Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota
Abstract
:1. Introduction
2. Methods and Aim of the Case Report
3. Case Presentation
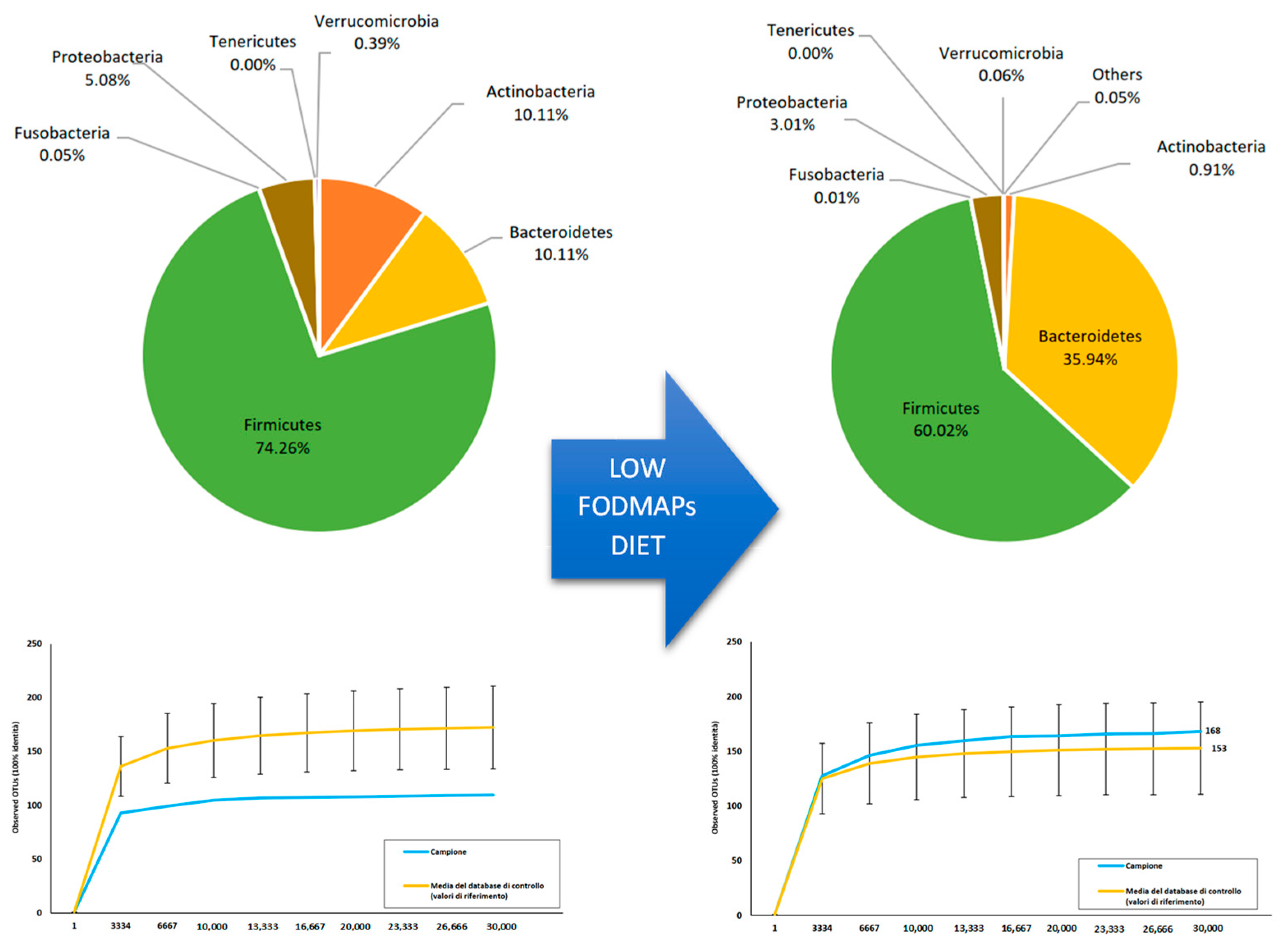
3.1. Microbiota Analysis: A Possible Turning Point?
3.2. Dietary Approaches following Stabilization of the Intestinal Framework
4. Discussion
4.1. The Possible Role of the Microbiota
4.2. Ketogenic Diet and Microbiota
4.3. Low-FODMAP Diet and Microbiota: Microbiota Pattern Change as a Possible Explanation?
4.4. Gut Microbiota Transplants: Could This Represent the Next Step?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karunakaran, S.; Menon, R.N.; Nair, S.S.; Santhakumar, S.; Nair, M.; Sundaram, S. Clinical and Genetic Profile of Autism Spectrum Disorder–Epilepsy (ASD-E) Phenotype: Two Sides of the Same Coin! Clin. EEG Neurosci. 2020, 51, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Nogay, N.H.; Walton, J.; Roberts, K.M.; Nahikian-Nelms, M.; Witwer, A.N. The Effect of the Low FODMAP Diet on Gastrointestinal Symptoms, Behavioral Problems and Nutrient Intake in Children with Autism Spectrum Disorder: A Randomized Controlled Pilot Trial. J. Autism Dev. Disord. 2021, 51, 2800–2811. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef] [Green Version]
- Orphanet. Available online: https://www.orpha.net/consor4.01/www/cgi-bin/Disease_Genes.php?lng=IT&data_id=16651&MISSING%20CONTENT=PGM1&search=Disease_Genes_Simple&title=PGM1 (accessed on 21 July 2022).
- Orphanet. Available online: https://www.orpha.net/consor/cgi-bin/Disease_Genes.php?lng=IT&data_id=23526&MISSING%20CONTENT=EEF1A2&search=Disease_Genes_Simple&title=EEF1A2 (accessed on 21 July 2022).
- Ilhan, Z.E.; Brochard, V.; Lapaque, N.; Auvin, S.; Lepage, P. Exposure to anti-seizure medications impact growth of gut bacterial species and subsequent host response. Neurobiol. Dis. 2022, 167, 105664. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; Van Sinderen, D.; et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amlerova, J.; Šroubek, J.; Angelucci, F.; Hort, J. Evidences for a Role of Gut Microbiota in Pathogenesis and Management of Epilepsy. Int. J. Mol. Sci. 2021, 22, 5576. [Google Scholar] [CrossRef]
- Catinean, A.; Neag, A.M.; Nita, A.; Buzea, M.; Buzoianu, A.D. Bacillus spp. Spores—A Promising Treatment Option for Patients with Irritable Bowel Syndrome. Nutrients 2019, 11, 1968. [Google Scholar] [CrossRef] [Green Version]
- Tagg, J.R.; Dierksen, K.P. Bacterial replacement therapy: Adapting ‘germ warfare’ to infection prevention. Trends Biotechnol. 2003, 21, 217–223. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Edwards, C.G.; Khan, N.A.; Holscher, H.D. Gastrointestinal Microbiota and Cognitive Function in Adult Females. FASEB J. 2017, 31, 965. [Google Scholar] [CrossRef]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Roussin, L.; Prince, N.; Perez-Pardo, P.; Kraneveld, A.D.; Rabot, S.; Naudon, L. Role of the Gut Microbiota in the Pathophysiology of Autism Spectrum Disorder: Clinical and Preclinical Evidence. Microorganisms 2020, 8, 1369. [Google Scholar] [CrossRef]
- Cotrina, M.L.; Ferreiras, S.; Schneider, P. High prevalence of self-reported autism spectrum disorder in the Propionic Acidemia Registry. JIMD Rep. 2020, 51, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Valiente-Pallejà, A.; Torrell, H.; Muntane, G.; Cortés, M.J.; Martínez-Leal, R.; Abasolo, N.; Alonso, Y.; Vilella, E.; Martorell, L. Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Hum. Mol. Genet. 2018, 27, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kaser, A.; Kiely, C.J.; Neville, B.A.; Powell, N.; et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut, 2021; ahead of print. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef]
- He, Z.; Cui, B.-T.; Zhang, T.; Li, P.; Long, C.; Ji, G.-Z.; Zhang, F. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccioli, A.; Cardinali, M.; Di Pierro, F.; Zonzini, G.B.; Matera, M.R. Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. Int. J. Mol. Sci. 2022, 23, 8829. https://doi.org/10.3390/ijms23158829
Bertuccioli A, Cardinali M, Di Pierro F, Zonzini GB, Matera MR. Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. International Journal of Molecular Sciences. 2022; 23(15):8829. https://doi.org/10.3390/ijms23158829
Chicago/Turabian StyleBertuccioli, Alexander, Marco Cardinali, Francesco Di Pierro, Giordano Bruno Zonzini, and Maria Rosaria Matera. 2022. "Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota" International Journal of Molecular Sciences 23, no. 15: 8829. https://doi.org/10.3390/ijms23158829
APA StyleBertuccioli, A., Cardinali, M., Di Pierro, F., Zonzini, G. B., & Matera, M. R. (2022). Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. International Journal of Molecular Sciences, 23(15), 8829. https://doi.org/10.3390/ijms23158829







