Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis
Abstract
1. Introduction
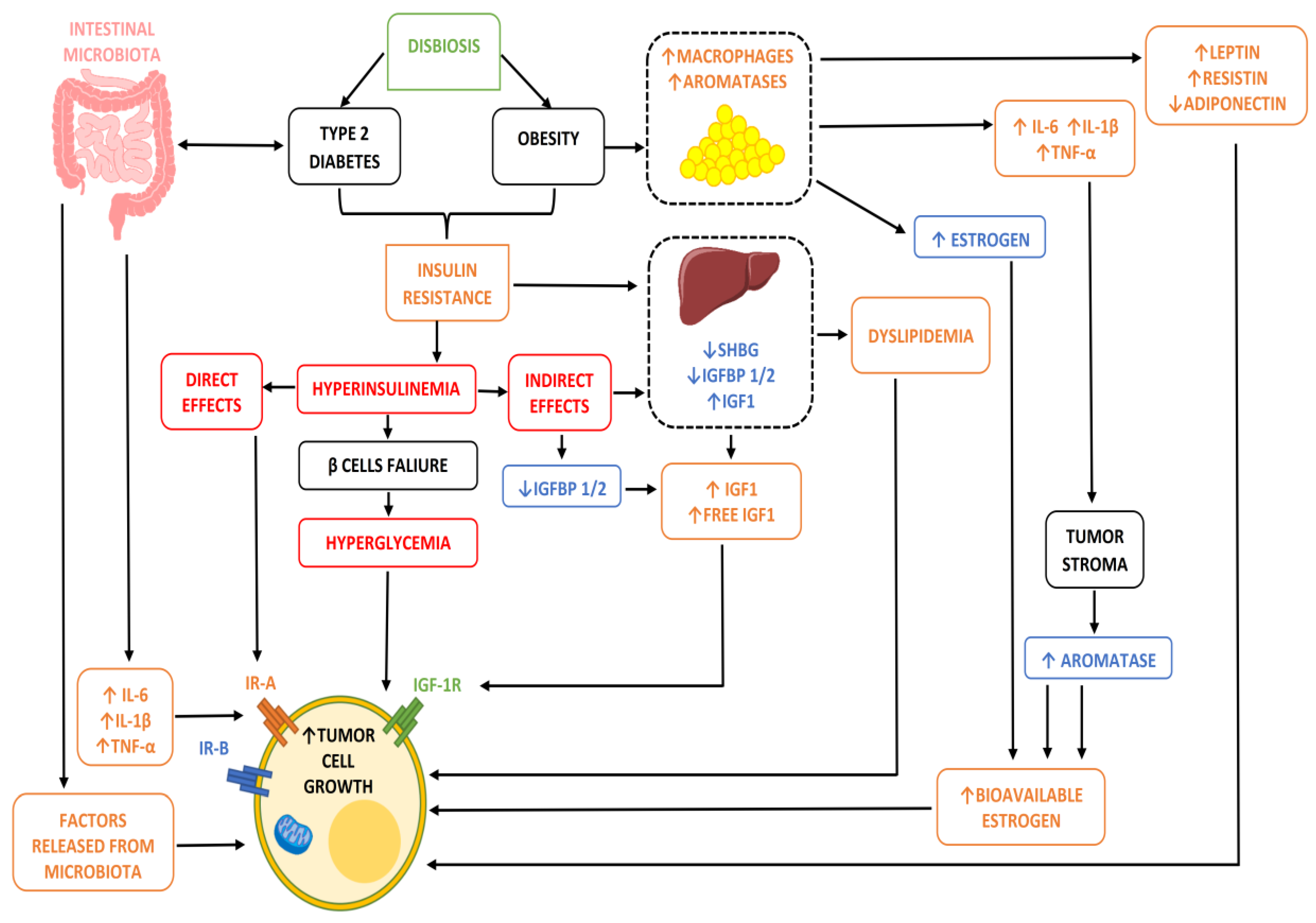
2. Metabolic Disorders as a Risk Factor for Breast Cancer
Mechanisms That Bind Metabolic Disorders and Carcinogenesis
- (1)
- In conditions of obesity or overweight, our adipocytes, due to their hypertrophy, undergo a state of hypoxia and necrosis and a consequent alteration of their metabolic activities. In particular, the alteration of the production of some adipocytokines such as adiponectin and leptin, and above all their relationship, is implemented, ultimately increasing the action of leptin which, being an element with proliferative and angiogenetic capacity, is potentially dangerous when present in high quantities and for a long time in our body [8]. Furthermore, this condition of adipocyte suffering also has the non-negligible effect of increasing the inflammatory response by causing chronic inflammation through the recall of immune cells (lymphocytes and macrophages) and the consequent response by cytokines. Clearly, it is not negligible that in these conditions of obesity/overweight, an increase in the adipose content ultimately also means a greater share of active aromatase enzyme and consequently a greater share of free estrogenic hormones potentially stimulating and proliferating on the mammary gland or on the other hormonally sensitive areas.
- (2)
- In the presence of obesity, MS or diabetes, insulin resistance (IR) is often established. This ultimately leads to hyperglycemia (glucose not being sequestered at the cellular level, and rather remaining in circulation) and hyperinsulinemia (the pancreas, feeling that hyperglycemia is present, is stimulated to produce new insulin), and these two situations are potentially one oncological bomb, being capable of intervening both directly and/or indirectly in the transformation and progression of a breast cell in oncology [9].
- (3)
- But why are hyperinsulinemia and hyperglycemia oncogenetics? That is, why do they stimulate the onset and progression of cancer cells? Insulin is a growth factor, and by its nature it stimulates cell growth and proliferation by acting either directly on the cell using its own receptors or by increasing the share of insulin-growth factor (IGF) thanks to the reduction in the number proteins which, by binding to this element, remain inactive (IGFBPs) [9]. In addition, insulin and hyperinsulinemia also have metabolic action by altering, for example, the adiponectin/leptin ratio seen above, increasing aromatase activity, and stimulating angiogenic activity (i.e., the ability of cancer cells to build their own blood vessels in order to guarantee the sustenance necessary to survive and grow).
- (4)
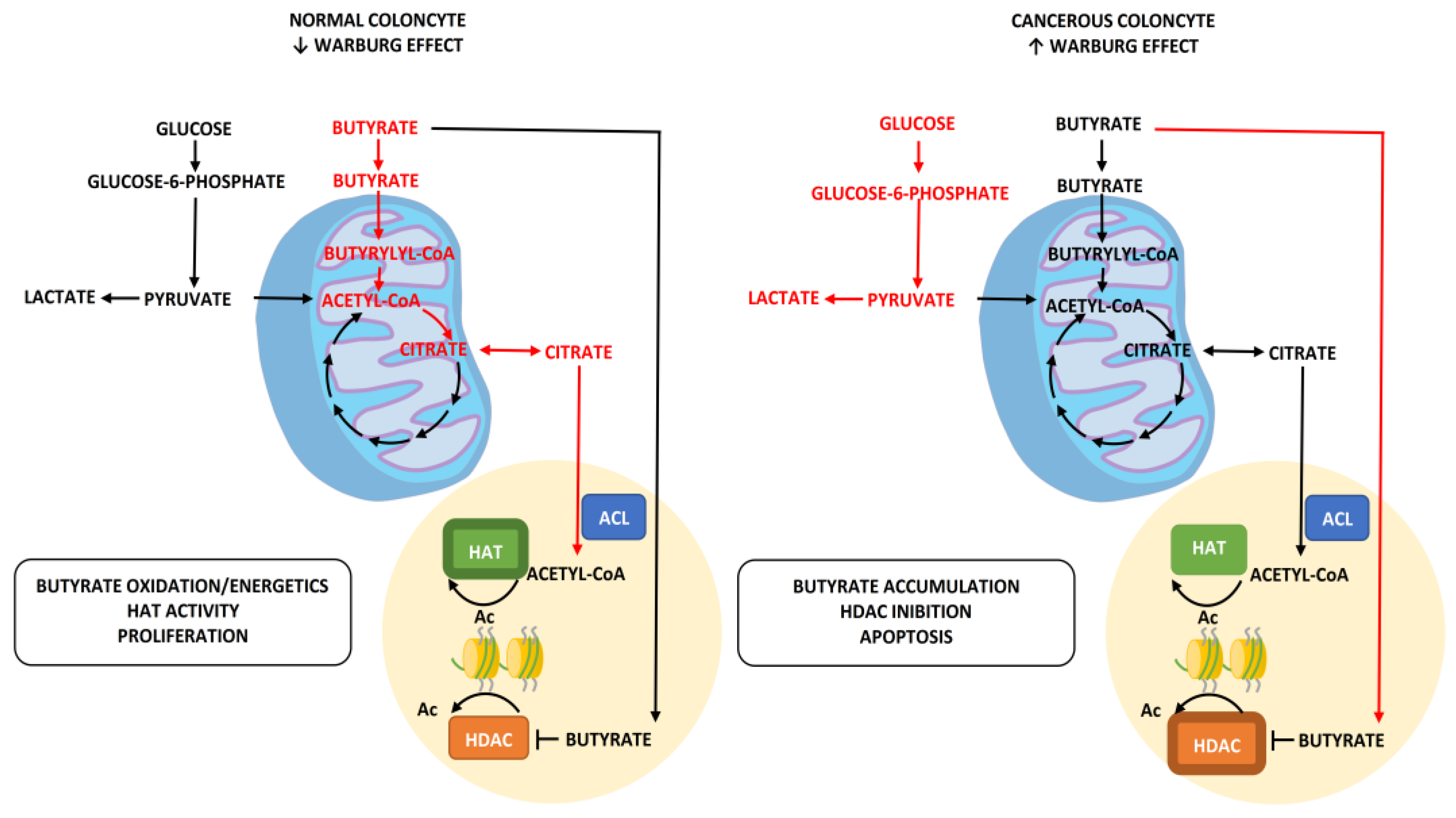
- Unlike insulin, blood sugar does not have any real oncological action, but rather indirectly favors the growth of a tumor, providing an ideal environment for its survival and growth. This occurs due to the metabolic reprogramming or Warburg effect. In practice, cancer cells guarantee themselves the energy to survive in a different way to normal ones, favoring the process of anaerobic glycolysis compared to that of oxidative phosphorylation (OXPHOS). The process of glycolysis is strictly interconnected with the amount of glucose available, as it is less efficient from an energy production point of view than phosphorylation and is therefore facilitated by the condition of hyperglycemia. Authoritative studies and meta-analyses have already highlighted and confirmed the relationship between IR/hyperglycemia and female tumors [10].
- (5)
- Alterations in the lipid profile often accompany dysmetabolic conditions, and hypercholesterolemia is now a recognized risk factor for various female cancers and in particular for breast cancer. Biologically speaking, hypercholesterolemia is now a recognized risk factor for cancer by virtue of both direct and indirect action on target tissues for hormonal activity. In fact, as we know, cholesterol is transformed in our body into estrogen hormones (this mechanism is all the more important when the aromatase enzyme is involved, as happens after menopause and as we have seen abundantly represented in the presence of excess adipose tissue) which, as we know, have a stimulatory and proliferative action on sensitive tissues. Not only that, but cholesterol in large quantities is also transformed by the enzyme CYP27A1 into its active metabolite called 27 hydroxycholesterol (27HC), which has a chemical structure very similar to that of estrogen and is therefore also capable (although it is not a real hormone) of binding to hormone receptors, igniting all of the proliferative pathways usually associated with this situation downstream [11]. These biological mechanisms therefore lead to hypercholesterolemia, which is a condition directly and indirectly affecting the incidence (little) and prognosis (a lot) of oncological disease thanks to its being a source of hormonal stimulation. This is further burdened by the fact that some of the key treatments involved in post-surgical adjuvant therapy, such as aromatase inhibitors (which aim at the total elimination of circulating estrogen hormones, therefore being substantially less effective in the presence of hypercholesterolemia), have the increase in lipid values among their main side effects, and in particular those of cholesterol (especially letrozole and anastrazole).
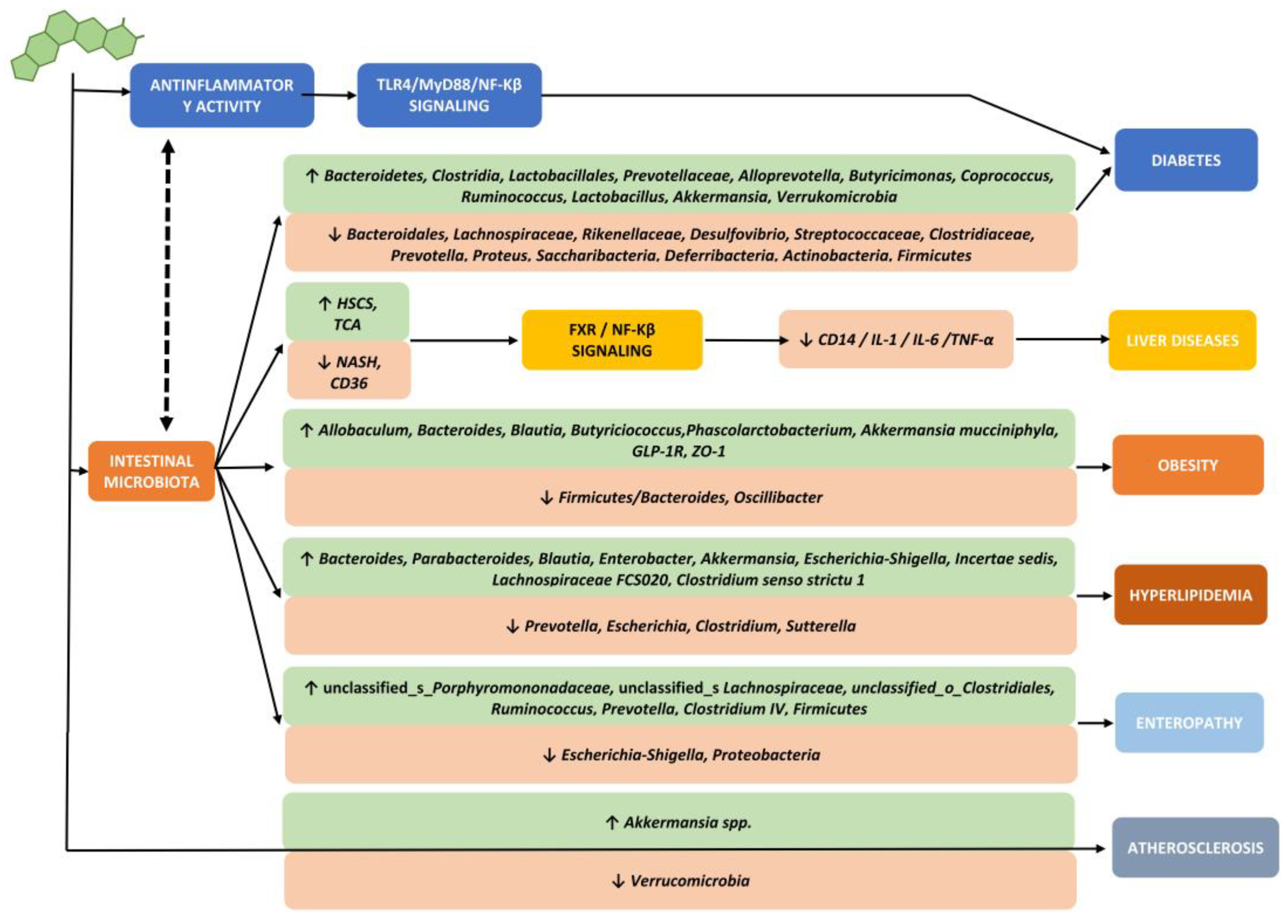
3. How Does the Gut Microbiota Determine Metabolic Disorders and Increase Tumor Risk?
- (1)
- Firmicutes/Bacteroidetes ratio;
- (2)
- Production and use of butyrate as an oncosuppressor;
- (3)
- Hormonal entero-hepatic circle.
3.1. Firmicutes/Bacteroidetes Ratio
- (1)
- The suppression of the expression of a lipoprotein with inhibitory functions of intestinal lipase (LPL), the so-called fasting-induced adipose factor (FIAF), with a consequent increase in the extraction capacity of fatty acids from postprandial fat particles and an increase in the accumulation of fat inside the adipocytes,
- (2)
- The increased ability to form short-chain fatty acids (SCFAs), in particular butyrate, by some bacterial populations (clostridiales) with consequent accumulation of calories in the form of body fat.
- (3)
- The increase in the permeation of lipopolysaccharides (LPS) due to a decrease in the share of SCFAs in the event of a decrease in clostridiales increases the release of inflammatory cytokines, primarily TNF-α, through a mechanism of activation of Toll-like receptor-4 (TLR4), its specific receptor, with an increase in peripheral inflammatory phenomena and the possible development of hepato-steatosis, insulin resistance, omental inflammation, metabolic syndrome and diabetes
3.2. Production and Use of Butyrate as Oncosuppressor
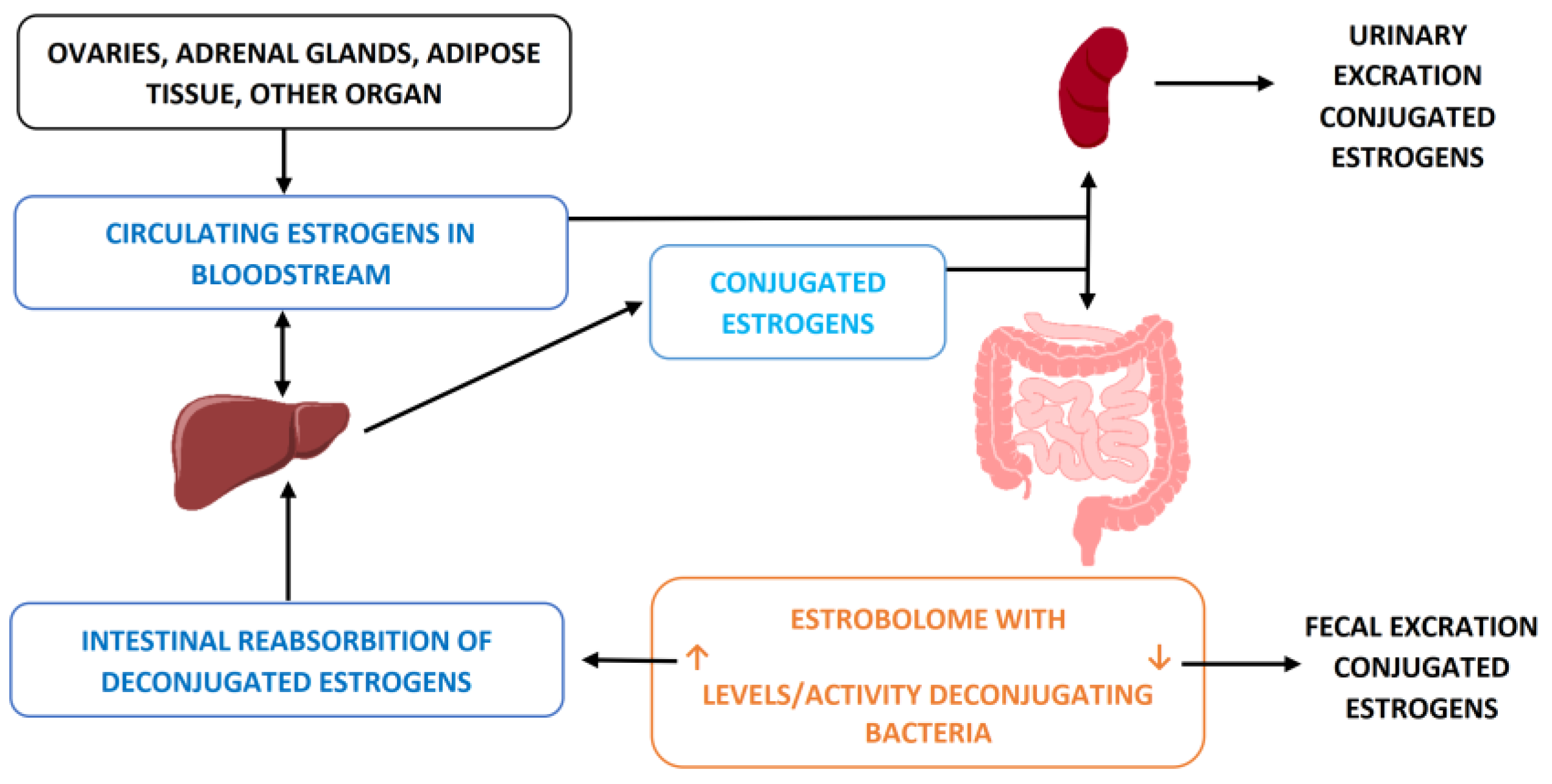
3.3. Hormonal Entero-Hepatic Circle
4. Possibility of Intervention Treatment of Metabolic Disorders: Expand Therapeutic Background
4.1. Berberine on Metabolic Factors
4.2. Berberine on Microbiota
4.3. Bacterial Therapies: Integrate the Potential of Berberine
5. Conclusions
- (1)
- Metabolic disorders (both those concerning the lipid and glucose profile of a patient) are recognized risk factors also for various types of cancer. In particular, given the profound relationship with the hormonal structure, many of the most important female neoplasms seem to be involved both in terms of incidence and progression and prognosis of the disease.
- (2)
- Dysbiosis of the intestinal microbiota contributes to generating metabolic disorders, sub-clinical inflammation and, therefore, indirectly influencing the onset and progression of oncological diseases related to them.
- (3)
- Berberine is certainly effective, both directly acting on the metabolic states and indirectly correcting and/or modulating the dysbiosis that generated them, ultimately working as a real anti-tumor compound both in a preventive sense (on risk factors) and a therapeutic one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.H. Honey and cancer: Sustainable inverse relationship particularly for developing nations-a review. Evid.-Based Complement. Altern. Med. 2012, 2012, 410406. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chlebowski, R.T. Obesity and Cancer: Insights for Clinicians. J. Clin. Oncol. 2016, 34, 4197–4202. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, N.S.; Scholz, C.; Friedl, T.W.P.; Zwingers, T.; Fasching, P.A.; Beckmann, M.W.; Fehm, T.; Mohrmann, S.; Salmen, J.; Ziegler, C.; et al. Prognostic Impact of Weight Change During Adjuvant Chemotherapy in Patients With High-Risk Early Breast Cancer: Results From the ADEBAR Study. Clin. Breast Cancer 2018, 18, 175–183. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef]
- Djiogue, S.; Nwabo Kamdje, A.H.; Vecchio, L.; Kipanyula, M.J.; Farahna, M.; Aldebasi, Y.; Seke Etet, P.F. Insulin resistance and cancer: The role of insulin and IGFs. Endocr. Relat. Cancer 2013, 20, R1–R17. [Google Scholar] [CrossRef]
- Sun, W.; Lu, J.; Wu, S.; Bi, Y.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am. J. Cancer Res. 2016, 6, 2334–2344. [Google Scholar]
- Kimbung, S.; Chang, C.Y.; Bendahl, P.O.; Dubois, L.; Thompson, J.W.; McDonnell, D.P.; Borgquist, S. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr. Relat. Cancer 2017, 24, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Simonetti, G.; Petruzzi, A.; Bertuccioli, A.; Botta, L.; Bruzzone, M.G.; Cuccarini, V.; Fariselli, L.; Lamperti, E. A novel lecithin-based delivery form of Boswellic acids as complementary treatment of radiochemotherapy-induced cerebral edema in patients with glioblastoma multiforme: A longitudinal pilot experience. J. Neurosurg. Sci. 2019, 63, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.M.; Lombardi, P.; Tillhon, M.; Scovassi, A.I. Berberine, an epiphany against cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.S.; Chen, W.; Liu, J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W.; Bin Zhang, K.; Tang, J.L.; Guang, L.X.; Ying, Y.; Xu, Y.; Zhang, L.; Li, D.D. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 2008, 31, 1169–1176. [Google Scholar] [CrossRef]
- Pan, G.Y.; Huang, Z.J.; Wang, G.J.; Fawcett, J.P.; Liu, X.D.; Zhao, X.C.; Sun, J.G.; Xie, Y.Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003, 69, 632–636. [Google Scholar]
- Zhou, L.; Yang, Y.; Wang, X.; Liu, S.; Shang, W.; Yuan, G.; Li, F.; Tang, J.; Chen, M.; Chen, J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism 2007, 56, 405–412. [Google Scholar] [CrossRef]
- Liu, L.Z.; Cheung, S.C.; Lan, L.L.; Ho, S.K.; Xu, H.X.; Chang, J.C.; Tong, P.C. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol. Cell. Endocrinol. 2010, 317, 148–153. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef]
- Bertuccioli, A.; Moricoli, S.; Amatori, S.; Rocchi, M.B.L.; Vici, G.; Sisti, D. Berberine and Dyslipidemia: Different Applications and Biopharmaceutical Formulations Without Statin-Like Molecules-A Meta-Analysis. J. Med. Food 2020, 23, 101–113. [Google Scholar] [CrossRef]
- Fogacci, F.; Grassi, D.; Rizzo, M.; Cicero, A.F. Metabolic effect of berberine-silymarin association: A meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Phytother. Res. 2019, 33, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the treatment of type 2 diabetes mellitus: A systemic review and meta-analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, A.K.; Shetty, C.; Rizvi, S.M.H.A.; Sharaf, J.; Williams, K.-A.D.; Tariq, M.; Acharekar, M.V.; Saldivia, S.E.G.; Unnikrishnan, S.; Chavarria, Y.Y.; et al. A Systematic Review of the Gastrointestinal Microbiome: A Game Changer in Colorectal Cancer. Cureus 2022, 14, e28545. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Wargo, J.A. The Microbiome in Gastrointestinal Cancers. Gastroenterol. Clin. N. Am. 2022, 51, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S. Anti-cancer activity of human gastrointestinal bacteria. Med. Oncol. 2022, 39, 220. [Google Scholar] [CrossRef] [PubMed]
- Deluce, J.; Maleki Vareki, S.; Fernandes, R. The role of gut microbiome in immune modulation in metastatic renal cell carcinoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221122714. [Google Scholar] [CrossRef]
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 949490. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. Effectiveness of berberine in bacillary dysentery. Zhonghua Nei Ke Za Zhi 1959, 7, 741–743. [Google Scholar]
- Homma, N.; Kono, M.; Kadohira, H.; Yoshihara, S.; Masuda, S. The effect of berberine chloride on the intestinal flora of infants. Arzneimittelforschung 1961, 11, 450–454. [Google Scholar] [PubMed]
- Mekawi, M. Effect of berberine alkaloid on cholera Vibro and its endotoxin. J. Egypt Med. Assoc. 1966, 49, 554–559. [Google Scholar]
- Lahiri, S.C.; Dutta, N.K. Berberine and chloramphenicol in the treatment of cholera and severe diarrhoea. J. Indian Med. Assoc. 1967, 48, 1–11. [Google Scholar] [PubMed]
- Subbaiah, T.V.; Amin, A.H. Effect of berberine sulphate on Entamoeba histolytica. Nature 1967, 215, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.K.; Jain, A.M.; Dube, M.K.; Bhandari, B. A combination of sulfadimidine, neomycin and berberine in the treatment of infectious diarrhoea. Indian J. Pediatr. 1969, 36, 242–244. [Google Scholar] [CrossRef]
- Sun, D.; Abraham, S.N.; Beachey, E.H. Influence of berberine sulfate on synthesis and expression of Pap fimbrial adhesin in uropathogenic Escherichia coli. Antimicrob. Agents Chemother. 1988, 32, 1274–1277. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hussain, A.; Attar, F.; Bloukh, S.H.; Edis, Z.; Sharifi, M.; Balali, E.; Nemati, F.; Derakhshankhah, H.; Zeinabad, H.A.; et al. A review of the berberine natural polysaccharide nanostructures as potential anticancer and antibacterial agents. Biomed. Pharmacother. 2022, 146, 112531. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Ji, B.; Jiang, Y.; Xiang, H. Association of low-grade inflammation caused by gut microbiota disturbances with osteoarthritis: A systematic review. Front. Vet. Sci. 2022, 9, 938629. [Google Scholar] [CrossRef] [PubMed]
- Pengrattanachot, N.; Thongnak, L.; Lungkaphin, A. The impact of prebiotic fructooligosaccharides on gut dysbiosis and inflammation in obesity and diabetes related kidney disease. Food Funct. 2022, 13, 5925–5945. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress (Thousand Oaks). 2022, 6, 24705470221076390. [Google Scholar] [CrossRef] [PubMed]
- Guido, G.; Ausenda, G.; Iascone, V.; Chisari, E. Gut permeability and osteoarthritis, towards a mechanistic understanding of the pathogenesis: A systematic review. Ann. Med. 2021, 53, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Grylls, A.; Seidler, K.; Neil, J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharmacother. 2021, 137, 111334. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z. Obesity-associated cancer risk: The role of intestinal microbiota in the etiology of the host proinflammatory state. Transl. Res. 2017, 179, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yu, J.; Yang, Y.; Zhang, F.; Su, W.; Fan, Q.; Wu, C.; Wu, S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 2021, 139, 111595. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sarvarian, P.; Gholipour, E.; Asenjan, K.S.; Aghebati-Maleki, L.; Motavalli, R.; Hojjat-Farsangi, M.; Yousefi, M. Berberine: A novel therapeutic strategy for cancer. IUBMB Life 2020, 72, 2065–2079. [Google Scholar] [CrossRef]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of P-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef]
- Petrangolini, G.; Corti, F.; Ronchi, M.; Arnoldi, L.; Allegrini, P.; Riva, A. Development of an Innovative Berberine Food-Grade Formulation with an Ameliorated Absorption: In Vitro Evidence Confirmed by Healthy Human Volunteers Pharmacokinetic Study. Evid. Based Complement. Altern. Med. 2021, 2021, 7563889. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M.; et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Peroni, G.; Perna, S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study. Nutrients 2021, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Y.; Zheng, Y. Probiotics for the Treatment of Bacterial Vaginosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3859. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Mumtaz, S.U.; Bertuccioli, A.; Recchia, M.; Zerbinati, N.; Khan, A. Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study. Microorganisms 2022, 10, 1926. [Google Scholar] [CrossRef]
- Bertuccioli, A.; Rocchi, M.B.L.; Morganti, I.; Vici, G.; Gervasi, M.; Amatori, S.; Sisti, D. Streptococcus salivarius K12 in pharyngotonsillitis and acute otitis media–A meta-analysis. Nutrafoods 2019, 2, 80–88. [Google Scholar] [CrossRef]
- Veiga, P.; Suez, J.; Derrien, M.; Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 2020, 5, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzaniga, M.; Zonzini, G.B.; Di Pierro, F.; Moricoli, S.; Bertuccioli, A. Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis. Int. J. Mol. Sci. 2022, 23, 12538. https://doi.org/10.3390/ijms232012538
Cazzaniga M, Zonzini GB, Di Pierro F, Moricoli S, Bertuccioli A. Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis. International Journal of Molecular Sciences. 2022; 23(20):12538. https://doi.org/10.3390/ijms232012538
Chicago/Turabian StyleCazzaniga, Massimiliano, Giordano Bruno Zonzini, Francesco Di Pierro, Sara Moricoli, and Alexander Bertuccioli. 2022. "Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis" International Journal of Molecular Sciences 23, no. 20: 12538. https://doi.org/10.3390/ijms232012538
APA StyleCazzaniga, M., Zonzini, G. B., Di Pierro, F., Moricoli, S., & Bertuccioli, A. (2022). Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis. International Journal of Molecular Sciences, 23(20), 12538. https://doi.org/10.3390/ijms232012538







