Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix
Abstract
1. Introduction
2. Results
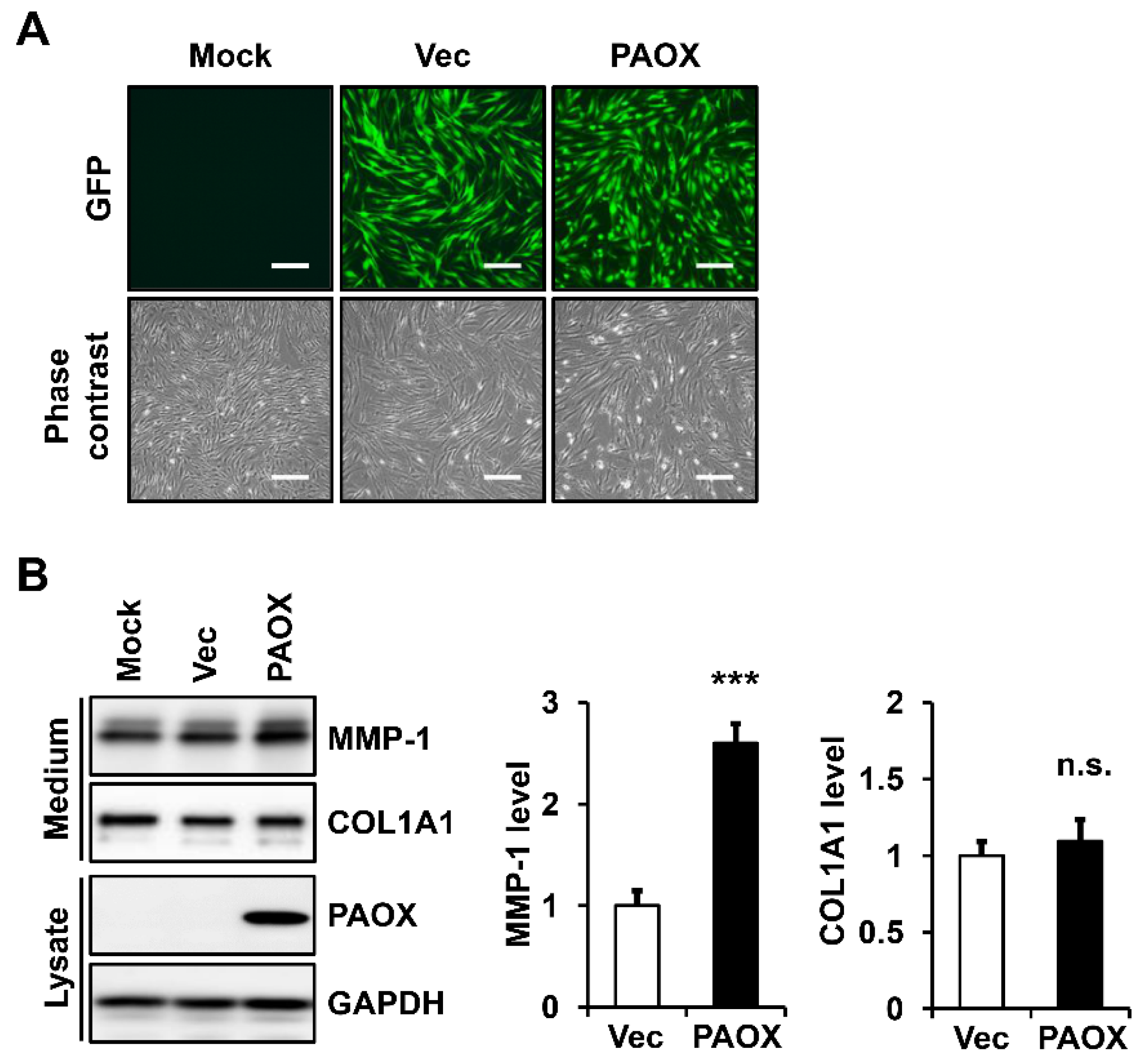
2.1. PAOX Expression Increases MMP-1 Secretion in Fibroblasts
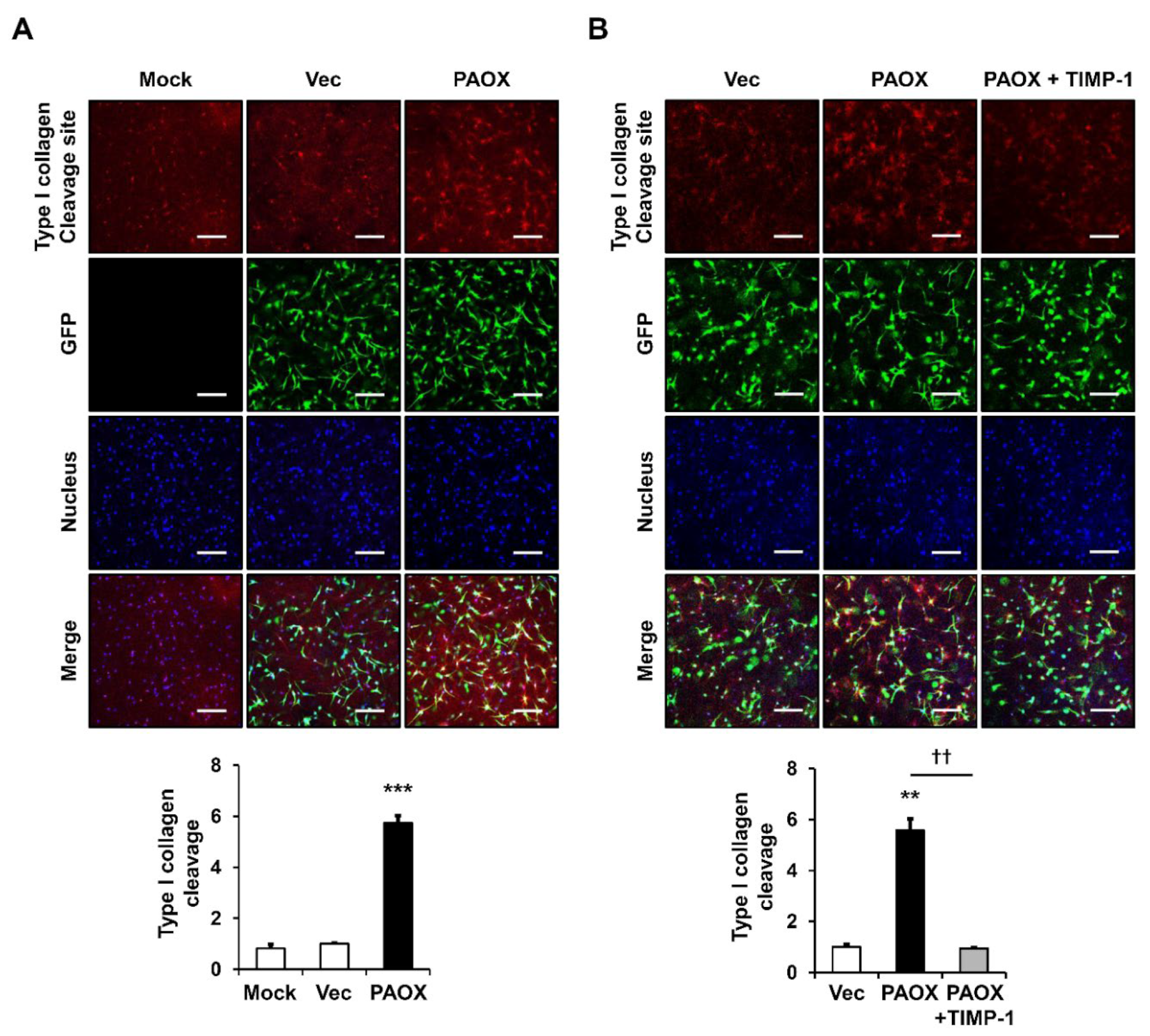
2.2. PAOX Expression Increases Type I Collagen Degradation in 3D Culture of Fibroblasts
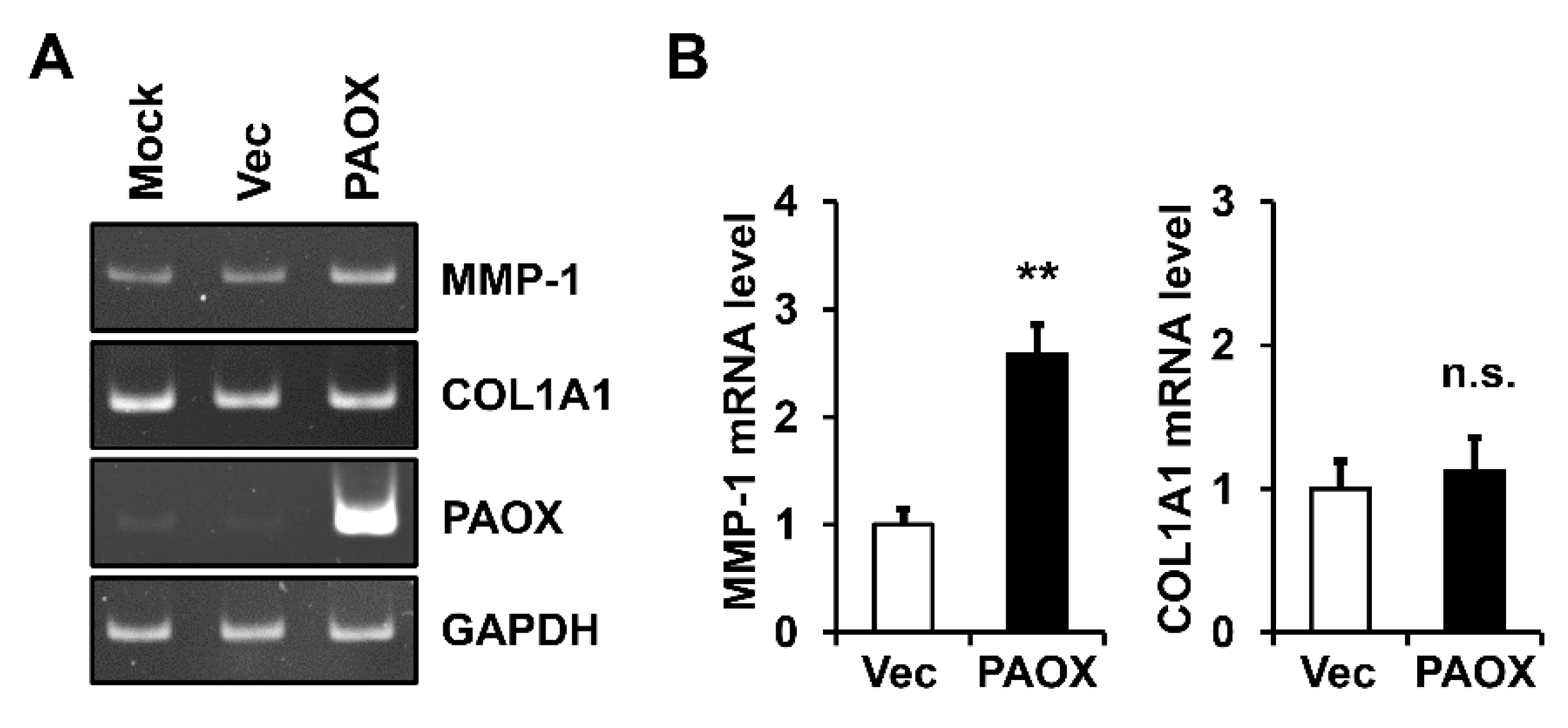
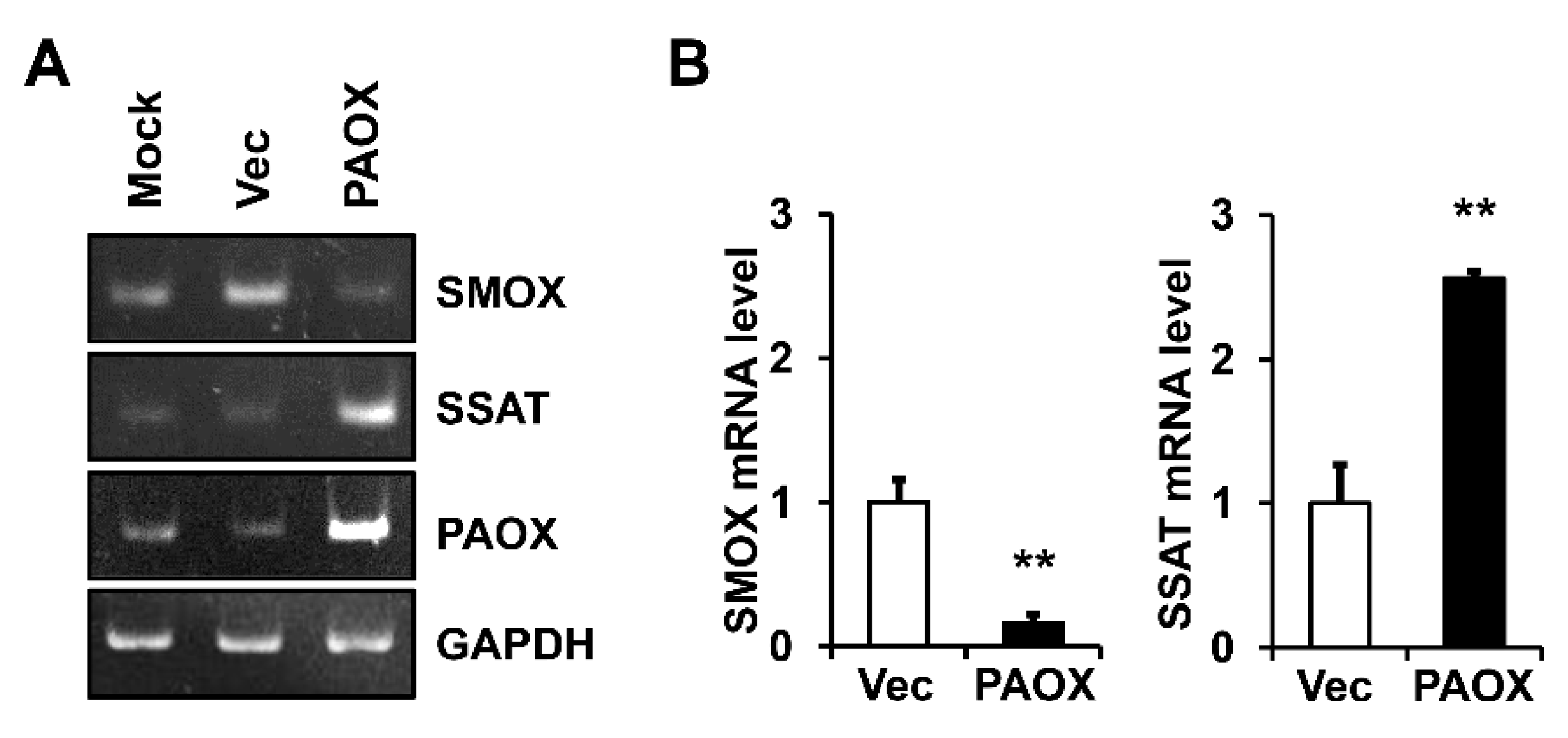
2.3. PAOX Expression Increases MMP-1 mRNA Level in Fibroblasts
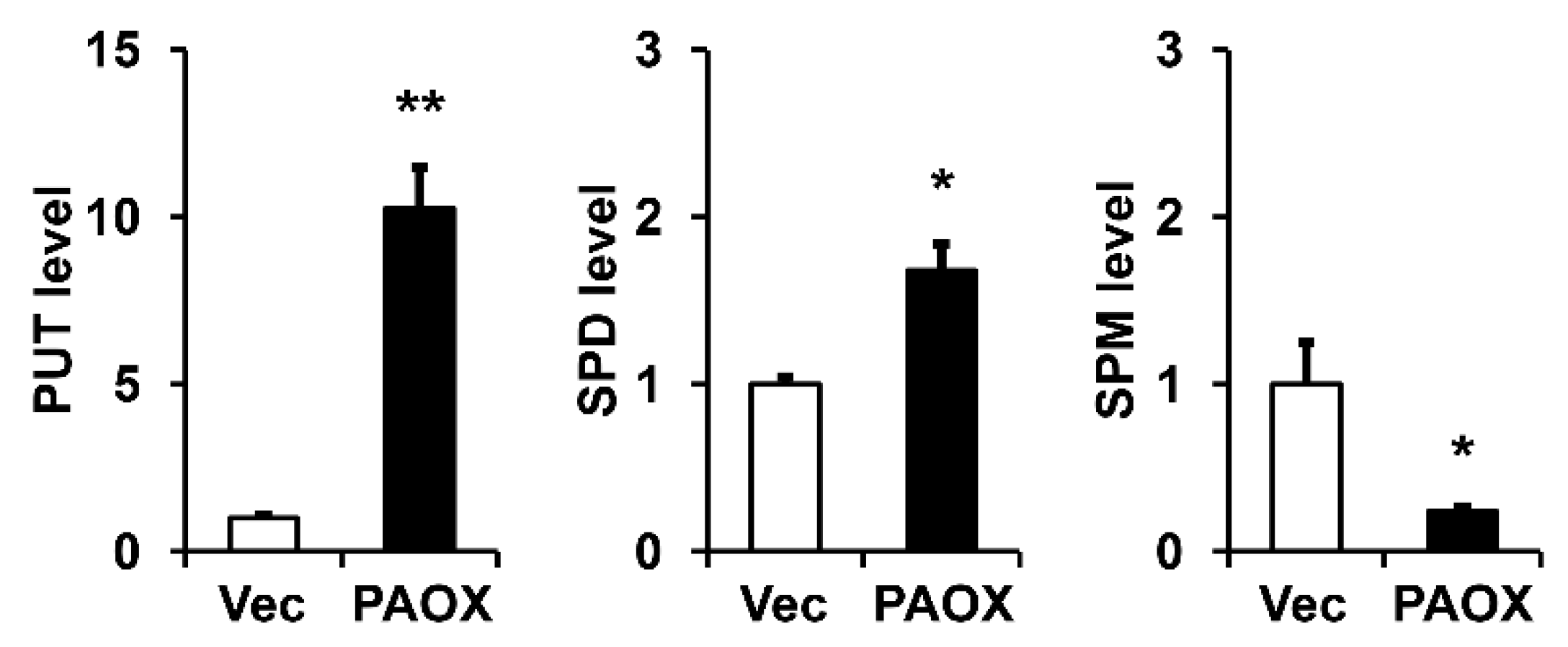
2.4. PAOX Expression Activates Polyamine Catabolism in Fibroblasts
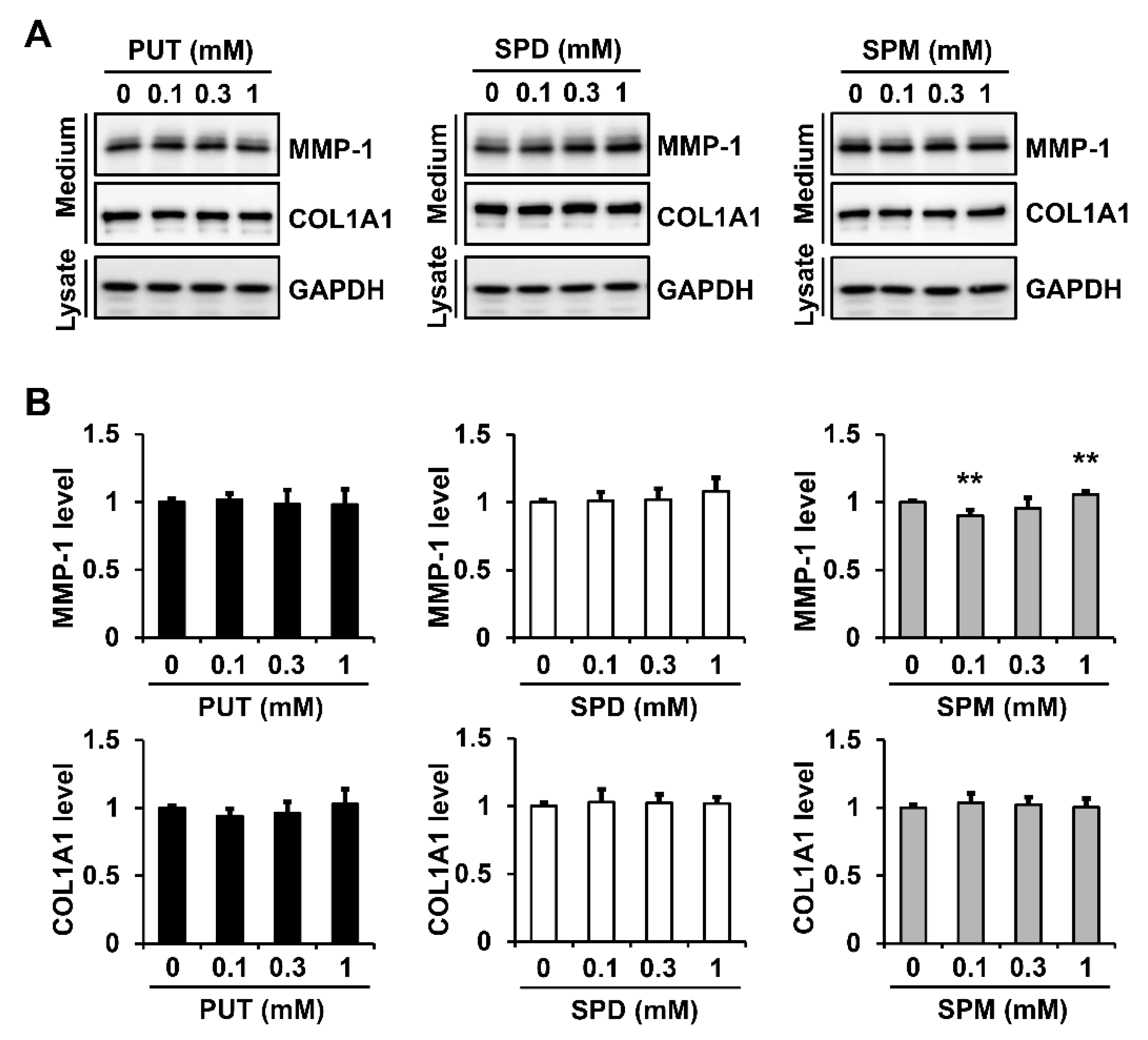
2.5. Exogenous Polyamines Have Little Effect on MMP-1 Secretion in Fibroblasts
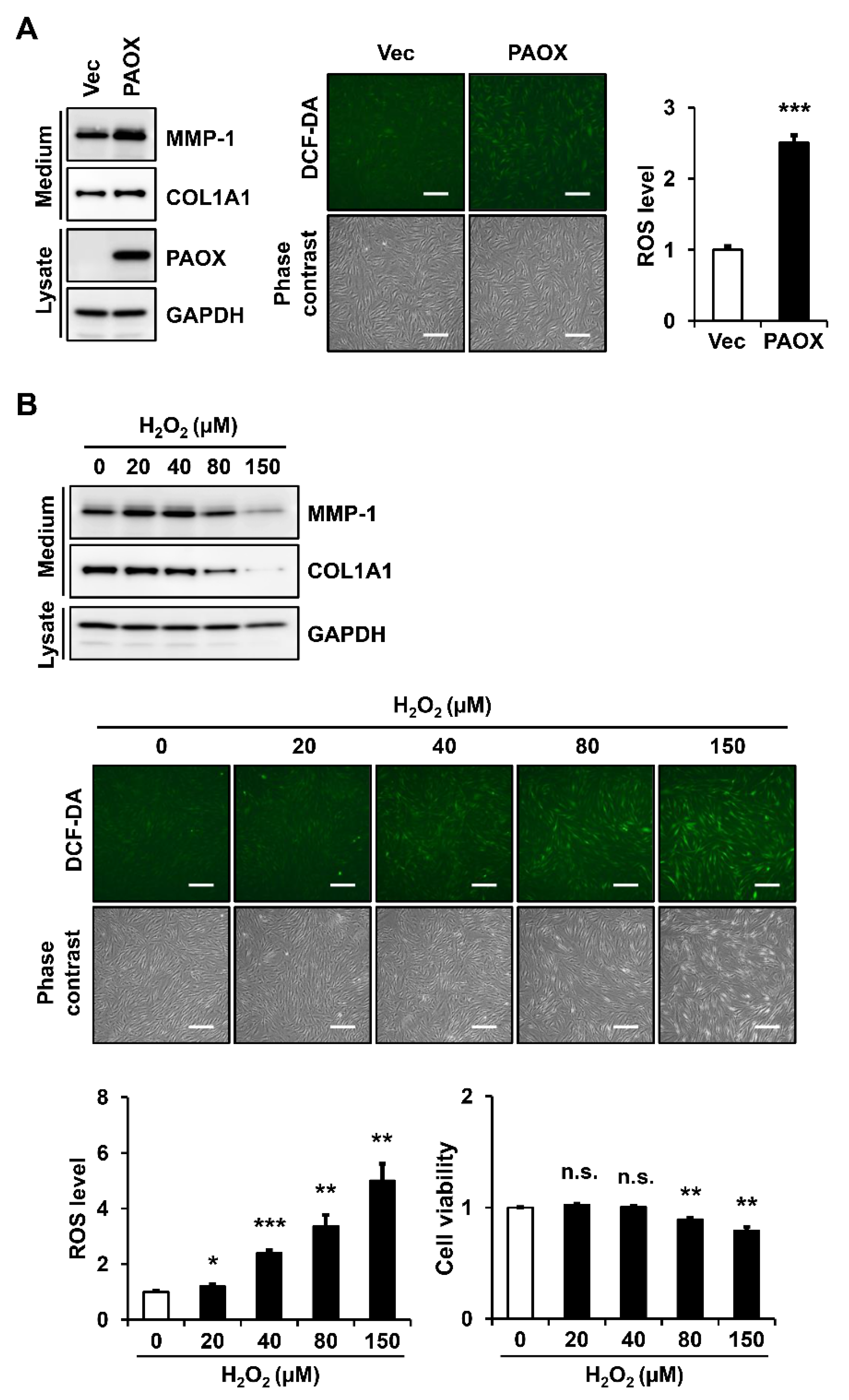
2.6. PAOX Expression Increases Intracellular ROS Levels in Fibroblasts
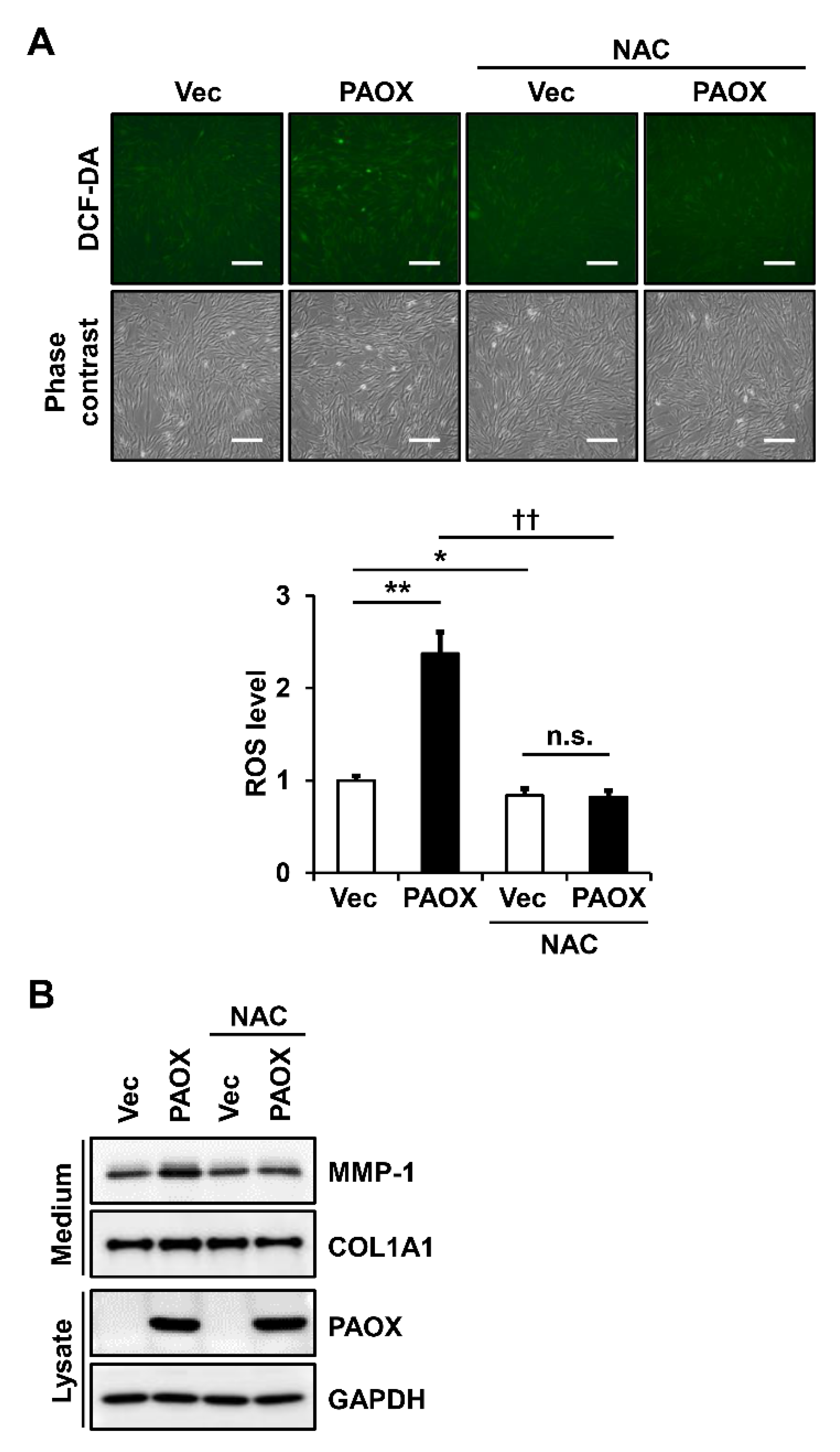
2.7. Antioxidants Decrease PAOX-Mediated Rise in ROS Levels and MMP-1 Secretion in Fibroblasts
2.8. PAOX Expression Enhances Activation of AP-1 and NF-κB Signaling Pathways
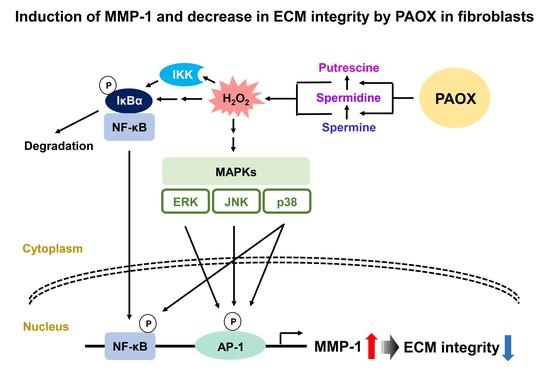
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture
4.3. Construction of Lentiviral Transfer Vectors Harboring Human PAOX cDNA
4.4. Production and Infection of Lentiviruses
4.5. Preparation of Conditioned Media and Cell Lysates for Western Blot Analysis
4.6. Analysis of Cleavage and Synthesis of Type I Collagen in a 3D-Culture System
4.7. RNA Isolation and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.8. Determination of Intracellular Polyamine Levels
4.9. Measurement of Cellular ROS Levels and Cell Viability
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kang, S.C. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm. Res. 2013, 62, 681–688. [Google Scholar] [CrossRef]
- Babbar, N.; Murray-Stewart, T.; Casero, R.A., Jr. Inflammation and polyamine catabolism: The good, the bad and the ugly. Biochem. Soc. Trans. 2007, 35, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Lagishetty, C.V.; Naik, S.R. Polyamines: Potential anti-inflammatory agents and their possible mechanism of action. Indian J. Pharmacol. 2008, 40, 121–125. [Google Scholar] [CrossRef] [PubMed]
- McCubbrey, A.L.; McManus, S.A.; McClendon, J.D.; Thomas, S.M.; Chatwin, H.B.; Reisz, J.A.; D’Alessandro, A.; Mould, K.J.; Bratton, D.L.; Henson, P.M.; et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. 2022, 38, 110222. [Google Scholar] [CrossRef]
- Brunton, V.G.; Grant, M.H.; Wallace, H.M. Spermine toxicity and glutathione depletion in BHK-21/C13 cells. Biochem. Pharmacol. 1990, 40, 1893–1900. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamine Catabolism in Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 4790. [Google Scholar] [CrossRef]
- Zahedi, K.; Huttinger, F.; Morrison, R.; Murray-Stewart, T.; Casero, R.A.; Strauss, K.I. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 2010, 27, 515–525. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, P.A.; Marques, M.R.; Santos, M.C. MMP-1 polymorphism and its relationship to pathological processes. J. Biosci. 2009, 34, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Kim, H.I.; Saldova, R.; Park, J.H.; Lee, Y.H.; Harvey, D.J.; Wormald, M.R.; Wynne, K.; Elia, G.; Kim, H.-J.; Rudd, P.M.; et al. The Presence of Outer Arm Fucose Residues on the N-Glycans of Tissue Inhibitor of Metalloproteinases-1 Reduces Its Activity. J. Proteome Res. 2013, 12, 3547–3560. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Cai, J.; Liu, K.; Liu, M.; Wang, H.; Zhang, H. N-acetylcysteine alleviates H2O2-induced damage via regulating the redox status of intracellular antioxidants in H9c2 cells. Int. J. Mol. Med. 2019, 43, 199–208. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Cortez, D.M.; Feldman, M.D.; Mummidi, S.; Valente, A.J.; Steffensen, B.; Vincenti, M.; Barnes, J.L.; Chandrasekar, B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3356–H3365. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.F.; Liu, T.; Wang, M.; Chen, S.; Chang, L.; Karisma, V.W.; Weixu; Diao, Q.; Xue, M.; Tang, X.; et al. Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B 2022, 226, 112350. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Noh, E.-M.; Jeong, E.-Y.; Yun, S.-K.; Jeong, Y.-J.; Kim, J.-H.; Kwon, K.-B.; Kim, B.-S.; Lee, S.-H.; Park, C.-S.; et al. Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-κB pathway in human dermal fibroblasts. Exp. Mol. Med. 2009, 41, 548–554. [Google Scholar] [CrossRef]
- Wittmann, C.; Chockley, P.; Singh, S.K.; Pase, L.; Lieschke, G.J.; Grabher, C. Hydrogen Peroxide in Inflammation: Messenger, Guide, and Assassin. Adv. Hematol. 2012, 2012, 541471. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Hammerberg, C.; Li, Y.; He, T.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell 2013, 12, 661–671. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, E.K.; Lee, S.T. Skullcapflavone II Inhibits Degradation of Type I Collagen by Suppressing MMP-1 Transcription in Human Skin Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.D.; Song, M.J.; Lee, D.H.; Chung, J.H.; Lee, S.-T. SOD3 Suppresses the Expression of MMP-1 and Increases the Integrity of Extracellular Matrix in Fibroblasts. Antioxidants 2022, 11, 928. [Google Scholar] [CrossRef]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef]
- Pietilä, M.; Parkkinen, J.J.; Alhonen, L.; Jänne, J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J. Investig. Dermatol. 2001, 116, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Suppola, S.; Heikkinen, S.; Parkkinen, J.J.; Uusi-Oukari, M.; Korhonen, V.-P.; Keinanen, T.; Alhonen, L.; Jänne, J. Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N1-acetyltransferase further accelerates the catabolism of hepatic polyamines in transgenic mice. Biochem. J. 2001, 358, 343–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zahedi, K.; Wang, Z.; Barone, S.; Prada, A.E.; Kelly, C.N.; Casero, R.A.; Yokota, N.; Porter, C.W.; Rabb, H.; Soleimani, M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2003, 284, F1046–F1055. [Google Scholar] [CrossRef]
- Løvaas, E.; Carlin, G. Spermine: An anti-oxidant and anti-inflammatory agent. Free Radic. Biol. Med. 1991, 11, 455–461. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine Inhibits Proinflammatory Cytokine Synthesis in Human Mononuclear Cells: A Counterregulatory Mechanism that Restrains the Immune Response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef]
- Soda, K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Qi, C.; Shen, L.; Wang, J.; Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 2018, 8, 10384. [Google Scholar] [CrossRef]
- Alge-Priglinger, C.S.; Kreutzer, T.; Obholzer, K.; Wolf, A.; Mempel, M.; Kernt, M.; Kampik, A.; Priglinger, S.G. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5495–5503. [Google Scholar] [CrossRef]
- Ho, B.Y.; Wu, Y.M.; Chang, K.J.; Pan, T.M. Dimerumic acid inhibits SW620 cell invasion by attenuating H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int. J. Biol. Sci. 2011, 7, 869–880. [Google Scholar] [CrossRef]
- Kamata, H.; Manabe, T.; Oka, S.; Kamata, K.; Hirata, H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS. Lett. 2002, 519, 231–237. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef] [PubMed]
- Alhonen, L.; Parkkinen, J.J.; Keinänen, T.; Sinervirta, R.; Herzig, K.-H.; Jänne, J. Activation of polyamine catabolism in transgenic rats induces acute pancreatitis. Proc. Natl. Acad. Sci. USA 2000, 97, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Hacker, A.; Huang, Y.; Casero, R.A., Jr. Tumor necrosis factor alpha induces spermidine/spermine N1-acetyltransferase through nuclear factor kappaB in non-small cell lung cancer cells. J. Biol. Chem. 2006, 281, 24182–24192. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Wines, N.; Willsteed, E. Menopause and the skin. Australas. J. Dermatol. 2001, 42, 149–160. [Google Scholar] [CrossRef]
- Devanathan, S.; Whitehead, T.; Schweitzer, G.G.; Fettig, N.; Kovacs, A.; Korach, K.S.; Finck, B.N.; Shoghi, K.I. An animal model with a cardiomyocyte-specific deletion of estrogen receptor alpha: Functional, metabolic, and differential network analysis. PLoS ONE 2014, 9, e101900. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.-T. Polyamine Oxidase Expression Is Downregulated by 17β-Estradiol via Estrogen Receptor 2 in Human MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7521. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, M.K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci. Rep. 2016, 6, 25616. [Google Scholar] [CrossRef]
- Shin, W.S.; Park, M.K.; Kim, J.H.; Oh, S.W.; Jang, J.Y.; Lee, H.; Lee, S.T. PTK7, a Catalytically Inactive Receptor Tyrosine Kinase, Increases Oncogenic Phenotypes in Xenograft Tumors of Esophageal Squamous Cell Carcinoma KYSE-30 Cells. Int. J. Mol. Sci. 2022, 23, 2391. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.T. Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Kim, K.R.; Chung, B.C. Determination of hair polyamines as N-ethoxycarbonyl-N-pentafluoropropionyl derivatives by gas chromatography-mass spectrometry. J. Chromatogr. A 2000, 897, 295–305. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.D.; Kim, J.H.; Kwon, G.E.; Lee, S.-T. Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 10487. https://doi.org/10.3390/ijms231810487
Jeong HD, Kim JH, Kwon GE, Lee S-T. Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix. International Journal of Molecular Sciences. 2022; 23(18):10487. https://doi.org/10.3390/ijms231810487
Chicago/Turabian StyleJeong, Hae Dong, Jin Hyung Kim, Go Eun Kwon, and Seung-Taek Lee. 2022. "Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix" International Journal of Molecular Sciences 23, no. 18: 10487. https://doi.org/10.3390/ijms231810487
APA StyleJeong, H. D., Kim, J. H., Kwon, G. E., & Lee, S.-T. (2022). Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix. International Journal of Molecular Sciences, 23(18), 10487. https://doi.org/10.3390/ijms231810487