Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications
Abstract
1. Introduction
2. Results and Discussion
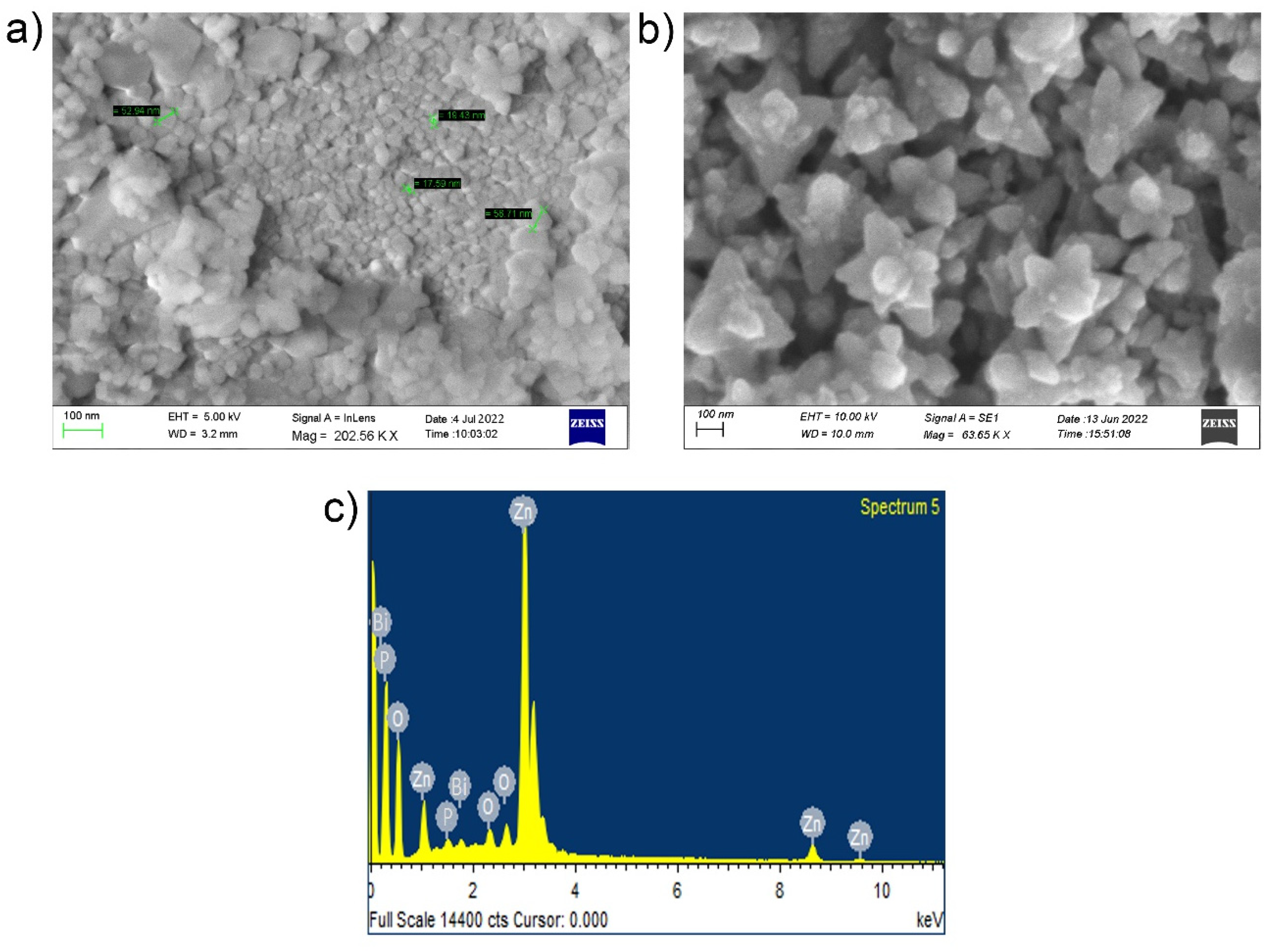
2.1. Characterization of As-Prepared NPs and NCs Catalysts
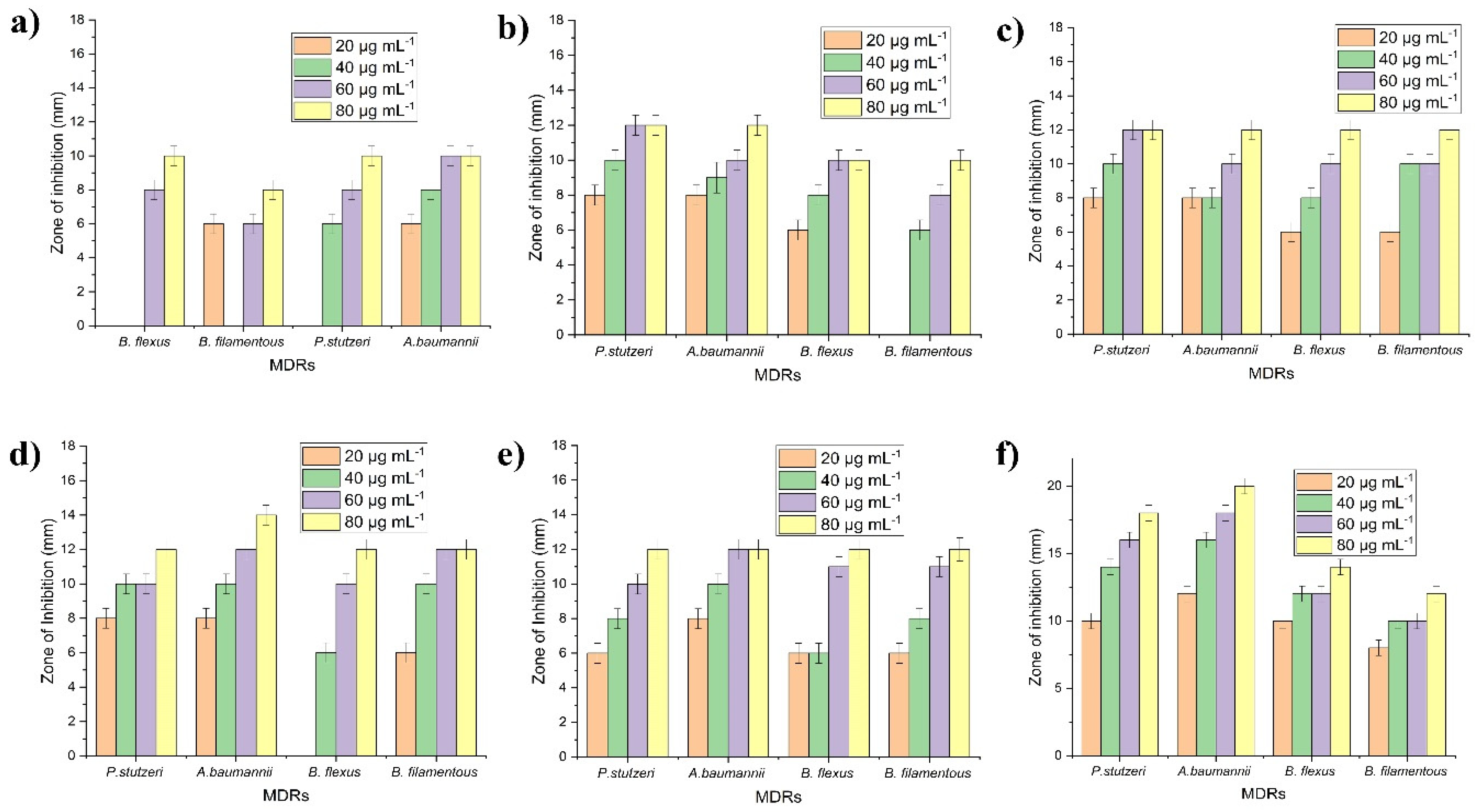
2.2. In Vitro Antibacterial Susceptibility Testing
2.3. SEM and Fe-SEM Characterization of NCs-4 Catalysts
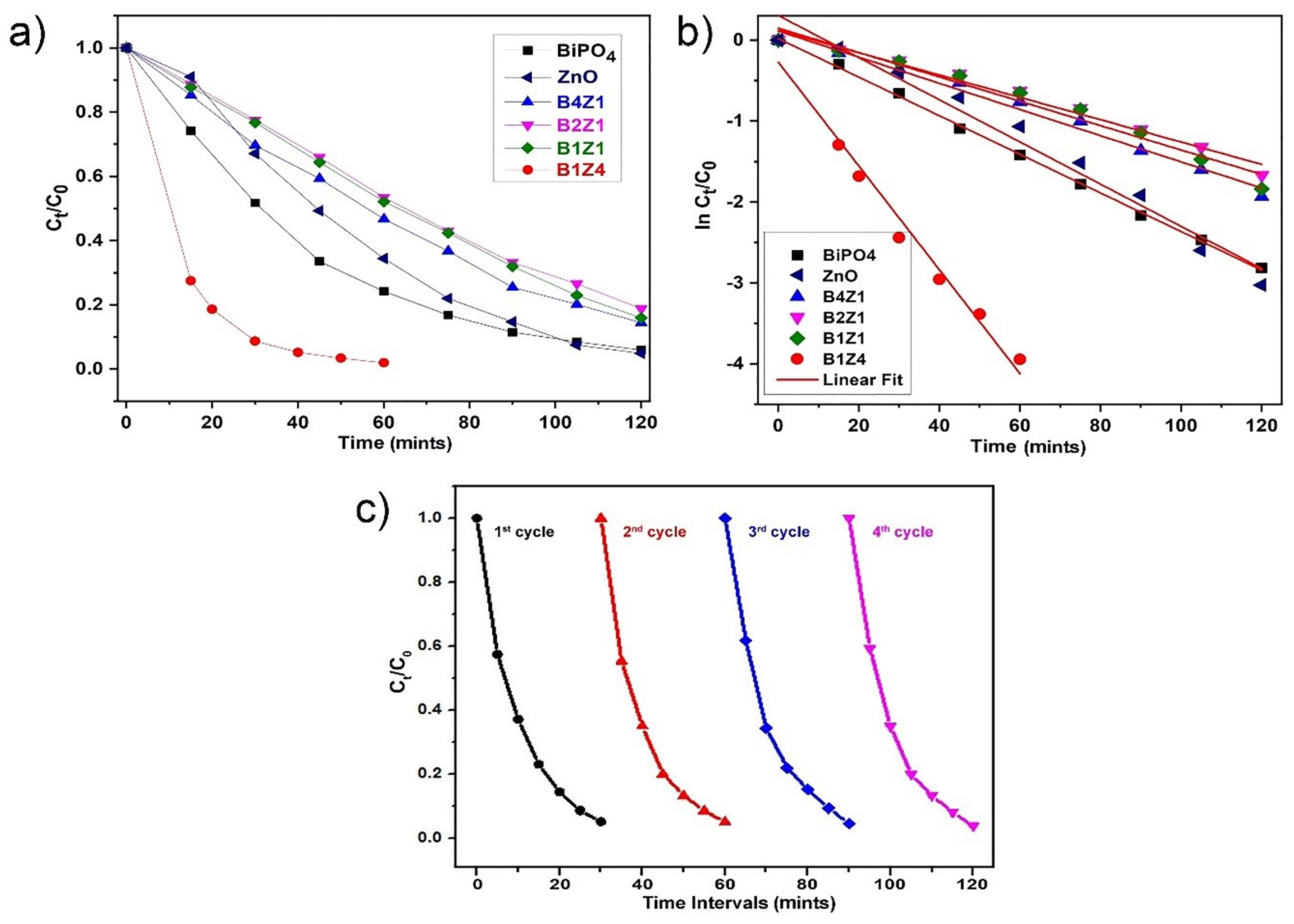
2.4. Photocatalytic Experiments
- Initial count—The test culture suspensions were prepared in sterile 0.85% saline matching an optical density of 0.5 McFarland standards corresponding to 107–108 (CFUs mL−1).
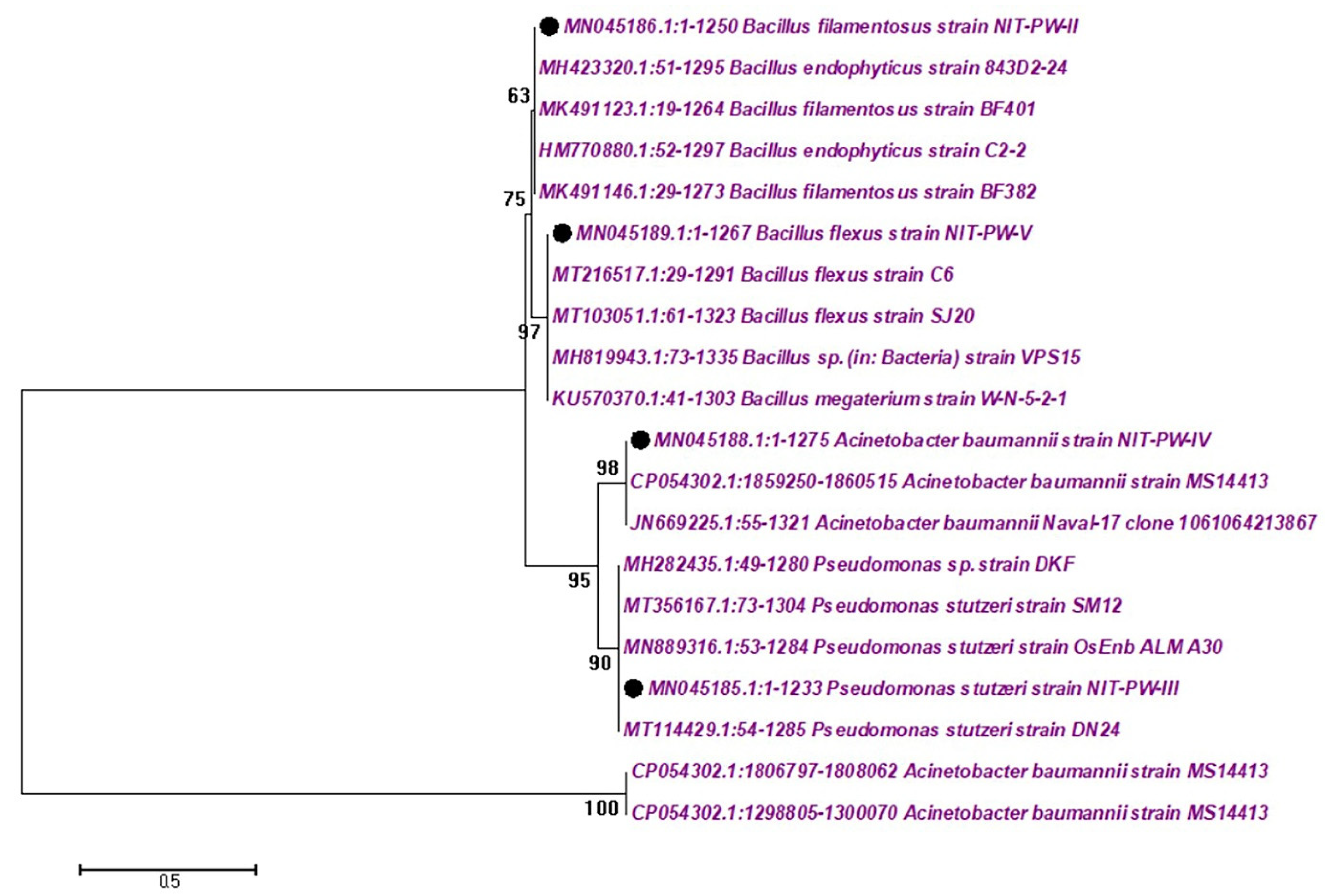
- Gram ve+ (n = 2)—Bacillus flexus—NCBI number: MN045189 (B. flexus), Bacillus filamentosus—NCBI number: MN045186 (B. filamentosus).
- Gram ve− (n = 2)—Pseudomonas stutzeri—NCBI number: MN045185 (P. stutzeri), and Acinetobacter baumannii—NCBI number: MN045188 (A. baumannii).
3. Experimental Section
3.1. Materials and Reagents
3.2. Collection of Microbial Cultures
3.3. Preparation of Catalysts
3.3.1. Synthesis of BiPO4 Nanoparticles (NPs)
3.3.2. The Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs)
3.3.3. Preparation of Bimetallic BiPO4/ZnO Nanocomposite (NCs)
3.4. Characterization and Property Measurements of As-Prepared NPs and NCs
3.5. In Vitro Antibacterial Susceptibility Testing
3.6. Statistical Analysis
3.7. Photocatalytic Activity Test
3.7.1. Photocatalytic Efficacies of NPs and NCs Catalyst
3.7.2. Photocatalytic Inactivation Experiments
3.8. Photocatalytic Cycle Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatesh, N.; Aravindan, S.; Ramki, K.; Murugadoss, G.; Thangamuthu, R.; Sakthivel, P. Sunlight-driven enhanced photocatalytic activity of bandgap narrowing Sn-doped ZnO nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 16792–16803. [Google Scholar] [CrossRef] [PubMed]
- Siva, N.; Sakthi, D.; Ragupathy, S.; Arun, V.; Kannadasan, N. Synthesis, structural, optical and photocatalytic behavior of Sn doped ZnO nanoparticles. Mater. Sci. Eng. B Solid. State. Mater. Adv. Technol. 2020, 253, 114497. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Ibrahim, S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2018, 16, 275–280. [Google Scholar] [CrossRef]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment-Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Wu, Q.; Tan, L.; Liu, X.; Li, Z.; Zhang, Y.; Zheng, Y.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. The enhanced near-infrared photocatalytic and photothermal effects of MXene-based heterojunction for rapid bacteria-killing. App. Catalyt. B Environ. 2021, 297, 120500. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.M.; Sin, J.C.; Mohamed, A.R. Constructing magnetic Pt-loaded BiFeO3 nanocomposite for boosted visible light photocatalytic and antibacterial activities. Environ. Sci. Pollut. Res. 2019, 26, 10204–10218. [Google Scholar] [CrossRef]
- Munisamy, M.; Yang, H.W.; Perumal, N.; Kang, N.; Kang, W.S.; Kim, S.J. A Flower-like In2O3Catalyst Derived via Metal–Organic Frameworks for Photocatalytic Applications. Int. J. Mol. Sci. 2022, 23, 4398. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Col. Surfac. B Biointer. 2014, 115, 359–367. [Google Scholar] [CrossRef]
- Mataseje, L.F.; Peirano, G.; Church, D.L.; Conly, J.; Mulvey, M.; Pitout, J.D. Colistin non-susceptible Pseudomonas aeruginosa ST654 with blaNDM-1 arrives in North America. Antimicrob. Agents. Chemotherpii AAC 2016, 15, 02591. [Google Scholar]
- UN-Water Annual Report 2020. Available online: https://www.unwater.org/sites/default/files/app/uploads/2021/11/Annual-Report-2020.pdf (accessed on 6 November 2022).
- Harinee, S.; Muthukumar, K.; Dahms, H.U.; Koperuncholan, M.; Vignesh, S.; Banu, R.J.; Ashok, M.; James, R.A. Biocompatible nanoparticles with enhanced photocatalytic and anti-microfouling potential. Int. Biodeterior. Biodegrad. 2019, 145, 104790. [Google Scholar] [CrossRef]
- Harinee, S.; Muthukumar, K.; James, R.A.; Arulmozhi, M.; Dahms, H.U.; Ashok, M. Bio-approach ZnO/Ag nano-flowers: Enhanced photocatalytic and photoexcited anti-microbial activities towards pathogenic bacteria. Mater. Today Sustain. 2022, 18, 100133. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharma. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Product. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Ling, Q.; Zhu, Y. Enhancement of full-spectrum photocatalytic activity over BiPO4/Bi2WO6 composites. Appl. Catal. B Environ. 2017, 200, 222–229. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Guo, S.; Wang, L.; Shi, W.; Huang, H.; Liu, Y.; Kang, Z. Carbon dot and BiVO4 quantum dot composites for overall water splitting via a two-electron pathway. Nanoscale 2008, 8, 17314. [Google Scholar] [CrossRef]
- Xue, J.; Li, J.; Bi, Q.; Tang, C.; Zhang, L.; Leng, Z. Yb-substitution triggered BiVO4-Bi2O3 heterojunction electrode for photoelectrocatalytic degradation of organics. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124640. [Google Scholar] [CrossRef]
- Cristino, V.; Pasti, L.; Marchetti, N.; Berardi, S.; Bignozzi, C.A.; Molinari, A.; Passabi, F.; Caramori, S.; Amidani, L.; Orlandi, M.; et al. Photoelectrocatalytic degradation of emerging contaminants at WO3/BiVO4 photoanodes in aqueous solution. Photochem. Photobiol. Sci. 2019, 18, 2150–2163. [Google Scholar] [CrossRef]
- Hameed, A.; Montini, T.; Gombac, V.; Fornasiero, P. Surface Phases and Photocatalytic Activity Correlation of Bi2O3/Bi2O4-x Nanocomposite. J. Am. Chem. Soc. 2018, 130, 9658–9659. [Google Scholar] [CrossRef] [PubMed]
- Orimolade, B.O.; Arotiba, O.A. Enhanced photoelectrocatalytic degradation of diclofenac sodium using a system of Ag-BiVO4/BiOI anode and Ag-BiOI cathode. Sci. Rep. 2022, 12, 4214. [Google Scholar] [CrossRef] [PubMed]
- Kalam, A.; Allami, S.A.S.; Al-Sehemi, A.G.; Assiri, M.A.; Yadav, P. Effect of stabilizer on optical band gap of ZnO and their performance in dye-sensitized solar cells. Bull. Chem. Soc. Ethiop. 2022, 36, 209–222. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Mazinani, B.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings Containing Chemically Resistant ZnO–SnOx Core–shell Nanoparticles for Photocatalytic Antifouling. Int. J. Mol. Sci. 2021, 22, 4513. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D.; Muresan, A.; Muresan, R.; Popescu, A. Textile wastewater treatment by homogeneous oxidation with hydrogen peroxide. Environ. Eng. Manag. J. 2009, 8, 1359–1369. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y. review of BiPO4 a highly efficient oxyacid-type photocatalyt for environmental applications. Catal. Sci. Technol. 2015, 5, 3071–3083. [Google Scholar] [CrossRef]
- Zong, S.; Wei, W.; Cui, H.; Jiang, Z.; Lu, X.; Zhang, M.; Xie, J. A novel synthesis of P/BiPO4 nanocomposites with enhanced visible-light photocatalysis. Mater. Res. Innov. 2015, 19, 361–367. [Google Scholar] [CrossRef]
- Osman, D.A.M.; Mustafa, M.A. Synthesis and characterization of zinc oxide nanoparticles using zinc acetate dihydrate and sodium hydroxide. J. Nanosci. Nanoeng. 2015, 1, 248–251. [Google Scholar]
- Wasly, H.S.; Abd El-Sadek, M.S.; Henini, M. Influence of reaction time and synthesis temperature on the physical properties of ZnO nanoparticles synthesized by the hydrothermal method. App. Phys. A 2018, 124, 76. [Google Scholar] [CrossRef]
- Elamin, N.; Elsanousi, A. Synthesis of ZnO Nanostructures and their Photocatalytic Activity. J. Appl. Ind. Sci. 2013, 1, 32–35. [Google Scholar]
- Lin, X.; Liu, D.; Guo, X.; Sun, N.; Zhao, S.; Chang, L.; Zhai, H.; Wang, Q. Fabrication and efficient visible light-induced photocatalytic activity of Bi2MoO6/BiPO4 composite. J. Phys. Chem. Solids. 2015, 76, 170–177. [Google Scholar] [CrossRef]
- Subramanian, H.; Krishnan, M.; Mahalingam, A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv. 2022, 12, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e036632. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Ivanova, K.; Ivanova, A.; Hoyo, J.; Perez-Rafael, S.; Tzanov, T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 7632. [Google Scholar] [CrossRef] [PubMed]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Pingmuang, K.; Nattestad, A.; Kangwansupamonkon, W.; Wallace, G.G.; Phanichphant, S.; Chen, J. Phase-controlled microwave synthesis of pure monoclinic BiVO4 nanoparticles for photocatalytic dye degradation. Appl. Mater. Today 2015, 1, 67–73. [Google Scholar] [CrossRef]
- Janani, B.; Al-Kheraif, A.A.; Thomas, A.M.; Syed, A.; Elgorban, A.M.; Raju, L.L.; Das, A.; Khan, S.S. Construction of nano-heterojunction AgFeO2/ZnO for boosted photocatalytic performance and its antibacterial applications. Mater. Sci. Semicond. Process. 2021, 133, 105924. [Google Scholar] [CrossRef]
- Chang, X.; Li, Z.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. Efficient synthesis of sunlight-driven ZnO-based heterogeneous photocatalysts. Mater. Des. 2016, 98, 324–332. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Gulce, H.; Eskizeybek, V.; Haspulat, B.; Sarl, F.; Gulce, A.; Avcl, A. Preparation of a new polyaniline/CdO nanocomposite and investigation of its photocatalytic activity: Comparative study under UV light and natural sunlight irradiation. Ind. Eng. Chem. Res. 2013, 52, 10924–10934. [Google Scholar] [CrossRef]
- Ju, P.; Wang, Y.; Sun, Y.; Zhang, D. Controllable one-pot synthesis of a nest-like Bi2WO6/BiVO4 composite with enhanced photocatalytic antifouling performance under visible ligh irradiation. Dalton Trans. 2016, 45, 4588–4602. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ju, P.; Wang, Y.; Sun, Y.; Zhang, D.; Yu, J. Chemical etching preparation of the Bi2WO6/BiOI p-n heterojunction with enhanced photocatalytic antifouling activity under visible light irradiation. Chem. Eng. J. 2016, 288, 264–275. [Google Scholar] [CrossRef]
- Krishnan, M.; Sivanandham, V.; Dahms, H.-U.; Murugaiah, S.G.; Seeni, P.; Gopalan, S.; Rathinam, A.J. Antifouling assessments on biogenic nanoparticles: A field study from polluted offshore platform. Mar. Pollut. Bull. 2015, 101, 816–825. [Google Scholar] [CrossRef]
- Dai, X.J.; Luo, Y.S.; Zhang, W.D.; Fu, S.Y. Facile hydrothermal synthesis and photocatalytic activity of bismuth tungstate hierarchical hollow spheres with an ultrahigh surface area. Dalton Trans. 2010, 39, 3426–3432. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysios, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef]
- Bae, K.N.; Noh, S.I.; Ahn, H.J.; Seong, T.Y. Effect of MnTiO3 surface treatment on the performance of dye-sensitized solar cells. Mater. Lett. 2013, 96, 67–70. [Google Scholar] [CrossRef]


| Concentration | DF | Sum of Squares | Mean Square | F-Value | Probability > F * |
|---|---|---|---|---|---|
| BiPO4-NPs (NPs-1) | |||||
| Model | 2 | 4.5 | 2.25 | 0.11947 | 0.88878 |
| Error | 9 | 169.5 | 18.83333 | ||
| Total | 11 | 174 | |||
| ZnO-NPs (NPs-2) | |||||
| Model | 2 | 8 | 4 | 1.09091 | 0.37652 |
| Error | 9 | 33 | 3.66667 | ||
| Total | 11 | 41 | |||
| BiPO4/ZnO-NCs (B1Z1-300:300; NCs-1) | |||||
| Model | 2 | 8 | 4 | 1.09091 | 0.37652 |
| Error | 9 | 33 | 3.66667 | ||
| Total | 11 | 41 | |||
| BiPO4/ZnO-NCs (B2Z1-300:150; NCs-2) | |||||
| Model | 2 | 8 | 4 | 1.5 | 0.27402 |
| Error | 9 | 24 | 2.66667 | ||
| Total | 11 | 32 | |||
| BiPO4/ZnO-NCs (B4Z1-300:75; NCs-3) | |||||
| Model | 2 | 8 | 4 | 0.6 | 0.56936 |
| Error | 9 | 60 | 6.66667 | ||
| Total | 11 | 68 | |||
| BiPO4/ZnO-NCs (B1Z4-75:300; NCs-4) | |||||
| Model | 2 | 8 | 4 | 0.34286 | 0.71862 |
| Error | 9 | 105 | 11.66667 | ||
| Total | 11 | 113 | |||
| Test Organisms | Photocatalytic Inactivation Experiments | ||||
|---|---|---|---|---|---|
| Catalyst NCs-4 and UV Light Irradiation at Different Time Intervals (CFUs mL−1) | Initial Count (Before Treatment) | ||||
| After 5 min | After 10 min | After 15 min | After 20 min | ||
| Multidrug-Resistant Strains (MDRs) | |||||
| Gram ve+ | 50.4 [×105] | 36.0 [×104] | 19.2 [×103] | 10.2 [×102] | 107–108 (CFUs mL−1) |
| Gram ve− | 44.2 [×105] | 52.4 [×104] | 22.8 [×103] | 8.6 [×102] | 107–108 (CFUs mL−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, M.; Subramanian, H.; Ramachandran, S.K.; Muthukumarasamy, A.; Ramadoss, D.; Mahalingam, A.; Rathinam, A.J.; Dahms, H.-U.; Hwang, J.-S. Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications. Int. J. Mol. Sci. 2023, 24, 1947. https://doi.org/10.3390/ijms24031947
Krishnan M, Subramanian H, Ramachandran SK, Muthukumarasamy A, Ramadoss D, Mahalingam A, Rathinam AJ, Dahms H-U, Hwang J-S. Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications. International Journal of Molecular Sciences. 2023; 24(3):1947. https://doi.org/10.3390/ijms24031947
Chicago/Turabian StyleKrishnan, Muthukumar, Harinee Subramanian, Sathish Kumar Ramachandran, Arulmozhi Muthukumarasamy, Dineshram Ramadoss, Ashok Mahalingam, Arthur James Rathinam, Hans-Uwe Dahms, and Jiang-Shiou Hwang. 2023. "Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications" International Journal of Molecular Sciences 24, no. 3: 1947. https://doi.org/10.3390/ijms24031947
APA StyleKrishnan, M., Subramanian, H., Ramachandran, S. K., Muthukumarasamy, A., Ramadoss, D., Mahalingam, A., Rathinam, A. J., Dahms, H.-U., & Hwang, J.-S. (2023). Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications. International Journal of Molecular Sciences, 24(3), 1947. https://doi.org/10.3390/ijms24031947








