparticles. Nanomater. Nanotechnol. 2016, 6, 29. [Google Scholar] [CrossRef]
- Costa Silva, L.P.; Oliveira, J.P.; Keijok, W.J.; Silva, A.R.; Aguiar, A.R.; Guimarães, M.C.C.; Ferraz, C.M.; Araújo, J.V.; Tobias, F.L.; Braga, F.R. Extracellular Biosynthesis of Silver Nanoparticles Using the Cell-Free Filtrate of Nematophagus Fungus Duddingtonia flagans. Int. J. Nanomed. 2017, 12, 6373–6381. [Google Scholar] [CrossRef]
- Ashrafi, S.J.; Rastegar, M.F.; Ashrafi, M.; Yazdian, F.; Pourrahim, R.; Suresh, A.K. Influence of External Factors on the Production and Morphology of Biogenic Silver Nanocrystallites. J. Nanosci. Nanotechnol. 2013, 13, 2295–2301. [Google Scholar] [CrossRef]
- Yahyaei, B.; Pourali, P. One Step Conjugation of Some Chemotherapeutic Drugs to the Biologically Produced Gold Nanoparticles and Assessment of Their Anticancer Effects. Sci. Rep. 2019, 9, 10242. [Google Scholar] [CrossRef]
- Benelli, G.; Lo Iacono, A.; Canale, A.; Mehlhorn, H. Mosquito Vectors and the Spread of Cancer: An Overlooked Connection? Parasitol. Res. 2016, 115, 2131–2137. [Google Scholar] [CrossRef]
- Almaary, K.S.; Sayed, S.R.M.; Abd-Elkader, O.H.; Dawoud, T.M.; El Orabi, N.F.; Elgorban, A.M. Complete Green Synthesis of Silver-Nanoparticles Applying Seed-Borne Penicillium duclauxii. Saudi J. Biol. Sci. 2020, 27, 1333–1339. [Google Scholar] [CrossRef]
- Benelli, G. Research in Mosquito Control: Current Challenges for a Brighter Future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Yadav, D.; Mohanta, T.K. Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark Extract Mediated Green Synthesis of Silver Nanoparticles: Evaluation of Antimicrobial Activity and Antiproliferative Response against Osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Smitha, S.L.; Nissamudeen, K.M.; Philip, D.; Gopchandran, K.G. Studies on Surface Plasmon Resonance and Photoluminescence of Silver Nanoparticles. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2008, 71, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Elamawi, R.M.; Al-harbi, R.E.; Hendi, A.A. Biosynthesis and Characterization of Silver Nanoparticles Using Trichoderma Longibrachiatum and Their Effect on Phytopathogenic Fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J. Nanobiotechnology 2005, 3, 8–14. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic Synthesis and Characterisation of Protein Capped Silver Nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and Characterization of Novel Silver Nanoparticles Using Chamaemelum nobile Extract for Antibacterial Application. In Proceedings of the Advances in Natural Sciences: Nanoscience and Nanotechnology; IOP Publishing: Bristol, UK, 2017; Volume 8. [Google Scholar]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-Mediated Green Synthesis of Silver Nanoparticles Using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, S.; Tayung, K.; Evidente, A. Funiculosone, a Substituted Dihydroxanthene-1,9-Dione with Two of Its Analogues Produced by an Endolichenic Fungus Talaromyces funiculosus and Their Antimicrobial Activity. Phytochemistry 2019, 157, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ran, H.; Zeng, J.; Liu, D.; Xin, Z. Tafuketide, a Phylogeny-Guided Discovery of a New Polyketide from Talaromyces funiculosus Salicorn 58. Appl. Microbiol. Biotechnol. 2016, 100, 5323–5338. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Biosynthesis, Characterisation and Antimicrobial Activity of Silver Nanoparticles Using Hibiscus Rosa-Sinensis Petals Extracts. Nanobiotechnology IET 2015, 9, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wójcik, A. Antimicrobial Properties of Gold, Silver, Copper and Platinum against Selected Microorganisms Isolated from Cases of Mastitis in Cattle. Med. Weter. 2014, 70, 4. [Google Scholar]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-Mediated Green Synthesis of Nano-Silver Using Aspergillus Sydowii and Its Antifungal/Antiproliferative Activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef]
- Echavarría, J.O.; Echavarría, J.O. Synthesis of Silver Nanoparticles Using White-Rot Fungus Anamorphous Bjerkandera sp. R1: Influence of Silver Nitrate Concentration and Fungus Growth Time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef] [PubMed]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Akintola, A.O.; Kehinde, B.D.; Ayoola, P.B.; Adewoyin, A.G.; Adedosu, O.T.; Ajayi, J.F.; Ogunsona, S.B. Antioxidant Properties of Silver Nanoparticles Biosynthesized from Methanolic Leaf Extract of Blighia sapida. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 805. [Google Scholar]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic Combination of Antioxidants, Silver Nanoparticles and Chitosan in a Nanoparticle Based Formulation: Characterization and Cytotoxic Effect on MCF-7 Breast Cancer Cell Lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Sarika, P.R.; Anil Kumar, P.R.; Raj, D.K.; James, N.R. Nanogels Based on Alginic Aldehyde and Gelatin by Inverse Miniemulsion Technique: Synthesis and Characterization. Carbohydr. Polym. 2015, 119, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.D.S.; De Almeida, P.D.O.; Aranha, E.S.P.; Boleti, A.P.D.A.; Newton, P.; De Vasconcellos, M.C.; Veiga, V.F.; Lima, E.S. Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins. Molecules 2015, 20, 6194–6210. [Google Scholar] [CrossRef] [PubMed]
- Chakrabartty, I.; Vijayasekhar, A.; Rangan, L. Therapeutic Potential of Labdane Diterpene Isolated from Alpinia nigra: Detailed Hemato- Compatibility and Antimicrobial Studies. Nat. Prod. Res. 2019, 35, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Green, B.J.; Beezhold, D.H. Fungal Hemolysins. Med. Mycol. 2013, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaveemongkonrat, S.; Duangsonk, K.; Houbraken, J.; Suwannaphong, P.; Vanittanakom, N.; Mekaprateep, M. Partial Characteristics of Hemolytic Factors Secreted from Airborne Aspergillus and Penicillium, and an Enhancement of Hemolysis by Aspergillus Micronesiensis CAMP-like Factor via Staphylococcus Aureus-Sphingomyelinase. J. Microbiol. 2019, 57, 1086–1094. [Google Scholar] [CrossRef]
- Nayak, D.; Kumari, M.; Rajachandar, S.; Ashe, S.; Thathapudi, N.C.; Nayak, B. Biofilm Impeding AgNPs Target Skin Carcinoma by Inducing Mitochondrial Membrane Depolarization Mediated through ROS Production. ACS Appl. Mater. Interfaces 2016, 8, 28538–28553. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The Potential Exposure and Hazards of Metal-Based Nanoparticles on Plants and Environment, with Special Emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A Review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Kaleghi, M.; Danaei, M.; Mozafari, M. Selective Cytotoxicity of Green Synthesized Silver Nanoparticles against the MCF-7 Tumor Cell Line and Their Enhanced Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Pannerselvam, B.; Thiyagarajan, D.; Pazhani, A.; Thangavelu, K.P.; Kim, H.J.; Rangarajulu, S.K. Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines. Processes 2021, 9, 888. [Google Scholar] [CrossRef]
- Nayak, D.; Thathapudi, N.C.; Ashe, S.; Nayak, B. Bioengineered Ethosomes Encapsulating AgNPs and Tasar Silk Sericin Proteins for Non Melanoma Skin Carcinoma (NMSC) as an Alternative Therapeutics. Int. J. Pharm. 2021, 596, 120265. [Google Scholar] [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J. Comparative Assessment of the Apoptotic Potential of Silver Nanoparticles Synthesized by Bacillus Tequilensis and Calocybe Indica in MDA-MB-231 Human Breast Cancer Cells: Targeting P53 for Anticancer Therapy. Int. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [PubMed]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical Applications of Reactive Oxygen Species Generation by Metal Nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sengupta, D.; Deb, M.; Shilpi, A.; Parbin, S.; Rath, S.K.; Pradhan, N.; Rakshit, M.; Patra, S.K. Expression Profiling of DNA Methylation-Mediated Epigenetic Gene-Silencing Factors in Breast Cancer. Clin. Epigenetics 2014, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.-S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon Citratus-Synthesized Gold Nanoparticles Boost the Predation Efficiency of Copepod Mesocyclops Aspericornis against Malaria and Dengue Mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Boothapandi, M.; Sultan Nasar, A. Nitric Oxide, DPPH and Hydrogen Peroxide Radical Scavenging Activity of TEMPO Terminated Polyurethane Dendrimers: Data Supporting Antioxidant Activity of Radical Dendrimers. Data Br. 2020, 28, 104972. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Deb, M.; Rath, S.K.; Kar, S.; Parbin, S.; Pradhan, N.; Patra, S.K. DNA Methylation and Not H3K4 Trimethylation Dictates the Expression Status of MiR-152 Gene Which Inhibits Migration of Breast Cancer Cells via DNMT1/CDH1 Loop. Exp. Cell Res. 2016, 346, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Mishra, A.K.; Nayak, D.; Patra, B.; Bratovcic, A.; Avula, S.K.; Mohanta, T.K.; Murugan, K.; Saravanan, M. Exploring Dose-Dependent Cytotoxicity Profile of Gracilaria Edulis- Mediated Green Synthesized Silver Nanoparticles against MDA-MB-231 Breast Carcinoma. Oxidative Med. Cell. Longev. 2022, 2022. [Google Scholar] [CrossRef]
- Chen, S.-K.; Hsu, C.-H.; Tsai, M.-L.; Chen, R.-H.; Drummen, G.P.C. Inhibition of Oxidative Stress by Low-Molecular-Weight Polysaccharides with Various Functional Groups in Skin Fibroblasts. Int. J. Mol. Sci. 2013, 14, 19399–19415. [Google Scholar] [CrossRef] [Green Version]





| Mosquito Species | Targeted Instar | Number of Consumed Prey | Total Predation (n) | Consumed Prey per Copepod per Day | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||
| Anopheles stephensi | Larva I | 0 | 36.6 ± 2.6 | 37.2 ± 1.0 | 35.6 ± 1.5 | 33.6 ± 3.5 | 30.5 ± 1.2 | 173.5 | 3.47 |
| Larva II | 0 | 22.8 ± 0.5 | 20.8 ± 0.8 | 19.2 ± 0.8 | 18.9 ± 2.1 | 20.4 ± 1.6 | 102.1 | 2.04 | |
| Larva III | 0 | 8.1 ± 1.9 | 7.6 ± 1.1 | 7.1 ± 0.4 | 7.5 ± 1.5 | 7.8 ± 1.0 | 38.1 | 0.76 | |
| Larva IV | 0 | 2.3 ± 2.1 | 2.2 ± 1.0 | 2.2 ± 1.7 | 2.6 ± 1.6 | 2.5 ± 1.3 | 11.8 | 0.23 | |
| Aedes aegypti | Larva I | 0 | 60.3 ± 1.8 | 58.1 ± 2.8 | 55.4 ± 1.0 | 57.9 ± 0.7 | 56.1 ± 2.4 | 287.8 | 5.75 |
| Larva II | 0 | 49.2 ± 1.4 | 47.6 ± 2.4 | 45.2 ± 0.9 | 47.8 ± 2.4 | 47.1 ± 1.8 | 236.9 | 4.73 | |
| Larva III | 0 | 26.1 ± 1.8 | 24.3 ± 0.5 | 22.4 ± 2.0 | 21.3 ± 3.1 | 20.9 ± 1.0 | 115 | 2.3 | |
| Larva IV | 0 | 9.5 ± 0.6 | 8.9 ± 1.8 | 8.6 ± 2.6 | 8.9 ± 2.4 | 9.2 ± 1.1 | 45.1 | 0.90 | |
| Mosquito Species | Targeted Instar | Number of Consumed Prey | Total Predation (n) | Consumed Prey per Copepod per Day | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||
| Anopheles stephensi | Larva I | 0 | 49.7 ± 1.5 | 50.4 ± 1.8 | 49.5 ± 2.5 | 47.9 ± 1.5 | 48.6 ± 3.1 | 246.1 | 4.92 |
| Larva II | 0 | 30.2 ± 0.9 | 27.6 ± 1.6 | 29.2 ± 3.1 | 31.4 ± 2.7 | 30.7 ± 2.1 | 149.1 | 2.98 | |
| Larva III | 0 | 14.6 ± 1.1 | 15.3 ± 1.9 | 14.7 ± 2.7 | 16.1 ± 1.6 | 15.5 ± 1.6 | 76.2 | 1.52 | |
| Larva IV | 0 | 8.8 ± 1.1 | 9.1 ± 0.4 | 7.4 ± 1.3 | 7.4 ± 1.8 | 8.2 ± 1.9 | 40.9 | 0.81 | |
| Aedes aegypti | Larva I | 0 | 72.3 ± 1.5 | 70.4 ± 0.7 | 73.1 ± 0.9 | 70.5 ± 0.5 | 71.9 ± 2.1 | 358.2 | 7.16 |
| Larva II | 0 | 59.1 ± 1.9 | 62.7 ± 1.8 | 59.0 ± 1.4 | 57.9 ± 1.4 | 60.1 ± 1.0 | 298.8 | 5.97 | |
| Larva III | 0 | 33.4 ± 2.4 | 35.1 ± 0.4 | 30.9 ± 1.4 | 33.4 ± 2.1 | 32.6 ± 1.2 | 165.4 | 3.30 | |
| Larva IV | 0 | 20.1 ± 0.9 | 18.9 ± 2.7 | 19.5 ± 0.8 | 20.1 ± 2.6 | 20.9 ± 2.3 | 99.5 | 1.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanta, Y.K.; Nayak, D.; Mishra, A.K.; Chakrabartty, I.; Ray, M.K.; Mohanta, T.K.; Tayung, K.; Rajaganesh, R.; Vasanthakumaran, M.; Muthupandian, S.; et al. Green Synthesis of Endolichenic Fungi Functionalized Silver Nanoparticles: The Role in Antimicrobial, Anti-Cancer, and Mosquitocidal Activities. Int. J. Mol. Sci. 2022, 23, 10626. https://doi.org/10.3390/ijms231810626
Mohanta YK, Nayak D, Mishra AK, Chakrabartty I, Ray MK, Mohanta TK, Tayung K, Rajaganesh R, Vasanthakumaran M, Muthupandian S, et al. Green Synthesis of Endolichenic Fungi Functionalized Silver Nanoparticles: The Role in Antimicrobial, Anti-Cancer, and Mosquitocidal Activities. International Journal of Molecular Sciences. 2022; 23(18):10626. https://doi.org/10.3390/ijms231810626
Chicago/Turabian StyleMohanta, Yugal Kishore, Debasis Nayak, Awdhesh Kumar Mishra, Ishani Chakrabartty, Manjit Kumar Ray, Tapan Kumar Mohanta, Kumananda Tayung, Rajapandian Rajaganesh, Murugan Vasanthakumaran, Saravanan Muthupandian, and et al. 2022. "Green Synthesis of Endolichenic Fungi Functionalized Silver Nanoparticles: The Role in Antimicrobial, Anti-Cancer, and Mosquitocidal Activities" International Journal of Molecular Sciences 23, no. 18: 10626. https://doi.org/10.3390/ijms231810626
APA StyleMohanta, Y. K., Nayak, D., Mishra, A. K., Chakrabartty, I., Ray, M. K., Mohanta, T. K., Tayung, K., Rajaganesh, R., Vasanthakumaran, M., Muthupandian, S., Murugan, K., Sharma, G., Dahms, H.-U., & Hwang, J.-S. (2022). Green Synthesis of Endolichenic Fungi Functionalized Silver Nanoparticles: The Role in Antimicrobial, Anti-Cancer, and Mosquitocidal Activities. International Journal of Molecular Sciences, 23(18), 10626. https://doi.org/10.3390/ijms231810626















