Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging
Abstract
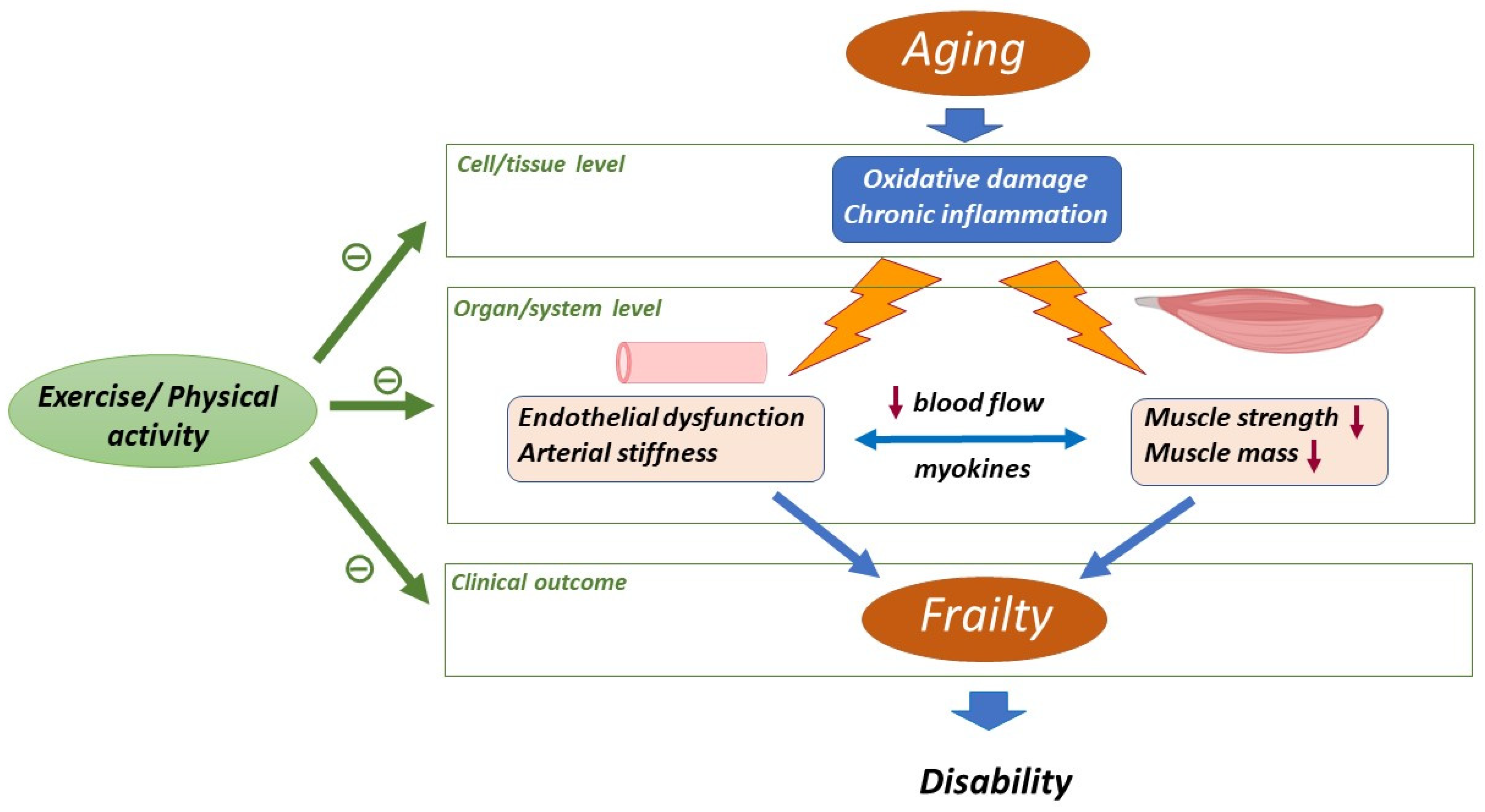
:1. Introduction
Oxidative Stress and Inflammation, Constituents of the Background of Aging
2. Aging-Related Changes in Skeletal Muscle and Cardiovascular System
2.1. Skeletal Muscle
2.1.1. Anabolic Resistance
2.1.2. Mitochondrial Dysfunction
2.1.3. Oxidative Stress
2.1.4. Inflammation
2.1.5. Myokines
2.2. Cardiovascular System
2.2.1. Oxidative Stress
2.2.2. Inflammation
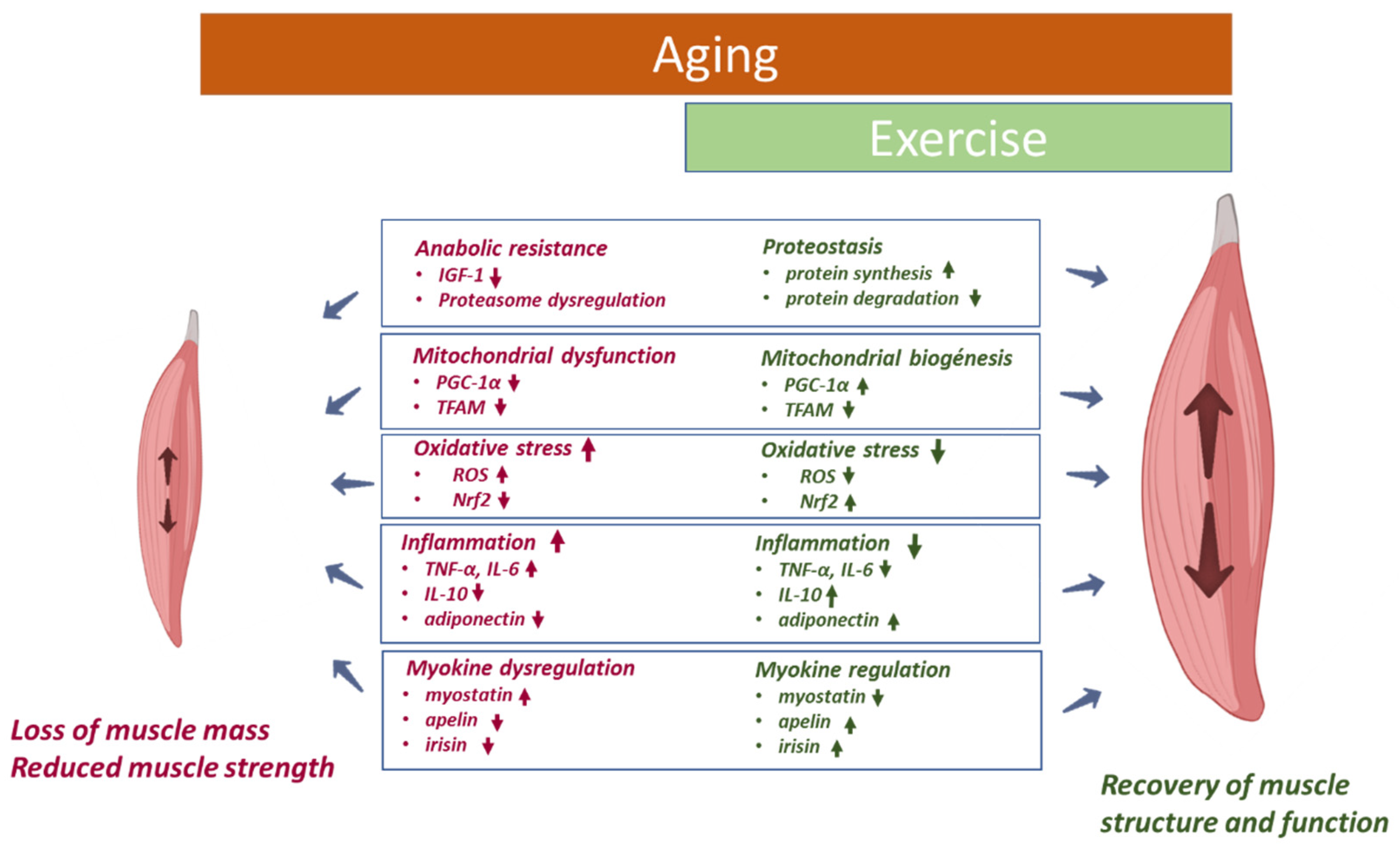
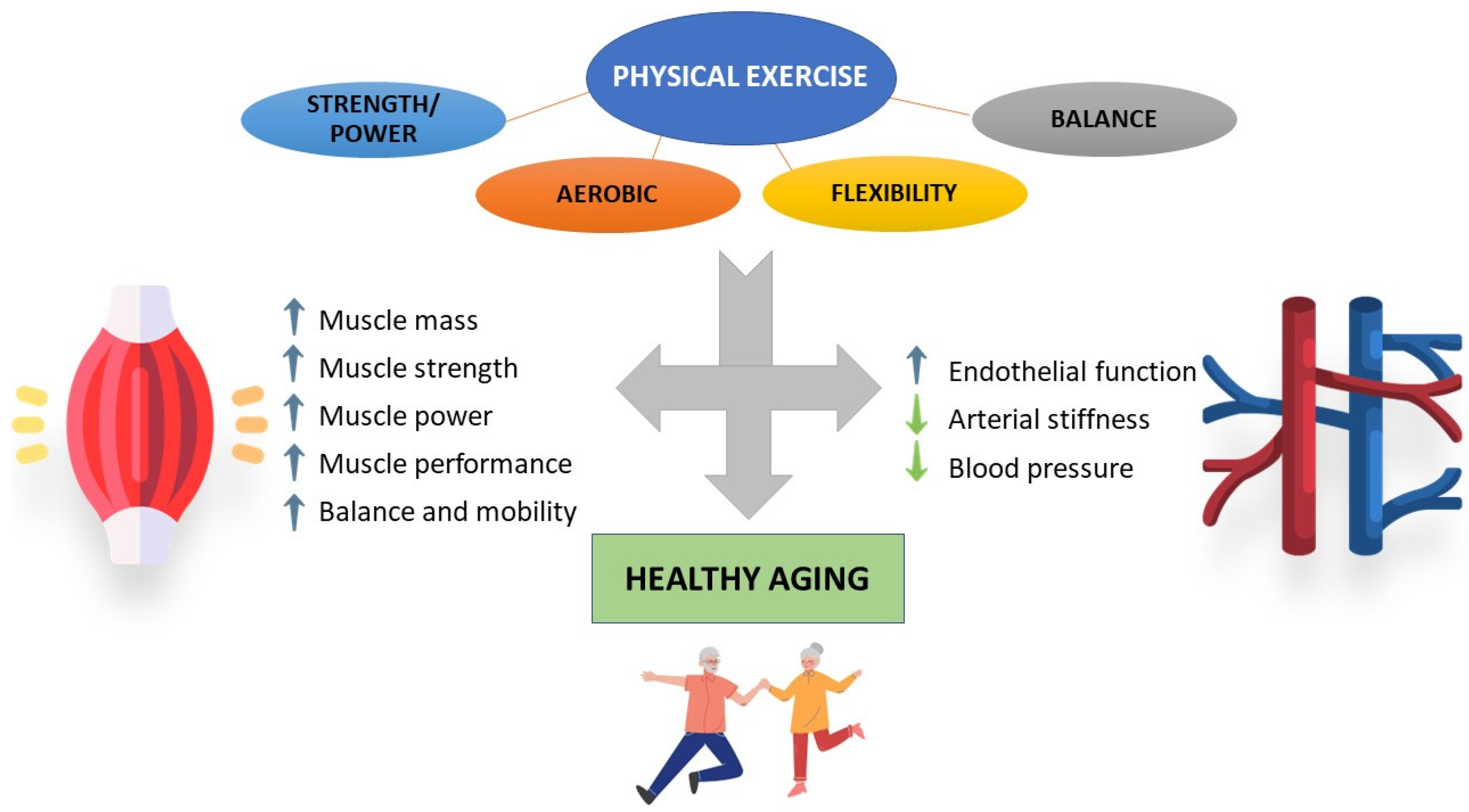
3. Impact of Physical Activity/Exercise on Aged Skeletal Muscle and Cardiovascular System
3.1. Skeletal Muscle
3.2. Cardiovascular System
4. Impact of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging
4.1. Skeletal Muscle
4.2. Cardiovascular System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzau, V.J.; Inouye, S.K.; Rowe, J.W.; Finkelman, E.; Yamada, T. Enabling Healthful Aging for All—The National Academy of Medicine Grand Challenge in Healthy Longevity. N. Engl. J. Med. 2019, 381, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Goodkind, D.; Kowal, P. An Aging World: 2015; U.S. Government Printing Office: Washington, DC, USA, 2016.
- Rodriguez-Mañas, L.; Rodríguez-Artalejo, F.; Sinclair, A.J. The Third Transition: The Clinical Evolution Oriented to the Contemporary Older Patient. J. Am. Med. Dir. Assoc. 2017, 18, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Debates: The Not-So-Close Relationship between Biological Aging and Age-Associated Pathologies in Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B547–B550. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Ageing and Health 2015; WHO: Geneva, Switzerland, 2015.
- Angulo, J.; el Assar, M.; Rodríguez-Mañas, L. Frailty and Sarcopenia as the Basis for the Phenotypic Manifestation of Chronic Diseases in Older Adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef]
- Arc-Chagnaud, C.; Salvador-Pascual, A.; Garcia-Dominguez, E.; Olaso-Gonzalez, G.; Correas, A.G.; Serna, E.; Brioche, T.; Chopard, A.; Fernandez-Marcos, P.J.; Serrano, M.; et al. Glucose 6-P Dehydrogenase Delays the Onset of Frailty by Protecting against Muscle Damage. J. Cachexia Sarcopenia Muscle 2021, 12, 1879–1896. [Google Scholar] [CrossRef]
- Angulo, J.; el Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical Activity and Exercise: Strategies to Manage Frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Shin, M.J.; Saini, S.K.; Custodero, C.; Aggarwal, M.; Anton, S.D.; Leeuwenburgh, C.; Mankowski, R.T. Vascular Dysfunction as a Potential Culprit of Sarcopenia. Exp. Gerontol. 2021, 145, 111220. [Google Scholar] [CrossRef]
- Davies, B.; García, F.; Ara, I.; Artalejo, F.R.; Rodriguez-Mañas, L.; Walter, S. Relationship Between Sarcopenia and Frailty in the Toledo Study of Healthy Aging: A Population Based Cross-Sectional Study. J. Am. Med. Dir. Assoc. 2018, 19, 282–286. [Google Scholar] [CrossRef]
- Davies, B.; Walter, S.; Rodríguez-Laso, A.; Carnicero Carreño, J.A.; García-García, F.J.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Differential Association of Frailty and Sarcopenia With Mortality and Disability: Insight Supporting Clinical Subtypes of Frailty. J. Am. Med. Dir. Assoc. 2022. [Google Scholar] [CrossRef]
- Palta, P.; Griswold, M.; Ranadive, R.; Bandeen-Roche, K.; Folsom, A.R.; Petruski-Ivleva, N.; Burgard, S.; Kucharska-Newton, A.; Windham, B.G. Midlife Cardiovascular Health and Robust Versus Frail Late-Life Status: The Atherosclerosis Risk in Communities Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Shrauner, W.; Lord, E.M.; Nguyen, X.M.T.; Song, R.J.; Galloway, A.; Gagnon, D.R.; Driver, J.A.; Gaziano, J.M.; Wilson, P.W.F.; Djousse, L.; et al. Frailty and Cardiovascular Mortality in More than 3 Million US Veterans. Eur. Heart J. 2022, 43, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.I.; Fukumoto, Y. Sarcopenia as a Comorbidity of Cardiovascular Disease. J. Cardiol. 2022, 79, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Oxidative Stress-Based Therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. The Role of Antioxidants in Cancer, Friends or Foes? Curr. Pharm. Des. 2019, 24, 5234–5244. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox Regulation of NF-Kappa B Activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)Ever after: Aging in the Context of Oxidative Stress, Proteostasis Loss and Cellular Senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative Stress Is Related to Frailty, Not to Age or Sex, in a Geriatric Population: Lipid and Protein Oxidation as Biomarkers of Frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Viña, J. The Free Radical Theory of Frailty: Mechanisms and Opportunities for Interventions to Promote Successful Aging. Free Radic. Biol. Med. 2019, 134, 690–694. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Frailty as a Phenotypic Manifestation of Underlying Oxidative Stress. Free Radic. Biol. Med. 2020, 149, 72–77. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J.; et al. Effect of Structured Physical Activity on Prevention of Major Mobility Disability in Older Adults: The LIFE Study Randomized Clinical Trial. JAMA 2014, 311, 2387–2396. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L.; Laosa, O.; Vellas, B.; Paolisso, G.; Topinkova, E.; Oliva-Moreno, J.; Bourdel-Marchasson, I.; Izquierdo, M.; Hood, K.; Zeyfang, A.; et al. Effectiveness of a Multimodal Intervention in Functionally Impaired Older People with Type 2 Diabetes Mellitus. J. Cachexia Sarcopenia Muscle 2019, 10, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent Intervention to Prevent Mobility Disability in Frail Older Adults: Randomised Controlled Trial (SPRINTT Project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rodriguez-Mañas, L.; Casas-Herrero, A.; Martinez-Velilla, N.; Cadore, E.L.; Sinclair, A.J. Is It Ethical Not to Precribe Physical Activity for the Elderly Frail? J. Am. Med. Dir. Assoc. 2016, 17, 779–781. [Google Scholar] [CrossRef]
- Dulac, M.; Leduc-Gaudet, J.P.; Reynaud, O.; Ayoub, M.B.; Guérin, A.; Finkelchtein, M.; Hussain, S.N.A.; Gouspillou, G. Drp1 Knockdown Induces Severe Muscle Atrophy and Remodelling, Mitochondrial Dysfunction, Autophagy Impairment and Denervation. J. Physiol. 2020, 598, 3691–3710. [Google Scholar] [CrossRef]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of Sarcopenia and the Relationship with Fat Mass: Descriptive Review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and Mirnas. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of Increased Risk of Insulin Resistance in Aging Skeletal Muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and Mechanisms of Primary Sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 1–8. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32, 2022. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of Disease Burden and Strategies to Improve Outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place? Int. J. Mol. Sci. 2020, 21, 5635. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Angulo, J.; Carnicero, J.A.; el Assar, M.; García-García, F.J.; Sinclair, A.J. Dual Effects of Insulin Resistance on Mortality and Function in Non-Diabetic Older Adults: Findings from the Toledo Study of Healthy Aging. Geroscience 2022, 44, 1095–1108. [Google Scholar] [CrossRef]
- Consitt, L.A.; Saxena, G.; Saneda, A.; Houmard, J.A. Age-Related Impairments in Skeletal Muscle PDH Phosphorylation and Plasma Lactate Are Indicative of Metabolic Inflexibility and the Effects of Exercise Training. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E145–E156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesketh, S.J.; Stansfield, B.N.; Stead, C.A.; Burniston, J.G. The Application of Proteomics in Muscle Exercise Physiology. Expert Rev. Proteom. 2020, 17, 813–825. [Google Scholar] [CrossRef]
- Hunt, L.C.; Schadeberg, B.; Stover, J.; Haugen, B.; Pagala, V.; Wang, Y.D.; Puglise, J.; Barton, E.R.; Peng, J.; Demontis, F. Antagonistic Control of Myofiber Size and Muscle Protein Quality Control by the Ubiquitin Ligase UBR4 during Aging. Nat. Commun. 2021, 12, 1418. [Google Scholar] [CrossRef]
- Cai, D.; Lee, K.K.H.; Li, M.; Tang, M.K.; Chan, K.M. Ubiquitin Expression Is Up-Regulated in Human and Rat Skeletal Muscles during Aging. Arch. Biochem. Biophys. 2004, 425, 42–50. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Husom, A.D.; Thompson, L.V. Altered Proteasome Structure, Function, and Oxidation in Aged Muscle. FASEB J. 2005, 19, 644–646. [Google Scholar] [CrossRef]
- Saez, I.; Vilchez, D. The Mechanistic Links Between Proteasome Activity, Aging and Age-Related Diseases. Curr. Genom. 2014, 15, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Musa, I.; Holm, L.; Lai, Y.C. Recent Advances in Measuring and Understanding the Regulation of Exercise-Mediated Protein Degradation in Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2021, 321, C276–C287. [Google Scholar] [CrossRef] [PubMed]
- Gaster, M.; Poulsen, P.; Handberg, A.; Schrøder, H.D.; Beck-Nielsen, H. Direct Evidence of Fiber Type-Dependent GLUT-4 Expression in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E910–E916. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.H.; Cortright, R.N.; Dohm, G.L.; Houmard, J.A. Effect of Aging on Response to Exercise Training in Humans: Skeletal Muscle GLUT-4 and Insulin Sensitivity. J. Appl. Physiol. 1999, 86, 2019–2025. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, T.; Mori, S.; Omura, T.; Noda, Y.; Fujita, Y.; Ohsawa, I.; Shigemoto, K. Muscle Fiber Type Specific Alterations of Mitochondrial Respiratory Function and Morphology in Aged Female Mice. Biochem. Biophys. Res. Commun. 2021, 540, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kadoguchi, T.; Shimada, K.; Miyazaki, T.; Kitamura, K.; Kunimoto, M.; Aikawa, T.; Sugita, Y.; Ouchi, S.; Shiozawa, T.; Yokoyama-Nishitani, M.; et al. Promotion of Oxidative Stress Is Associated with Mitochondrial Dysfunction and Muscle Atrophy in Aging Mice. Geriatr. Gerontol. Int. 2020, 20, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Leeuwenburgh, C.; Coelho-Junior, H.J.; Bernabei, R.; Landi, F.; Marzetti, E. Targeting Mitochondrial Quality Control for Treating Sarcopenia: Lessons from Physical Exercise. Expert Opin Targets 2019, 23, 153–160. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Bossola, M.; Allocca, E.; Menghi, A.; Pesce, V.; Lezza, A.M.S.; Bernabei, R.; Landi, F.; Marzetti, E. Update on Mitochondria and Muscle Aging: All Wrong Roads Lead to Sarcopenia. Biol. Chem. 2018, 399, 421–436. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Y.B.; Tao, X.H.; Pan, W.L.; Wu, Y.X.; Wang, X.H.; He, Y.Q.; Xiao, W.F.; Li, Y.S. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2021, 12, 2016–2030. [Google Scholar] [CrossRef]
- Viña, J.; Salvador-Pascual, A.; Tarazona-Santabalbina, F.J.; Rodriguez-Mañas, L.; Gomez-Cabrera, M.C. Exercise Training as a Drug to Treat Age Associated Frailty. Free Radic. Biol. Med. 2016, 98, 159–164. [Google Scholar] [CrossRef]
- Liu, H.W.; Chang, Y.C.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. Dysregulations of Mitochondrial Quality Control and Autophagic Flux at an Early Age Lead to Progression of Sarcopenia in SAMP8 Mice. Biogerontology 2020, 21, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Hu, F.; Li, J.; Song, J.; Liang, J.; Li, L.; Song, Y.; Chen, Z.; Li, Q.; Ke, L. Qiangji Jianli Decoction Promotes Mitochondrial Biogenesis in Skeletal Muscle of Myasthenia Gravis Rats via AMPK/PGC-1α Signaling Pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 129, 110482. [Google Scholar] [CrossRef] [PubMed]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial Oxidative Capacity and NAD+ Biosynthesis Are Reduced in Human Sarcopenia across Ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-H.; Singh, S.P.; Raffaele, M.; Waldman, M.; Hochhauser, E.; Ospino, J.; Arad, M.; Peterson, S.J. Adipocyte-Specific Expression of PGC1α Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion. Antioxidants 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Å.B.; Dorn, G.W. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev. 2019, 99, 853–892. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, P.; Ren, J. Physical Exercise, Autophagy and Cardiometabolic Stress in Aging. Aging 2019, 11, 5287–5288. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol Prevents Sarcopenic Obesity by Reversing Mitochondrial Dysfunction and Oxidative Stress via the PKA/LKB1/AMPK Pathway. Aging 2019, 11, 2217. [Google Scholar] [CrossRef]
- Viña, J.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; de La Rosa, A.; Gomez-Cabrera, M.C. Modulating Oxidant Levels to Promote Healthy Aging. Antioxid Redox Signal 2020, 33, 570–579. [Google Scholar] [CrossRef]
- Delrieu, L.; Martin, A.; Touillaud, M.; Pérol, O.; Morelle, M.; Febvey-Combes, O.; Freyssenet, D.; Friedenreich, C.; Dufresne, A.; Bachelot, T.; et al. Sarcopenia and Serum Biomarkers of Oxidative Stress after a 6-Month Physical Activity Intervention in Women with Metastatic Breast Cancer: Results from the ABLE Feasibility Trial. Breast Cancer Res. Treat. 2021, 188, 601. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the Major Contributors to Superoxide Production in Contracting Skeletal Muscle. The Role of NAD(P)H Oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.J. Reactive Oxygen Species in Sarcopenia: Should We Focus on Excess Oxidative Damage or Defective Redox Signalling? Mol. Asp. Med. 2016, 50, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. MTORC1 Underlies Age-related Muscle Fiber Damage and Loss by Inducing Oxidative Stress and Catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, S.S.; Van Remmen, H.; Brooks, S.V.; Faulkner, J.A.; Larkin, L.; McArdle, A.; Jackson, M.J.; Vasilaki, A.; Richardson, A. Accelerated Sarcopenia in Cu/Zn Superoxide Dismutase Knockout Mice. Free Radic. Biol. Med. 2019, 132, 19–23. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The Contribution of Reactive Oxygen Species to Sarcopenia and Muscle Ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L. v Age-Induced Oxidative Stress: How Does It Influence Skeletal Muscle Quantity and Quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, Y.; Tamura, Y.; Takahashi, K.; Takeda, K.; Takemasa, T.; Hatta, H. Effects of Nrf2 Deficiency on Mitochondrial Oxidative Stress in Aged Skeletal Muscle. Physiol. Rep. 2019, 7, e13998. [Google Scholar] [CrossRef]
- Bose, C.; Alves, I.; Singh, P.; Palade, P.T.; Carvalho, E.; Børsheim, E.; Jun, S.R.; Cheema, A.; Boerma, M.; Awasthi, S.; et al. Sulforaphane Prevents Age-Associated Cardiac and Muscular Dysfunction through Nrf2 Signaling. Aging Cell 2020, 19, e13261. [Google Scholar] [CrossRef]
- el Assar, M.; Angulo, J.; Carnicero, J.A.; Walter, S.; García-García, F.J.; López-Hernández, E.; Sánchez-Puelles, J.M.; Rodríguez-Mañas, L. Frailty Is Associated With Lower Expression of Genes Involved in Cellular Response to Stress: Results From the Toledo Study for Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 734.e1–734.e7. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshikawa, M.; Harada, M.; Noyama, S.; Kiyono, K.; Motoike, Y.; Nomura, Y.; Nishimura, A.; Izawa, H.; Watanabe, E.; Ozaki, Y. Association between Inflammation and Skeletal Muscle Proteolysis, Skeletal Mass and Strength in Elderly Heart Failure Patients and Their Prognostic Implications. BMC Cardiovasc Disord 2020, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Brzeszczyńska, J.; Meyer, A.; McGregor, R.; Schilb, A.; Degen, S.; Tadini, V.; Johns, N.; Langen, R.; Schols, A.; Glass, D.J.; et al. Alterations in the in Vitro and in Vivo Regulation of Muscle Regeneration in Healthy Ageing and the Influence of Sarcopenia. J. Cachexia Sarcopenia Muscle 2018, 9, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baylis, D.; Bartlett, D.B.; Syddall, H.E.; Ntani, G.; Gale, C.R.; Cooper, C.; Lord, J.M.; Sayer, A.A. Immune-Endocrine Biomarkers as Predictors of Frailty and Mortality: A 10-Year Longitudinal Study in Community-Dwelling Older People. Age 2013, 35, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Gamberale, R.; Monno, A.; Citterio, L.; Lanzani, C.; De Lorenzo, R.; Ramirez, G.A.; Esposito, A.; Manunta, P.; Manfredi, A.A.; et al. Pharmacological Blockade of TNFα Prevents Sarcopenia and Prolongs Survival in Aging Mice. Aging 2020, 12, 23497–23508. [Google Scholar] [CrossRef] [PubMed]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of Inflammation and Their Association with Muscle Strength and Mass: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Pedersen, M.; Bruunsgaard, H.; Weis, N.; Hendel, H.W.; Andreassen, B.U.; Eldrup, E.; Dela, F.; Pedersen, B.K. Circulating Levels of TNF-Alpha and IL-6-Relation to Truncal Fat Mass and Muscle Mass in Healthy Elderly Individuals and in Patients with Type-2 Diabetes. Mech. Ageing Dev. 2003, 124, 495–502. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory Markers and Physical Performance in Older Persons: The InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on Relationship between Elderly Sarcopenia and Inflammatory Cytokine IL-6, Anti-Inflammatory Cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [Green Version]
- Bergens, O.; Nilsson, A.; Kadi, F. Associations between Circulating Inflammatory Biomarkers and Indicators of Muscle Health in Older Men and Women. J. Clin. Med. 2021, 10, 5316. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, S.; Jung, D.Y.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X.; et al. IL-10 Prevents Aging-Associated Inflammation and Insulin Resistance in Skeletal Muscle. FASEB J. 2017, 31, 701–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, A.E.; Hilmer, S.N.; Mach, J.; Mitchell, S.J.; de Cabo, R.; Howlett, S.E. Animal Models of Frailty: Current Applications in Clinical Research. Clin. Interv. Aging 2016, 11, 1519–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; de Alvares Goulart, R.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchain, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef] [PubMed]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The Exerkine Apelin Reverses Age-Associated Sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Yakabe, M.; Hosoi, T.; Akishita, M.; Ogawa, S. Updated Concept of Sarcopenia Based on Muscle-Bone Relationship. J. Bone Min. Metab. 2020, 38, 7–13. [Google Scholar] [CrossRef]
- Liaw, F.Y.; Kao, T.W.; Fang, W.H.; Han, D.S.; Chi, Y.C.; Yang, W.S. Increased Follistatin Associated with Decreased Gait Speed among Old Adults. Eur. J. Clin. Investig. 2016, 46, 321–327. [Google Scholar] [CrossRef]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef]
- Abou-Samra, M.; Selvais, C.M.; Boursereau, R.; Lecompte, S.; Noel, L.; Brichard, S.M. AdipoRon, a New Therapeutic Prospect for Duchenne Muscular Dystrophy. J. Cachexia Sarcopenia Muscle 2020, 11, 518–533. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Schaar, A.E.; Gustafson, G.E.; Smith, A.B.; Howell, P.R.; Greenman, A.; Baum, S.; Colman, R.J.; Lamming, D.W.; Diffee, G.M.; et al. Adiponectin Receptor Agonist AdipoRon Improves Skeletal Muscle Function in Aged Mice. Elife 2022, 11, e71282. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Kaplon, R.E.; Hill, S.D.; McNamara, M.N.; Santos-Parker, J.R.; Pierce, G.L.; Seals, D.R.; Donato, A.J. Endothelial Cell Senescence with Aging in Healthy Humans: Prevention by Habitual Exercise and Relation to Vascular Endothelial Function. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H890–H895. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and Atherosclerosis: Vascular Intrinsic and Extrinsic Factors and Potential Role of IL-6. Nat. Rev. Cardiol. 2020, 18, 58–68. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; García-Rojo, E.; Sevilleja-Ortiz, A.; García-Gómez, B.; Fernández, A.; Sánchez-Ferrer, A.; la Fuente, J.M.; Romero-Otero, J.; Rodríguez-Mañas, L. Early Manifestation of Aging-Related Vascular Dysfunction in Human Penile Vasculature-A Potential Explanation for the Role of Erectile Dysfunction as a Harbinger of Systemic Vascular Disease. Geroscience 2022, 44, 485–501. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Fernández, A.; Sánchez-Ferrer, A.; Angulo, J.; Rodríguez-Mañas, L. Multivessel Analysis of Progressive Vascular Aging in the Rat: Asynchronous Vulnerability among Vascular Territories. Mech. Ageing Dev. 2018, 173, 39–49. [Google Scholar] [CrossRef] [PubMed]
- de Rezende Mikael, L.; de Paiva, A.M.G.; Gomes, M.M.; Sousa, A.L.L.; Jardim, P.C.B.V.; de Oliveira Vitorino, P.V.; Euzébio, M.B.; de Moura Sousa, W.; Barroso, W.K.S. Vascular Aging and Arterial Stiffness. Arq. Bras. De Cardiol. 2017, 109, 253. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative Stress and Vascular Inflammation in Aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Gao, D.; Zuo, Z.; Tian, J.; Ali, Q.; Lin, Y.; Lei, H.; Sun, Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension 2016, 68, 1191–1199. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.; Sun, Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension 2015, 66, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Canestro, C.; Puspitasari, Y.M.; Liberale, L.; Guzik, T.J.; Flammer, A.J.; Bonetti, N.R.; Wüst, P.; Costantino, S.; Paneni, F.; Akhmedov, A.; et al. MMP-2 Knockdown Blunts Age-Dependent Carotid Stiffness by Decreasing Elastin Degradation and Augmenting ENOS Activation. Cardiovasc. Res. 2021, 118, 2385–2396. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise Benefits in Cardiovascular Disease: Beyond Attenuation of Traditional Risk Factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Bouzón, C.; Carcaillon, L.; García-García, F.J.; Amor-Andrés, M.S.; El Assar, M.; Rodríguez-Mañas, L. Association between Endothelial Dysfunction and Frailty: The Toledo Study for Healthy Aging. Age 2014, 36, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tap, L.; Kirkham, F.A.; Mattace-Raso, F.; Joly, L.; Rajkumar, C.; Benetos, A. Unraveling the Links Underlying Arterial Stiffness, Bone Demineralization, and Muscle Loss. Hypertension 2020, 76, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Bielecka-Dabrowa, A.; Ebner, N.; dos Santos, M.R.; Ishida, J.; Hasenfuss, G.; von Haehling, S. Cachexia, Muscle Wasting, and Frailty in Cardiovascular Disease. Eur. J. Heart Fail 2020, 22, 2314–2326. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, G.; Song, Y.; Yoon, H.; Jeong, H.M.; Gu, J.E.; Han, M.; Heo, J.; Yoo, J.J.; Yoon, J.W.; et al. Low Muscle Mass in Patients Receiving Hemodialysis: Correlations with Vascular Calcification and Vascular Access Failure. J. Clin. Med. 2021, 10, 3698. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Lewis, J.R.; Scott, D.S.; Kiel, D.P.; Schousboe, J.T.; Ebeling, P.R.; Prince, R.L. Aortic Calcification Is Associated with Five-Year Decline in Handgrip Strength in Older Women. Calcif Tissue Int. 2018, 103, 589–598. [Google Scholar] [CrossRef]
- Minn, Y.K.; Suk, S.H. Higher Skeletal Muscle Mass May Protect against Ischemic Stroke in Community-Dwelling Adults without Stroke and Dementia: The PRESENT Project. BMC Geriatr. 2017, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Gori, T. Exogenous NO Therapy for the Treatment and Prevention of Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial Dysfunction in Aged Humans Is Related with Oxidative Stress and Vascular Inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar] [CrossRef]
- Ma, L.; Wang, K.; Shang, J.; Cao, C.; Zhen, P.; Liu, X.; Wang, W.; Zhang, H.; Du, Y.; Liu, H. Anti-Peroxynitrite Treatment Ameliorated Vasorelaxation of Resistance Arteries in Aging Rats: Involvement with NO-SGC-CGKs Pathway. PLoS ONE 2014, 9, e104788. [Google Scholar] [CrossRef]
- Barcena, M.L.; Aslam, M.; Pozdniakova, S.; Norman, K.; Ladilov, Y. Cardiovascular Inflammaging: Mechanisms and Translational Aspects. Cells 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Meditz, A.; Deane, K.D.; Kohrt, W.M. Tetrahydrobiopterin Improves Endothelial Function and Decreases Arterial Stiffness in Estrogen-Deficient Postmenopausal Women. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1211–H1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial Dysfunction and Angiogenesis Impairment in the Ageing Vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwon, O.S.; Andtbacka, R.H.I.; Hyngstrom, J.R.; Reese, V.; Murphy, M.P.; Richardson, R.S. Age-Related Endothelial Dysfunction in Human Skeletal Muscle Feed Arteries: The Role of Free Radicals Derived from Mitochondria in the Vasculature. Acta Physiol. 2018, 222, e12893. [Google Scholar] [CrossRef] [Green Version]
- Canugovi, C.; Stevenson, M.D.; Vendrov, A.E.; Hayami, T.; Robidoux, J.; Xiao, H.; Zhang, Y.Y.; Eitzman, D.T.; Runge, M.S.; Madamanchi, N.R. Increased Mitochondrial NADPH Oxidase 4 (NOX4) Expression in Aging Is a Causative Factor in Aortic Stiffening. Redox Biol. 2019, 26, 101288. [Google Scholar] [CrossRef]
- Nosalski, R.; Mikolajczyk, T.; Siedlinski, M.; Saju, B.; Koziol, J.; Maffia, P.; Guzik, T.J. Nox1/4 Inhibition Exacerbates Age Dependent Perivascular Inflammation and Fibrosis in a Model of Spontaneous Hypertension. Pharmacol. Res. 2020, 161, 1043–6618. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Walker, S.J.; Dworakowski, R.; Lakatta, E.G.; Shah, A.M. Involvement of NADPH Oxidase in Age-Associated Cardiac Remodeling. J. Mol. Cell. Cardiol. 2010, 48, 765. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Sevilleja-Ortiz, A.; Fernández, A.; Sánchez-Ferrer, A.; Romero-Otero, J.; Martínez-Salamanca, J.I.; La Fuente, J.M.; Rodríguez-Mañas, L. Short-Term Pharmacological Activation of Nrf2 Ameliorates Vascular Dysfunction in Aged Rats and in Pathological Human Vasculature. A Potential Target for Therapeutic Intervention. Redox Biol. 2019, 26, 101271. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, V.E.; Ikoba, A.P.; Ziemba, B.P.; Ballak, D.B.; Hoischen, A.; Dinarello, C.A.; Ehringer, M.A.; Seals, D.R. Circulating Interleukin-37 Declines with Aging in Healthy Humans: Relations to Healthspan Indicators and IL37 Gene SNPs. Geroscience 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-Ageing: The Role of Inflammation in Age-Dependent Cardiovascular Disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Marín-Aguilar, F.; Lechuga-Vieco, A.V.; Alcocer-Gómez, E.; Castejón-Vega, B.; Lucas, J.; Garrido, C.; Peralta-Garcia, A.; Pérez-Pulido, A.J.; Varela-López, A.; Quiles, J.L.; et al. NLRP3 Inflammasome Suppression Improves Longevity and Prevents Cardiac Aging in Male Mice. Aging Cell 2020, 19, e13050. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The Protective Effect of Resveratrol on Vascular Aging by Modulation of the Renin-Angiotensin System. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Rocco, E.; Grimaldi, M.C.; Maino, A.; Cappannoli, L.; Pedicino, D.; Liuzzo, G.; Biasucci, L.M. Advances and Challenges in Biomarkers Use for Coronary Microvascular Dysfunction: From Bench to Clinical Practice. J. Clin. Med. 2022, 11, 2055. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Maino, A.; Grimaldi, M.C.; Cappannoli, L.; Aspromonte, N. Novel Biomarkers in Heart Failure: New Insight in Pathophysiology and Clinical Perspective. J. Clin. Med. 2021, 10, 2771. [Google Scholar] [CrossRef]
- Rafiei, A.; Ahmadi, R.; Kazemian, S.; Rahimzadeh-Fallah, T.; Mohammad-Rezaei, M.; Azadegan-Dehkordi, F.; Sanami, S.; Mirzaei, Y.; Aghaei, F.; Bagheri, N. Serum Levels of IL-37 and Correlation with Inflammatory Cytokines and Clinical Outcomes in Patients with Coronary Artery Disease. J. Investig. Med. 2022. [Google Scholar] [CrossRef]
- Ballak, D.B.; Brunt, V.E.; Sapinsley, Z.J.; Ziemba, B.P.; Richey, J.J.; Zigler, M.C.; Johnson, L.C.; Gioscia-Ryan, R.A.; Culp-Hill, R.; Eisenmesser, E.Z.; et al. Short-Term Interleukin-37 Treatment Improves Vascular Endothelial Function, Endurance Exercise Capacity, and Whole-Body Glucose Metabolism in Old Mice. Aging Cell 2019, 19, e13074. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise Effects on Physiological Function during Aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Med. Sci. Sports Exerc 2009, 41, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. The Economic Burden of Physical Inactivity: A Global Analysis of Major Non-Communicable Diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Alkandari, J.R.; Andersen, L.B.; Bauman, A.E.; Brownson, R.C.; et al. Effect of Physical Inactivity on Major Non-Communicable Diseases Worldwide: An Analysis of Burden of Disease and Life Expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Strain, T.; Brage, S.; Sharp, S.J.; Richards, J.; Tainio, M.; Ding, D.; Benichou, J.; Kelly, P. Use of the Prevented Fraction for the Population to Determine Deaths Averted by Existing Prevalence of Physical Activity: A Descriptive Study. Lancet Glob. Health 2020, 8, e920–e930. [Google Scholar] [CrossRef]
- Piggin, J. What Is Physical Activity? A Holistic Definition for Teachers, Researchers and Policy Makers. Front. Sports Act. Living 2020, 2, 72. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.L.; Mañas, A.; García-García, F.J.; Ara, I.; Carnicero, J.A.; Walter, S.; Rodríguez-Mañas, L. Sedentary Behaviour, Physical Activity, and Sarcopenia among Older Adults in the TSHA: Isotemporal Substitution Model. J. Cachexia Sarcopenia Muscle 2019, 10, 188–198. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise Training and Nutritional Supplementation for Physical Frailty in Very Elderly People. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas-Herrero, Á.; Sáez de Asteasu, M.L.; Antón-Rodrigo, I.; Sánchez-Sánchez, J.L.; Montero-Odasso, M.; Marín-Epelde, I.; Ramón-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail Multicomponent Intervention on Functional Capacity: A Multicentre, Randomized Controlled Trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Investigators, L.S.; Pahor, M.; Primero, S.N.B. Effects of a Physical Activity Intervention on Measures of Physical Performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1157–1165. [Google Scholar]
- Dautzenberg, L.; Beglinger, S.; Tsokani, S.; Zevgiti, S.; Raijmann, R.C.M.A.; Rodondi, N.; Scholten, R.J.P.M.; Rutjes, A.W.S.; di Nisio, M.; Emmelot-Vonk, M.; et al. Interventions for Preventing Falls and Fall-Related Fractures in Community-Dwelling Older Adults: A Systematic Review and Network Meta-Analysis. J. Am. Geriatr. Soc. 2021, 69, 2973–2984. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramirez-Vélez, R.; Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Valenzuela, P.L.; Lucia, A.; Izquierdo, M. Safety and Effectiveness of Long-Term Exercise Interventions in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 1095–1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, L.; Chen, S.T.; Bae, J.H.; Kim, D.Y.; Liu, X.; Song, W. Effects and Moderators of Exercise on Sarcopenic Components in Sarcopenic Elderly: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 649748. [Google Scholar] [CrossRef]
- Saeidi, A.; Haghighi, M.M.; Kolahdouzi, S.; Daraei, A.; Abderrahmane, A.B.; Essop, M.F.; Laher, I.; Hackney, A.C.; Zouhal, H. The Effects of Physical Activity on Adipokines in Individuals with Overweight/Obesity across the Lifespan: A Narrative Review. Obes. Rev. 2021, 22, e13090. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451. [Google Scholar] [CrossRef]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended Physical Activity and All Cause and Cause Specific Mortality in US Adults: Prospective Cohort Study. BMJ 2020, 370, 2031. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Orssatto, L.B.d.R.; Wiest, M.J.; Diefenthaeler, F. Neural and Musculotendinous Mechanisms Underpinning Age-Related Force Reductions. Mech. Ageing Dev. 2018, 175, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of Resistance Exercise on Lean Body Mass in Aging Adults: A Meta-Analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.M.; Bemben, D.A.; Bemben, M.G. Skeletal Muscle Adaptations Following 80 Weeks of Resistance Exercise in Older Adults. J. Geriatr. Phys. 2021, 45, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.M.; Lee, J.; Hamidian, R.; Jones, C.A.; Khadaroo, R.G. Management of Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. J. Am. Med. Dir. Assoc. 2022, 23, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Talar, K.; Hernández-belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-ibáñez, J. Benefits of Resistance Training in Early and Late Stages of Frailty and Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2021, 10, 1630. [Google Scholar] [CrossRef]
- Cadore, E.L.; Sáez de Asteasu, M.L.; Izquierdo, M. Multicomponent Exercise and the Hallmarks of Frailty: Considerations on Cognitive Impairment and Acute Hospitalization. Exp. Gerontol. 2019, 122, 10–14. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Hu, S.; Chen, H.; Zhang, M.; Yang, Y.; Liu, Y. Effects of Multicomponent Exercise on the Muscle Strength, Muscle Endurance and Balance of Frail Older Adults: A Meta-Analysis of Randomised Controlled Trials. J. Clin. Nurs. 2022, 1–11. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Reid, K.F.; Fielding, R.A. Skeletal Muscle Power: A Critical Determinant of Physical Functioning In Older Adults. Exerc. Sport Sci. Rev. 2012, 40, 4. [Google Scholar] [CrossRef]
- Alcazar, J.; Alegre, L.M.; van Roie, E.; Magalhães, J.P.; Nielsen, B.R.; González-Gross, M.; Júdice, P.B.; Casajús, J.A.; Delecluse, C.; Sardinha, L.B.; et al. Relative Sit-to-stand Power: Aging Trajectories, Functionally Relevant Cut-off Points, and Normative Data in a Large European Cohort. J. Cachexia Sarcopenia Muscle 2021, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Fernandes, O.; Pereira, A.; Oliveira, R.; Goñi, F.D.A.; Leite, N.J.C.; Brito, J.P. The Effects of High-Speed Resistance Training on Health Outcomes in Independent Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5390. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Izquierdo, M. Muscle Power Training: A Hallmark for Muscle Function Retaining in Frail Clinical Setting. J. Am. Med. Dir. Assoc. 2018, 19, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Uchida, M.C.; Picca, A.; Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Marzetti, E. Evidence-Based Recommendations for Resistance and Power Training to Prevent Frailty in Community-Dwellers. Aging Clin. Exp. Res. 2021, 33, 2069–2086. [Google Scholar] [CrossRef]
- Orssatto, L.B.R.; Bezerra, E.S.; Shield, A.J.; Trajano, G.S. Is Power Training Effective to Produce Muscle Hypertrophy in Older Adults? A Systematic Review and Meta-Analysis. Appl. Physiol. Nutr. Metab. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Rosa Orssatto, L.B.; de la Rocha Freitas, C.; Shield, A.J.; Silveira Pinto, R.; Trajano, G.S. Effects of Resistance Training Concentric Velocity on Older Adults’ Functional Capacity: A Systematic Review and Meta-Analysis of Randomised Trials. Exp. Gerontol. 2019, 127, 110731. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Uchida, M.C. Effects of Low-Speed and High-Speed Resistance Training Programs on Frailty Status, Physical Performance, Cognitive Function, and Blood Pressure in Prefrail and Frail Older Adults. Front. Med. 2021, 8, 702436. [Google Scholar] [CrossRef]
- Solomon, A.; Borodulin, K.; Ngandu, T.; Kivipelto, M.; Laatikainen, T.; Kulmala, J. Self-Rated Physical Fitness and Estimated Maximal Oxygen Uptake in Relation to All-Cause and Cause-Specific Mortality. Scand. J. Med. Sci. Sports 2018, 28, 532–540. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-Analysis. JAMA-J. Am. Med. Assoc. 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Madssen, E.; Skaug, E.A.; Wisløff, U.; Ellingsen, Ø.; Videm, V. Inflammation Is Strongly Associated With Cardiorespiratory Fitness, Sex, BMI, and the Metabolic Syndrome in a Self-Reported Healthy Population: HUNT3 Fitness Study. Mayo Clin. Proc. 2019, 94, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ezzatvar, Y.; Ramírez-Vélez, R.; Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Lobelo, F.; Izquierdo, M.; García-Hermoso, A. Cardiorespiratory Fitness and All-Cause Mortality in Adults Diagnosed with Cancer Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2021, 31, 1745–1752. [Google Scholar] [CrossRef]
- Fardman, A.; Banschick, G.D.; Rabia, R.; Percik, R.; Fourey, D.; Segev, S.; Klempfner, R.; Grossman, E.; Maor, E. Cardiorespiratory Fitness and Survival Following Cancer Diagnosis. Eur. J. Prev. Cardiol. 2021, 28, 1242–1249. [Google Scholar] [CrossRef]
- Jackson, A.S.; Sui, X.; Hébert, J.R.; Church, T.S.; Blair, S.N. Role of Lifestyle and Aging on the Longitudinal Change in Cardiorespiratory Fitness. Arch. Intern. Med. 2009, 169, 1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Hummel, M.; Hantikainen, E.; Adami, H.O.; Ye, W.; Bellocco, R.; Bonn, S.E.; Lagerros, Y.T. Association between Total and Leisure Time Physical Activity and Risk of Myocardial Infarction and Stroke–a Swedish Cohort Study. BMC Public Health 2022, 22, 532. [Google Scholar] [CrossRef]
- Gonzalez-Jaramillo, N.; Wilhelm, M.; Arango-Rivas, A.M.; Gonzalez-Jaramillo, V.; Mesa-Vieira, C.; Minder, B.; Franco, O.H.; Bano, A. Systematic Review of Physical Activity Trajectories and Mortality in Patients With Coronary Artery Disease. J. Am. Coll Cardiol. 2022, 79, 1690–1700. [Google Scholar] [CrossRef]
- Ochi, M.; Kohara, K.; Tabara, Y.; Kido, T.; Uetani, E.; Ochi, N.; Igase, M.; Miki, T. Arterial Stiffness Is Associated with Low Thigh Muscle Mass in Middle-Aged to Elderly Men. Atherosclerosis 2010, 212, 327–332. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Arterial Stiffness and Frailty-A Systematic Review and Metaanalysis. Exp. Gerontol. 2021, 153, 111480. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Tudor-Locke, C.; Álvarez-Bueno, C.; Cunha, P.G.; Aguiar, E.J.; Martínez-Vizcaíno, V. Steps per Day and Arterial Stiffness. Hypertension 2019, 73, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension: Erratum. J. Hypertens 2019, 37, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the Management of Arterial HypertensionThe Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, P.C.; Friedenreich, C.M.; Leitzmann, M.F.; Buman, M.P.; Lambert, E.; Willumsen, J.; Bull, F. Global Public Health Guidelines on Physical Activity and Sedentary Behavior for People Living With Chronic Conditions: A Call to Action. J. Phys. Act. Health 2020, 18, 76–85. [Google Scholar] [CrossRef]
- Sosner, P.; Guiraud, T.; Gremeaux, V.; Arvisais, D.; Herpin, D.; Bosquet, L. The Ambulatory Hypotensive Effect of Aerobic Training: A Reappraisal through a Meta-Analysis of Selected Moderators. Scand. J. Med. Sci. Sports 2017, 27, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Saco-Ledo, G.; Valenzuela, P.L.; Ruiz-Hurtado, G.; Ruilope, L.M.; Lucia, A. Exercise Reduces Ambulatory Blood Pressure in Patients with Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e018487. [Google Scholar] [CrossRef]
- Pierce, G.L.; Eskurza, I.; Walker, A.E.; Fay, T.N.; Seals, D.R. Sex-Specific Effects of Habitual Aerobic Exercise on Brachial Artery Flow-Mediated Dilation in Middle-Aged and Older Adults. Clin. Sci. 2011, 120, 13–23. [Google Scholar] [CrossRef] [Green Version]
- DeSouza, C.A.; Shapiro, L.F.; Clevenger, C.M.; Dinenno, F.A.; Monahan, K.D.; Tanaka, H.; Seals, D.R. Regular Aerobic Exercise Prevents and Restores Age-Related Declines in Endothelium-Dependent Vasodilation in Healthy Men. Circulation 2000, 102, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Bouaziz, W.; Lang, P.O.; Schmitt, E.; Leprêtre, P.M.; Lefebvre, F.; Momas, C.; Kaltenbach, G.; Geny, B.; Vogel, T. Effects of a Short-Term Interval Aerobic Training Program with Recovery Bouts on Vascular Function in Sedentary Aged 70 or over: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2019, 82, 217–225. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different Exercise Training Modalities Produce Similar Endothelial Function Improvements in Individuals with Prehypertension or Hypertension: A Randomized Clinical Trial. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef]
- Carpes, L.; Costa, R.; Schaarschmidt, B.; Reichert, T.; Ferrari, R. High-Intensity Interval Training Reduces Blood Pressure in Older Adults: A Systematic Review and Meta-Analysis. Exp. Gerontol. 2022, 158, 111657. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, J.P.D.; Mensink, R.P.; Ivanov, D.; Adam, J.J.; Uludağ, K.; Joris, P.J. Aerobic Exercise Training Improves Cerebral Blood Flow and Executive Function: A Randomized, Controlled Cross-Over Trial in Sedentary Older Men. Front. Aging Neurosci. 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahin, O.; Moraes-Ferreira, R.; Cortinhas-Alves, E.A.; Guerreiro, J.F. Is Resistance Training Alone an Antihypertensive Therapy? A Meta-Analysis. J. Hum. Hypertens. 2021, 35, 769–775. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hotta, K.; Ota, E.; Mori, R.; Matsunaga, A. Effects of Resistance Training on Muscle Strength, Exercise Capacity, and Mobility in Middle-Aged and Elderly Patients with Coronary Artery Disease: A Meta-Analysis. J. Cardiol. 2016, 68, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saz-Lara, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Reina-Gutiérrez, S.; Sequí-Domínguez, I.; Ruiz, J.R.; Martínez-Vizcaíno, V. What Type of Physical Exercise Should Be Recommended for Improving Arterial Stiffness on Adult Population? A Network Meta-Analysis. Eur. J. Cardiovasc. Nurs. 2021, 20, 696–716. [Google Scholar] [CrossRef]
- Kato, M.; Green, F.N.; Hotta, K.; Tsukamoto, T.; Kurita, Y.; Kubo, A.; Takagi, H. The Efficacy of Stretching Exercises on Arterial Stiffness in Middle-Aged and Older Adults: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2020, 17, 5643. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. Do Non-Responders to Exercise Exist—and If So, What Should We Do About Them? Sports Med. 2019, 49, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Cadore, E.L.; Ramirez-Velez, R.; Izquierdo, M. Inter-individual Variability in Response to Exercise Intervention or Usual Care in Hospitalized Older Adults. J. Cachexia Sarcopenia Muscle 2019, 10, 1266. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S.; et al. Adverse Metabolic Response to Regular Exercise: Is It a Rare or Common Occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef]
- Seo, D.Y.; Hwang, B.G. Effects of Exercise Training on the Biochemical Pathways Associated with Sarcopenia. Phys. Act. Nutr. 2020, 24, 32–38. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Padilha, C.; Nunes, J.P.; Schoenfeld, B.J.; Cyrino, L.T.; Tomeleri, C.M.; Nascimento, M.A.; Antunes, M.; Fernandes, R.R.; et al. Improvement of Oxidative Stress in Older Women Is Dependent on Resistance Training Volume: Active Aging Longitudinal Study. J. Strength Cond Res. 2022, 36, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal Muscle Aging: Influence of Oxidative Stress and Physical Exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial Effects of Exercise on Age-Related Mitochondrial Dysfunction and Oxidative Stress in Skeletal Muscle. J. Physiol. 2016, 594, 5105–5123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic Exercise Training and Vascular Function with Ageing in Healthy Men and Women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef]
- Alcazar, J.; Losa-Reyna, J.; Rodriguez-Lopez, C.; Navarro-Cruz, R.; Alfaro-Acha, A.; Ara, I.; García-García, F.J.; Alegre, L.M.; Guadalupe-Grau, A. Effects of Concurrent Exercise Training on Muscle Dysfunction and Systemic Oxidative Stress in Older People with COPD. Scand. J. Med. Sci. Sports 2019, 29, 1591–1603. [Google Scholar] [CrossRef]
- Cobley, J.N.; Sakellariou, G.K.; Owens, D.J.; Murray, S.; Waldron, S.; Gregson, W.; Fraser, W.D.; Burniston, J.G.; Iwanejko, L.A.; McArdle, A.; et al. Lifelong Training Preserves Some Redox-Regulated Adaptive Responses after an Acute Exercise Stimulus in Aged Human Skeletal Muscle. Free Radic. Biol. Med. 2014, 70, 23–32. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Filaire, E.; Matran, R.; Robin, S.; Fabre, C. Lifelong Voluntary Exercise Modulates Age-Related Changes in Oxidative Stress. Int. J. Sports Med. 2018, 39, 21–28. [Google Scholar] [CrossRef]
- Jackson, M.J.; Pollock, N.; Staunton, C.; Jones, S.; McArdle, A. Redox Control of Signalling Responses to Contractile Activity and Ageing in Skeletal Muscle. Cells 2022, 11, 1698. [Google Scholar] [CrossRef]
- McArdle, A.; Pollock, N.; Staunton, C.A.; Jackson, M.J. Aberrant Redox Signalling and Stress Response in Age-Related Muscle Decline: Role in Inter- and Intra-Cellular Signalling. Free Radic. Biol. Med. 2019, 132, 50–57. [Google Scholar] [CrossRef]
- Pugh, J.N.; Stretton, C.; McDonagh, B.; Brownridge, P.; McArdle, A.; Jackson, M.J.; Close, G.L. Exercise Stress Leads to an Acute Loss of Mitochondrial Proteins and Disruption of Redox Control in Skeletal Muscle of Older Subjects: An Underlying Decrease in Resilience with Aging? Free Radic. Biol. Med. 2021, 177, 88–99. [Google Scholar] [CrossRef]
- Moreira, O.C.; Estébanez, B.; Martínez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Mitochondrial Function and Mitophagy in the Elderly: Effects of Exercise. Oxid. Med. Cell. Longev. 2017, 2017, 2012798. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Ringholm, S.; Olesen, J.; Prats, C.; Pilegaard, H. Exercise Training Protects against Aging-Induced Mitochondrial Fragmentation in Mouse Skeletal Muscle in a PGC-1α Dependent Manner. Exp. Gerontol. 2017, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand-Moghadam, B.; Eskandari, M.; Golestani, F.; Rezae, S.; Mahmoudi, N.; Gaeini, A.A. The Effect of 12-Week Resistance Exercise Training on Serum Levels of Cellular Aging Process Parameters in Elderly Men. Exp. Gerontol. 2020, 141, 111090. [Google Scholar] [CrossRef]
- Yan, X.; Shen, Z.; Yu, D.; Zhao, C.; Zou, H.; Ma, B.; Dong, W.; Chen, W.; Huang, D.; Yu, Z. Nrf2 Contributes to the Benefits of Exercise Interventions on Age-Related Skeletal Muscle Disorder via Regulating Drp1 Stability and Mitochondrial Fission. Free Radic. Biol. Med. 2022, 178, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-De-castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef] [Green Version]
- Bronisz-Budzyńska, I.; Kozakowska, M.; Podkalicka, P.; Kachamakova-Trojanowska, N.; Łoboda, A.; Dulak, J. The Role of Nrf2 in Acute and Chronic Muscle Injury. Skelet Muscle 2020, 10, 35. [Google Scholar] [CrossRef]
- Done, A.J.; Gage, M.J.; Nieto, N.C.; Traustadóttir, T. Exercise-Induced Nrf2-Signaling Is Impaired in Aging. Free Radic. Biol. Med. 2016, 96, 130–138. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Villareal, D.T. Physical Exercise as Therapy for Frailty. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Lambert, C.P.; Wright, N.R.; Finck, B.N.; Villareal, D.T. Exercise but Not Diet-Induced Weight Loss Decreases Skeletal Muscle Inflammatory Gene Expression in Frail Obese Elderly Persons. J. Appl. Physiol. 2008, 105, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Qiu, P.; Xia, R.; Lin, H.; Ye, B.; Tao, J.; Chen, L. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Aging Neurosci. 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The Impact of Exercise Training on Inflammatory Markers in Postmenopausal Women: A Systemic Review and Meta-Analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef] [PubMed]
- Gomarasca, M.; Micielska, K.; Faraldi, M.; Flis, M.; Perego, S.; Banfi, G.; Ziemann, E.; Lombardi, G. Impact of 12-Week Moderate-Intensity Aerobic Training on Inflammasome Complex Activation in Elderly Women. Front. Physiol. 2022, 13, 792859. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of Aging and Lifelong Aerobic Exercise on Basal and Exercise-Induced Inflammation in Women. J. Appl. Physiol. 2020, 129, 1493–1504. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Shreef, F.M. Inflammatory Cytokines and Immune System Modulation by Aerobic versus Resisted Exercise Training for Elderly. Afr. Health Sci. 2018, 18, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.K.; Jensen, S.M.; Schjerling, P.; Mackey, A.L.; Andersen, J.L.; Kjaer, M. The Effect of Resistance Exercise upon Age-Related Systemic and Local Skeletal Muscle Inflammation. Exp. Gerontol. 2019, 121, 19–32. [Google Scholar] [CrossRef]
- Hangelbroek, R.W.J.; Knuiman, P.; Tieland, M.; de Groot, L.C.P.G.M. Attenuated Strength Gains during Prolonged Resistance Exercise Training in Older Adults with High Inflammatory Status. Exp. Gerontol. 2018, 106, 154–158. [Google Scholar] [CrossRef]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine Irisin-Induced Protection against Oxidative Stress in Vitro. Involvement of Heme Oxygenase-1 and Antioxidazing Enzymes Superoxide Dismutase-2 and Glutathione Peroxidase. J. Physiol. Pharm. 2018, 69, 117–125. [Google Scholar] [CrossRef]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafi, F.; Biglari, S.; Afousi, A.G.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C. Vascular Ageing and Aerobic Exercise. Int. J. Environ. Res. Public Health 2021, 18, 666. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Palta, P.; Folsom, A.R.; Meyer, M.L.; Matsushita, K.; Evenson, K.R.; Aguilar, D.; Heiss, G. Habitual Physical Activity and Central Artery Stiffening in Older Adults: The Atherosclerosis Risk in Communities Study. J. Hypertens 2018, 36, 1889–1894. [Google Scholar] [CrossRef]
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re Only as Old as Your Arteries: Translational Strategies for Preserving Vascular Endothelial Function with Aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef] [Green Version]
- Lesniewski, L.A.; Durrant, J.R.; Connell, M.L.; Henson, G.D.; Black, A.D.; Donato, A.J.; Seals, D.R. Aerobic Exercise Reverses Arterial Inflammation with Aging in Mice. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1025–H1032. [Google Scholar] [CrossRef]
- Pierce, G.L.; Donato, A.J.; Larocca, T.J.; Eskurza, I.; Silver, A.E.; Seals, D.R. Habitually Exercising Older Men Do Not Demonstrate Age-Associated Vascular Endothelial Oxidative Stress. Aging Cell 2011, 10, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, T.; Maeda, S.; Miyauchi, T.; Iemitsu, M.; Takanashi, M.; Irukayama-Tomobe, Y.; Yokota, T.; Ohmori, H.; Matsuda, M. Exercise Training Improves Ageing-Induced Decrease in ENOS Expression of the Aorta. Acta Physiol. Scand. 2003, 178, 3–10. [Google Scholar] [CrossRef]
- Shimomura, M.; Fujie, S.; Sanada, K.; Kajimoto, H.; Hamaoka, T.; Iemitsu, M. Relationship between Plasma Asymmetric Dimethylarginine and Nitric Oxide Levels Affects Aerobic Exercise Training-Induced Reduction of Arterial Stiffness in Middle-Aged and Older Adults. Phys. Act. Nutr. 2021, 25, 16–22. [Google Scholar] [CrossRef]
- Sindler, A.L.; Delp, M.D.; Reyes, R.; Wu, G.; Muller-Delp, J.M. Effects of Ageing and Exercise Training on ENOS Uncoupling in Skeletal Muscle Resistance Arterioles. J. Physiol. 2009, 587, 3885–3897. [Google Scholar] [CrossRef]
- Byun, K.; Lee, S. The Potential Role of Irisin in Vascular Function and Atherosclerosis: A Review. Int. J. Mol. Sci 2020, 21, 7184. [Google Scholar] [CrossRef] [PubMed]
- Fujie, S.; Sato, K.; Miyamoto-Mikami, E.; Hasegawa, N.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Reduction of Arterial Stiffness by Exercise Training Is Associated with Increasing Plasma Apelin Level in Middle-Aged and Older Adults. PLoS ONE 2014, 9, e93545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Parker, J.R.; Strahler, T.R.; Vorwald, V.M.; Pierce, G.L.; Seals, D.R. Habitual Aerobic Exercise Does Not Protect against Micro-or Macrovascular Endothelial Dysfunction in Healthy Estrogen-Deficient Postmenopausal Women. J. Appl. Physiol. 2017, 122, 11–19. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; Clayton, Z.S.; Zigler, M.C.; Richey, J.J.; Cuevas, L.M.; Rossman, M.J.; Battson, M.L.; Ziemba, B.P.; Hutton, D.A.; VanDongen, N.S.; et al. Lifelong Voluntary Aerobic Exercise Prevents Age- and Western Diet- Induced Vascular Dysfunction, Mitochondrial Oxidative Stress and Inflammation in Mice. J. Physiol. 2021, 599, 911–925. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Robinson, A.T.; Rossman, M.J.; Seals, D.R.; Edwards, D.G. Mitochondrial Contributions to Vascular Endothelial Dysfunction, Arterial Stiffness, and Cardiovascular Diseases. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H2080–H2100. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, B.; Zhang, X.F.; Ma, Y.P.; Liu, J.D.; Wang, X.Z. Chronic Aerobic Exercise Training Attenuates Aortic Stiffening and Endothelial Dysfunction through Preserving Aortic Mitochondrial Function in Aged Rats. Exp. Gerontol. 2014, 56, 37–44. [Google Scholar] [CrossRef]
- Lighthouse, J.K.; Burke, R.M.; Velasquez, L.S.; Dirkx, R.A.; Aiezza, A.; Moravec, C.S.; Alexis, J.D.; Rosenberg, A.; Small, E.M. Exercise Promotes a Cardioprotective Gene Program in Resident Cardiac Fibroblasts. JCI Insight 2019, 4, e92098. [Google Scholar] [CrossRef] [Green Version]

| Biological System | Signaling Pathway | Tissue Effect | Evidence in Animals | Evidence in Humans | Effect of Exercise |
|---|---|---|---|---|---|
| Skeletal muscle | Increased ROS/RNS | Cellular damage Defective redox signaling Increased proteolysis | SOD-1 KO mice lost muscle mass and function [77] | Signals of increased oxidative stress was related to functional outcomes in the elderly [26] | Increased catalase expression in trained older subjects [218] Exercise increased SOD and GPX [219] Decreased oxidative stress by exercise was associated with muscle size and strength in older individuals with COPD [217] |
| Reduced PGC-1α | Reduced mitochondrial biogenesis Mitochondrial dysfunction | Associated with sarcopenia in mice [59] | PGC-1α was related to reduced physical activity and muscle atrophy in older humans [57,61] | Endurance exercise induced PGC-1α expression in skeletal muscle in aged animals and humans [223] 12-weeks resistance training increased PGC-1α levels in elderly men [225] | |
| Reduced Nrf2 | Increased oxidative stress | Reduced muscle strength and increased fatigue in mice [75,76] | Positive association of Nrf2 with gait speed in older subjects [82] | Long-term exercise increased Nrf2 expression in aged mice related to attenuation of sarcopenia phenotype in vivo [226] Physical activity increased Nrf2 expression promoting protection against ROS-induced damage in skeletal muscle [227,228] | |
| Vascular system | Increased ROS/RNS | Reduced NO availability | Inhibition of peroxynitrite normalized vasorelaxation in resistance arteries of aged rats [122] | Increased superoxide anion and peroxynitrite were detected in aged human vessels with defective endothelial vasodilation [121] | Expression of SOD2 and activity of SOD3 were greater in exercising vs. sedentary older subjects [249] |
| Uncoupled eNOS/BH4 deficiency | Reduced NO availability Increased ROS production | Reduced levels of BH4 were associated with impaired endothelial vasodilation in aged rat arterioles [123] | Vascular reduction in BH4 was related to arterial stiffness and endothelial dysfunction in postmenopausal women [124] | Old age reduced and exercise training restored levels of BH4 in rat soleus feed arterioles related to improved flow-mediated dilation [252] | |
| Increased NOX | Increased ROS production | Age-dependent increase in blood pressure, cardiomyocyte hypertrophy, coronary remodeling and cardiac fibrosis was associated with increased NOX2 activity [130] Attenuation of NOX activity improved endothelial dysfunction in aged coronary arteries [126] | NOX was overexpressed in arteries from older subjects while NOX inhibition improved endothelial vasodilation [121] | Exercise was related to reduced endothelial NOX expression in elderly subjects [249] | |
| Reduced Nrf2 | Defective antioxidant response Increased oxidative damage | Increased oxidative stress in hearts from old mice correlates with Nrf2 dysregulation and is reversed by sulforaphane [81] | Short-term pharmacological activation decreased age-related impairment of endothelium-dependent and ROS-induced vasodilation in rat and human vascular tissues [132] | Exercise increases Nrf2 expression in mouse cardiac fibroblasts [259] and in human peripheral blood mononuclear cells [230] |
| Biological System | Signaling Pathway | Tissue Effect | Evidence in Animals | Evidence in Humans | Effect of Exercise |
|---|---|---|---|---|---|
| Skeletal muscle | Increased pro-inflammatory cytokines | Muscle inflammation (increased NF-κB) Increased proteolysis | Blockade of TNF-α prevents sarcopenia in aged mice [82] | Increased TNF-α and IL-6 correlates with muscle mass loss in older subjects [90]. Associated with poor physical performance and reduced muscular strength in older subjects [85] | Multicomponent exercise program downregulated expressions of IL-6 and TNF-α in frail obese elderly subjects [232] Physical activity resulted in a reduction in systemic concentrations of IL-6, TNF-α, CRP in middle aged and older subjects and postmenopausal women [233,234] Moderate-intensity aerobic exercise reduced the expression of inflammasome constituents (NLRP3, TLR4) and IL-1β, IL-6, and TNF-α [235] |
| Reduced anti-inflammatory cytokines | Muscle inflammation | Muscle overexpression of IL-10 was associated with a low level of muscle inflammation and insulin resistance [94] IL-10 KO mice was proposed as a model of frailty with reduced muscle strength [95] | Increased IL-6/IL-10 ratio in older subjects with sarcopenia [92] | Physical activity was associated with higher levels of IL-10 and adiponectin [236] | |
| Myokine alteration | Apelin increases mitocondriogenesis and reduces inflammation Myostatin inhibits muscle synthesis and promotes muscle catabolism and is inhibited by follistatin Adiponectin increases mitochondrial function and augments oxidative fibers Irisin improves mitochondrial function decreases ROS production and protects skeletal muscle from metabolic stresses | Apelin restoration prevented muscle wasting in aged mice [92] Adiponectin signaling improves skeletal muscle function in aged mice [97] | Elevated myostatin has been related to sarcopenia in humans [43] Circulating irisin was decreased in older subjects losing muscle strength [243] | Serum irisin levels increased in response to exercise in aged humans [243] Improvement in muscle strength by resistance training was related to decreased myostatin and increased follistatin in sarcopenic older adults [244] Production of apelin in response to muscle contraction contributes to the positive feedback of physical activity and muscle function [98] | |
| Vascular system | Activated NF-κB | Vascular inflammation Transcription of inflammatory cytokines and mediators of inflammation Vascular remodeling | Inhibition of vascular NF-κB improved blood flow regulation and decreased systemic inflammation [126] | Enhanced activation of NF-κB in vessels from aged humans which correlated with endothelial dysfunction [121] | Voluntary wheel running by old mice decreased aortic NF-κB activation [248] |
| Increased pro-inflammatory cytokines | Endothelial dysfunction Vascular remodeling | Abrogation of inflammasome (NLRP3) preserved cardiac function in old mice and increased the lifespan [137] Anti-inflammatory cytokine, IL-37, improved vascular endothelial function in old mice [142] | CRP and IL-6 have been positively related to aortic stiffness and inversely correlated to endothelial function in older adults [125] | Voluntary wheel running by old mice decreased aortic expression of inflammatory cytokines and macrophage infiltration and reversed impaired NO-mediated endothelial dilation [248] | |
| Myokine alteration | Irisin improves vascular function Apelin could increase NO production | Higher concentrations of irisin were detected in centenarian people without CVD [113] | Aerobic exercise training increased apelin concentrations along with higher NO production and lower aortic stiffness in middle-aged and older subjects [254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. https://doi.org/10.3390/ijms23158713
El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. International Journal of Molecular Sciences. 2022; 23(15):8713. https://doi.org/10.3390/ijms23158713
Chicago/Turabian StyleEl Assar, Mariam, Alejandro Álvarez-Bustos, Patricia Sosa, Javier Angulo, and Leocadio Rodríguez-Mañas. 2022. "Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging" International Journal of Molecular Sciences 23, no. 15: 8713. https://doi.org/10.3390/ijms23158713






