Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Structure and Properties of Composites
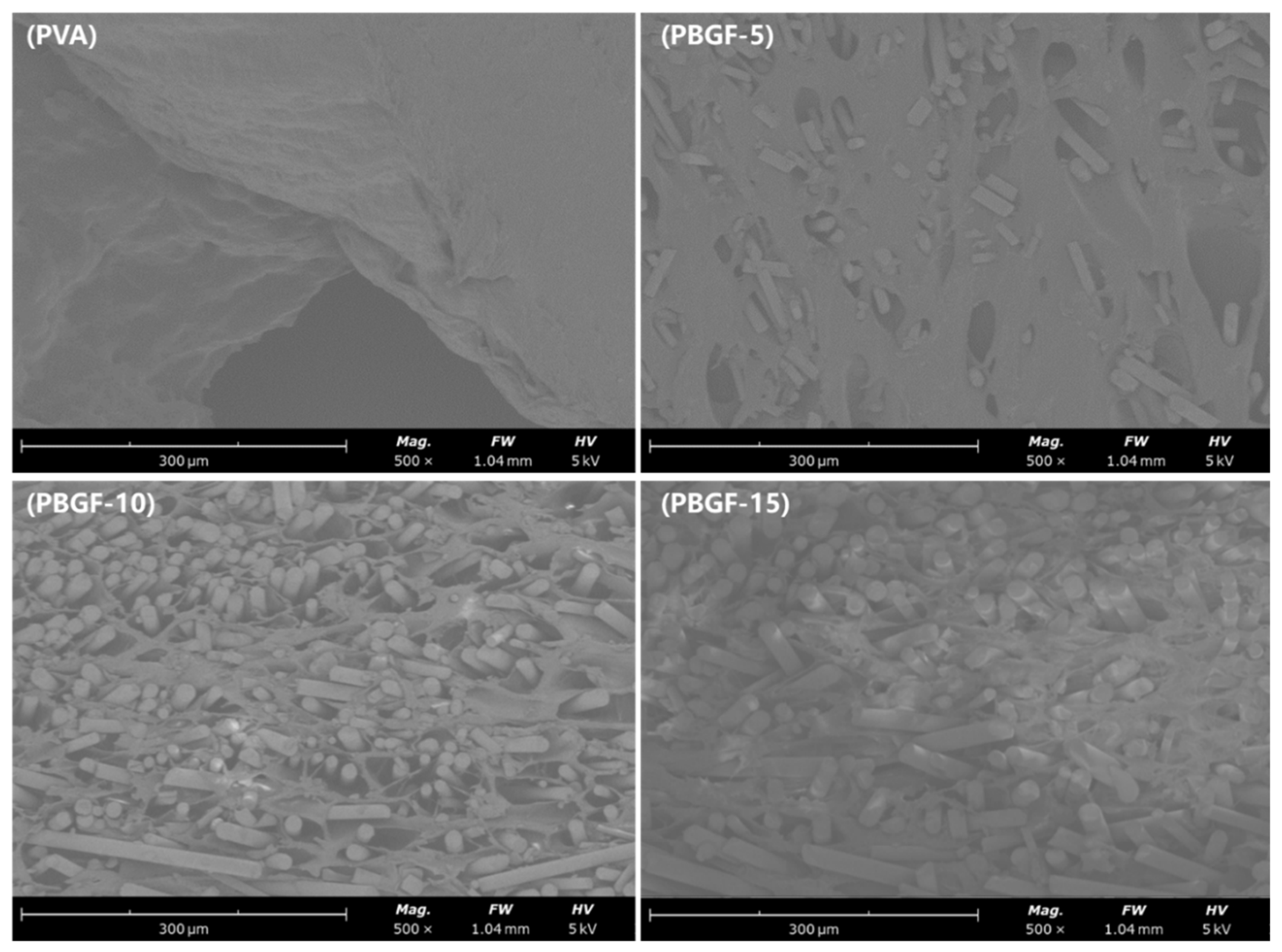
2.1.1. Composite Structure Analysis
2.1.2. Thermal Properties Analysis
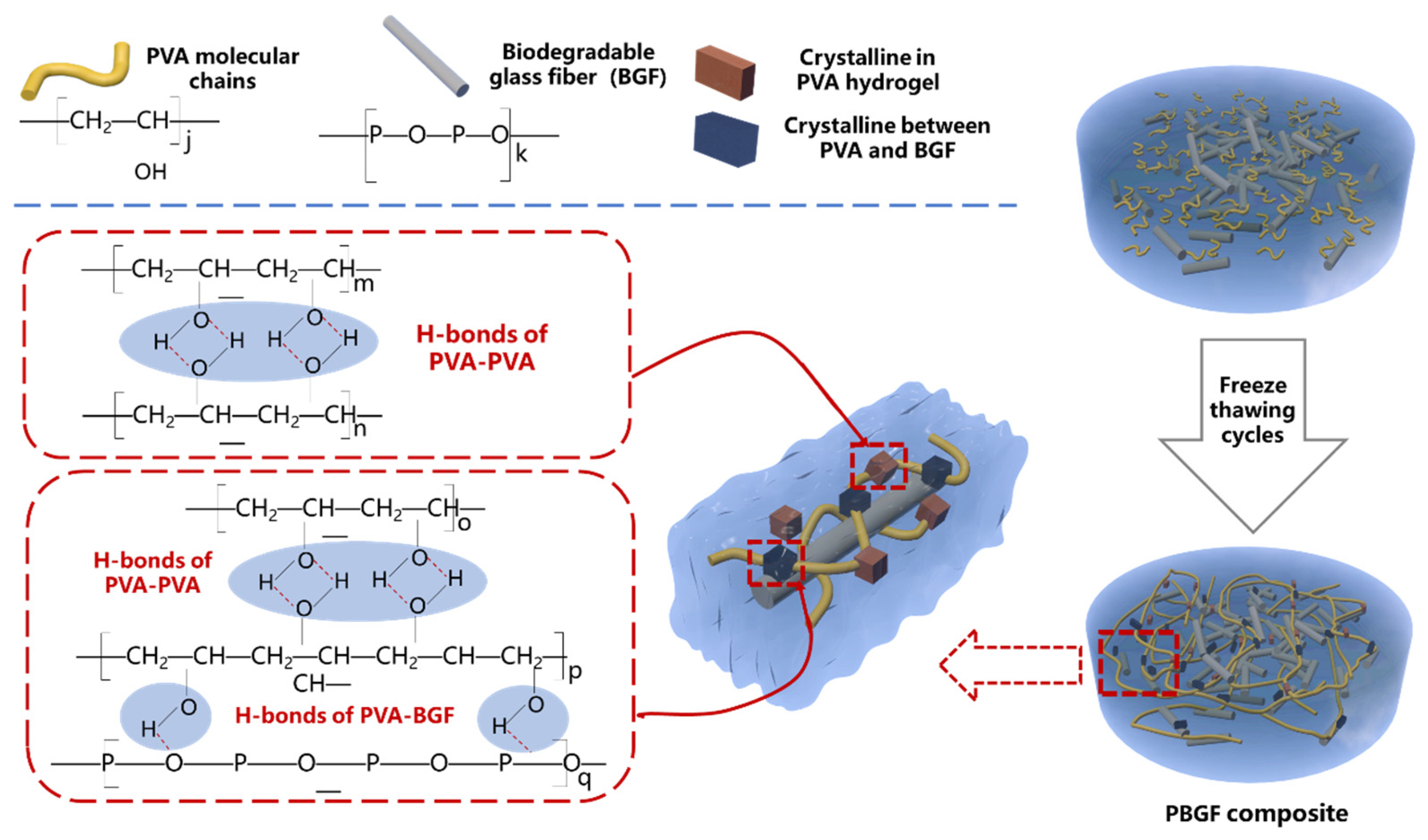
2.1.3. The Mechanism of Composite Formation
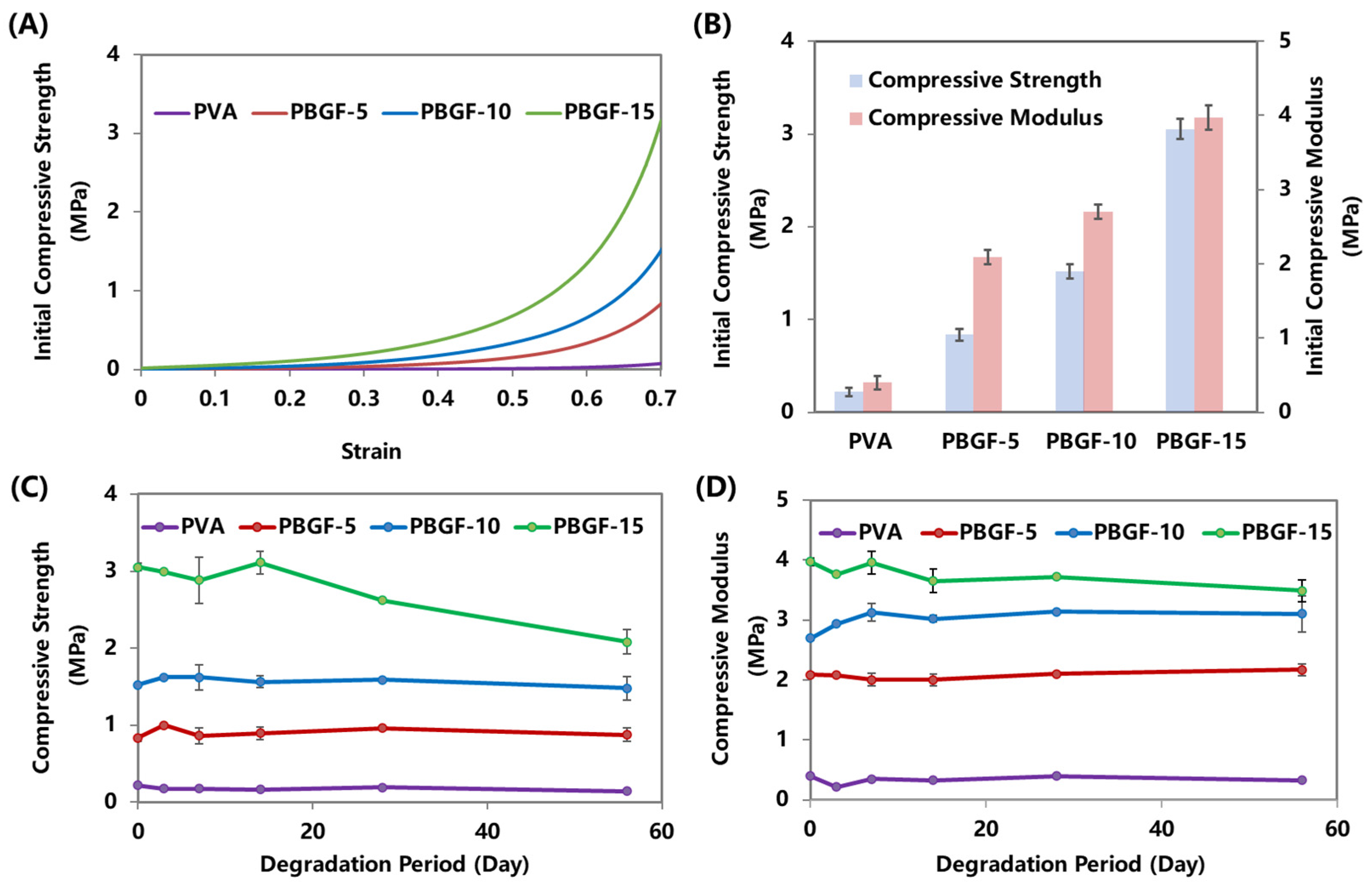
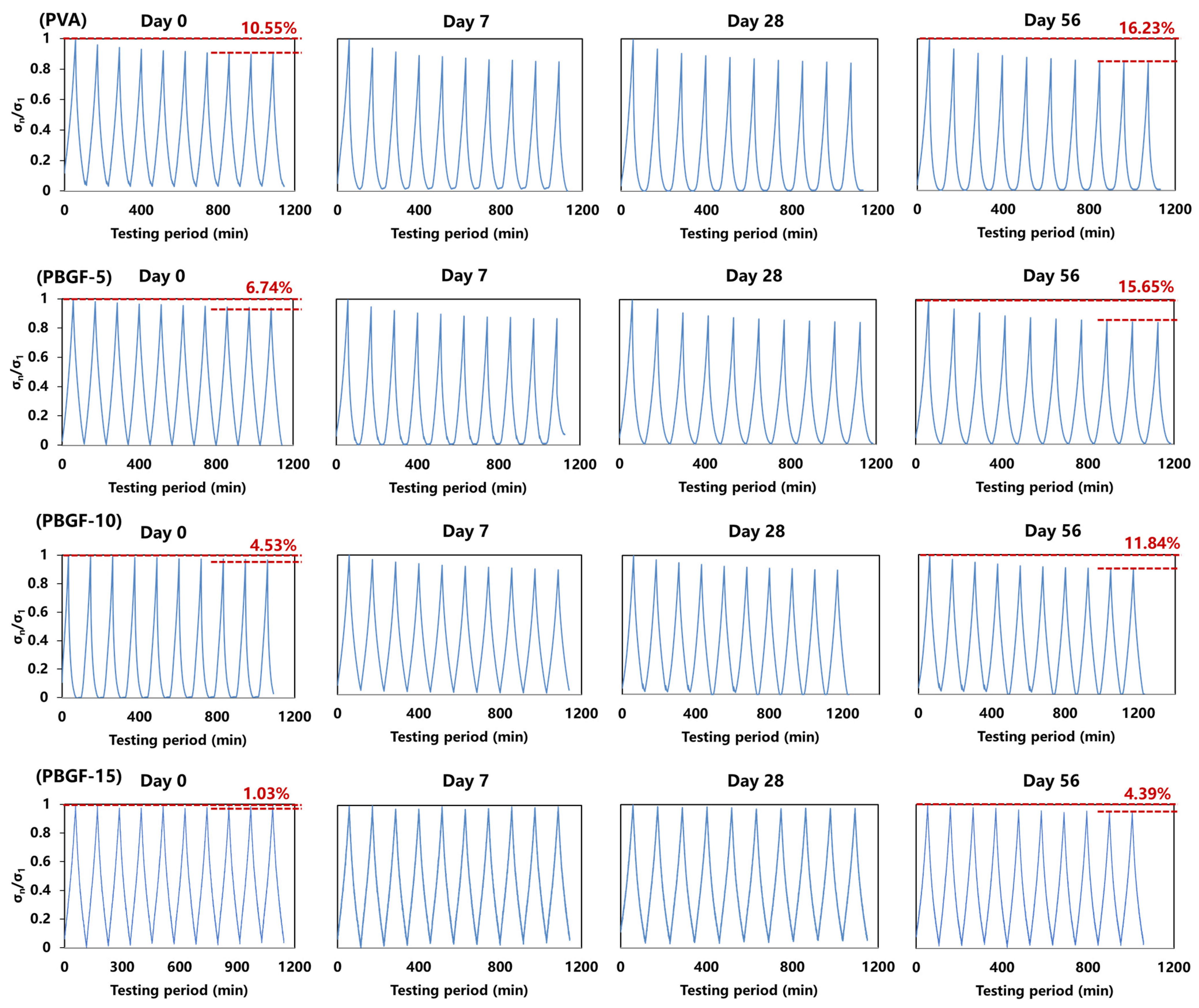
2.2. Mechanical Performance of Composites during Degradation
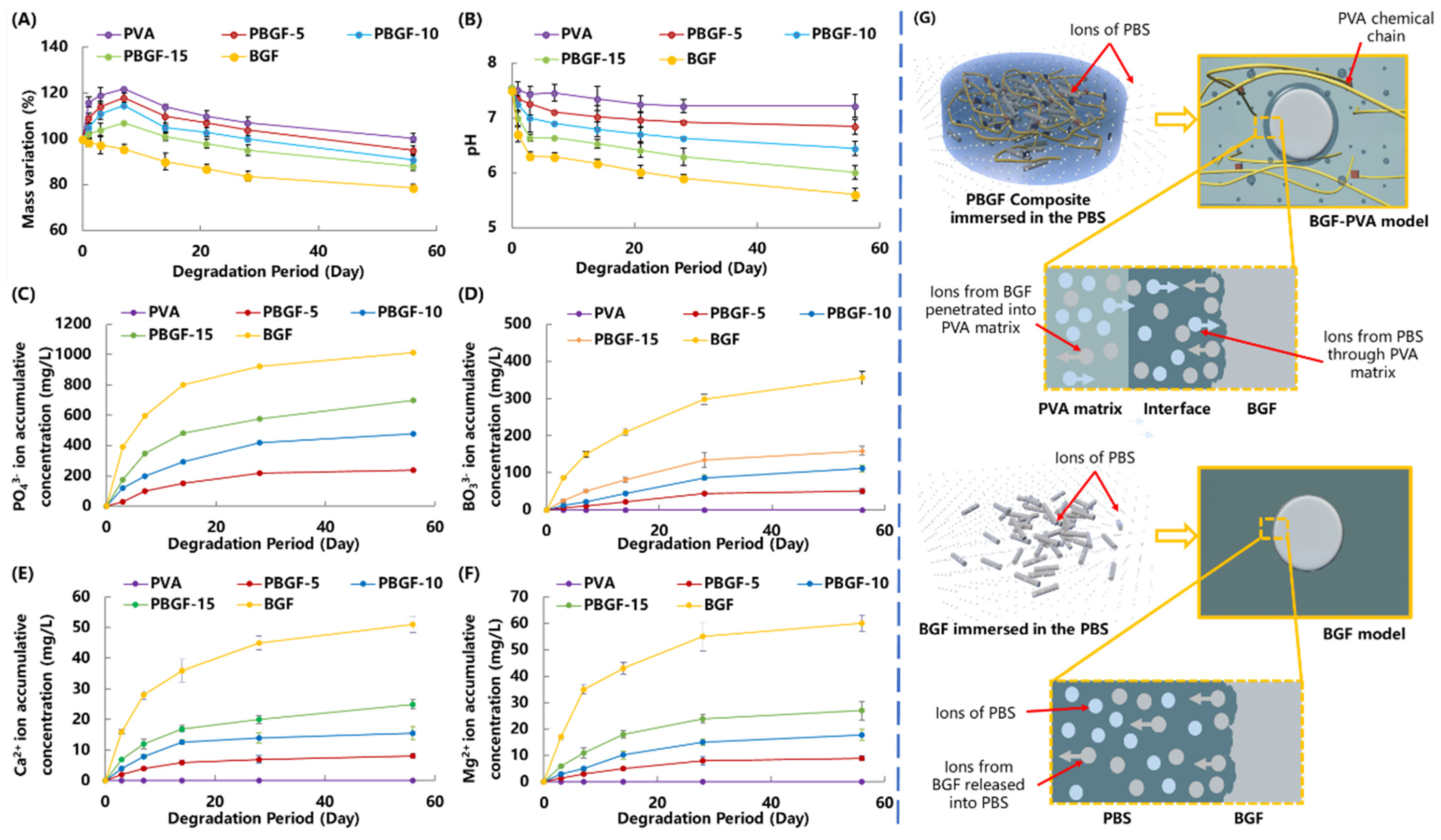
2.3. Degradation Behavior of Composites
2.3.1. Mass Variation and Ion Release Behavior
2.3.2. Dynamic Compressive Behavior during Degradation
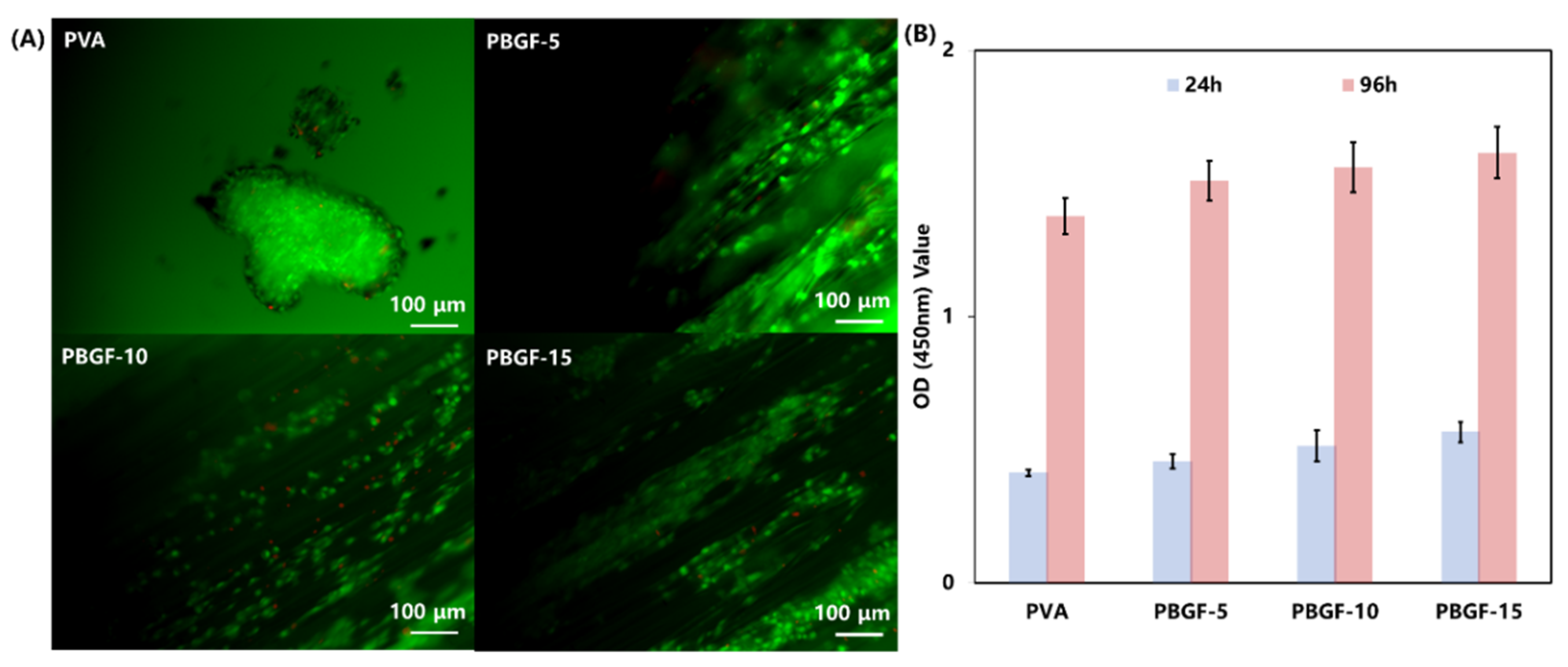
2.4. Cell Study In Vitro
3. Materials and Methods
3.1. Material Preparation
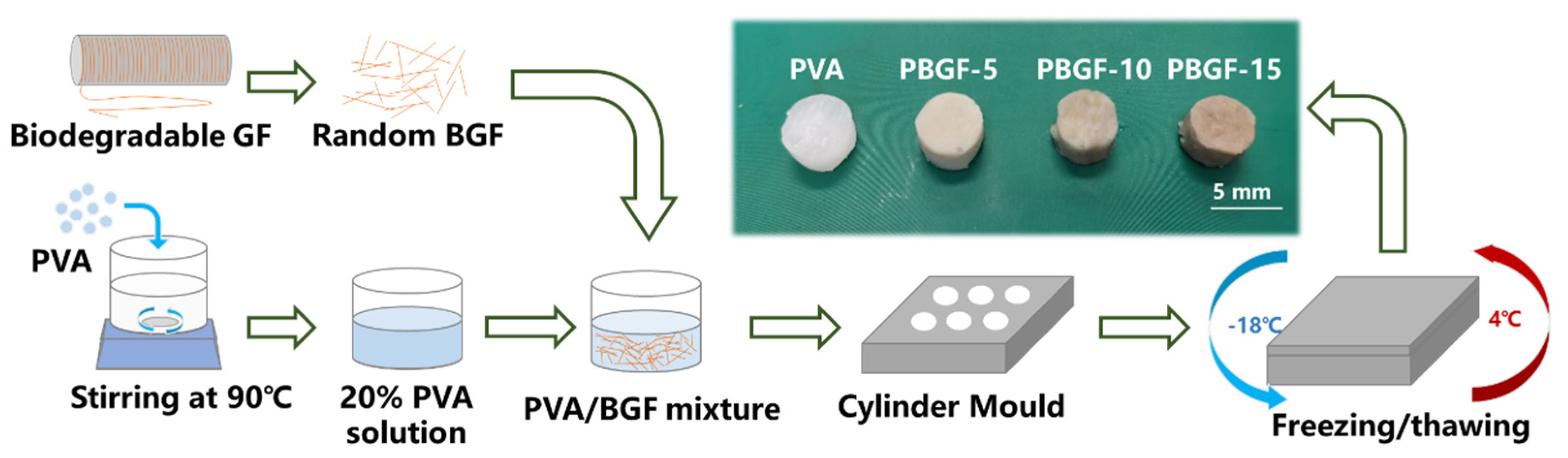
3.1.1. Biodegradable Glass Fiber Manufacture
3.1.2. PVA-BGF Composite (PBGF) Preparation
3.2. Characterization
3.2.1. Swelling Tests
3.2.2. ATR-IR Spectroscopy
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Thermogravimetric Analysis (TGA)
3.2.5. Mechanical Behavior
3.2.6. Dynamic Compressive Behavior
3.2.7. Degradation Behavior In Vitro
3.2.8. Scanning Electron Microscope (SEM)
3.2.9. Cell Study In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, H.D.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar] [CrossRef]
- Marchiori, G.; Berni, M.; Boi, M.; Filardo, G. Cartilage mechanical tests: Evolution of current standards for cartilage repair and tissue engineering. A literature review. Clin. Biomech. 2019, 68, 58–72. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Kumar, J.V.; Sharma, V.; Gupta, P.K.; Verma, R.S. Meniscal tissue engineering via 3d printed pla monolith with carbohydrate based self-healing interpenetrating network hydrogel. Int. J. Biol. Macromol. 2020, 162, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, C.-Y.; Liu, Z.; Phoenix, S.L.; King, D.R.; Cui, W.; Huang, Y.; Gong, J.P. Mechanical behavior of unidirectional fiber reinforced soft composites. Extrem. Mech. Lett. 2020, 35, 100642. [Google Scholar] [CrossRef]
- Cui, W.; King, D.R.; Huang, Y.; Chen, L.; Sun, T.L.; Guo, Y.; Saruwatari, Y.; Hui, C.Y.; Kurokawa, T.; Gong, J.P. Fiber-reinforced viscoelastomers show extraordinary crack resistance that exceeds metals. Adv. Mater. 2020, 32, 1907180. [Google Scholar] [CrossRef]
- Koc, U.; Aykut, Y.; Eren, R. Natural fibers woven fabric reinforced hydrogel composites for enhanced mechanical properties. J. Ind. Text. 2020, 51, 6315–6332. [Google Scholar] [CrossRef]
- Lin, S.; Cao, C.; Wang, Q.; Gonzalez, M.; Dolbow, J.E.; Zhao, X. Design of stiff, tough and stretchy hydrogel composites via nanoscale hybrid crosslinking and macroscale fiber reinforcement. Soft Matter 2014, 10, 7519–7527. [Google Scholar] [CrossRef]
- No, J.Y.; Tarafder, S.; Reischl, B.; Ramaswamy, Y.; Dunstan, C.; Friedrich, O.; Lee, C.H.; Zreiqat, H. High-strength fiber-reinforced composite hydrogel scaffolds as biosynthetic tendon graft material. ACS Biomater. Sci. Eng. 2020, 6, 1887–1898. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, L. The story of bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Lapa, A.; Cresswell, M.; Jackson, P.; Boccaccini, A.R. Phosphate glass fibres with therapeutic ions release capability—A review. Adv. Appl. Ceram. 2020, 119, 1–14. [Google Scholar] [CrossRef]
- Hasan, M.S.; Ahmed, I.; Parsons, A.J.; Rudd, C.D.; Walker, G.S.; Scotchford, A.C. Investigating the use of coupling agents to improve the interfacial properties between a resorbable phosphate glass and polylactic acid matrix. J. Biomater. Appl. 2013, 28, 354–366. [Google Scholar] [CrossRef]
- Chen, M.; Parsons, A.J.; Felfel, R.M.; Rudd, C.D.; Irvine, D.J.; Ahmed, I. In-situ polymerisation of fully bioresorbable polycaprolactone/phosphate glass fibre composites: In vitro degradation and mechanical properties. J. Mech. Behav. Biomed. Mater. 2016, 59, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Hu, H.; Deng, Z.; Pang, L.; Jiang, H.; Wang, D.; Li, J.; Liu, Z.; Wang, H.; Zeng, X. Novel bioactive glass cross-linked pva hydrogel with enhanced chondrogenesis properties and application in mice chondrocytes for cartilage repair. J. Non-Cryst. Solids 2020, 529, 119594. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Callcott, T. Development of an Artificial Meniscus Implant; Georgia Institute of Technology: Atlanta, GA, USA, 2020. [Google Scholar]
- Xu, Z.; Li, J.; Zhou, H.; Jiang, X.; Yang, C.; Wang, F.; Pan, Y.; Li, N.; Li, X.; Shi, L. Morphological and swelling behavior of cellulose nanofiber (cnf)/poly(vinyl alcohol)(pva) hydrogels: Poly (ethylene glycol)(peg) as porogen. RSC Adv. 2016, 6, 43626–43633. [Google Scholar] [CrossRef]
- Huang, M.; Cai, D.; Liu, Y.; Sun, J.; Wang, J.; Qin, C.; Dai, L.; Kazuo, Y. Investigation of a-pva/s-pva hydrogels prepared by freezing-thawing method. Fibers Polym. 2012, 13, 955–962. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Azami, M.; Mozafari, M. Controllable synthesis and characterization of porous polyvinyl alcohol/hydroxyapatite nanocomposite scaffolds via an in situ colloidal technique. Colloids Surf. B Biointerfaces 2011, 84, 310–316. [Google Scholar] [CrossRef]
- Mansur, H.S.; Costa, H.S. Nanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem. Eng. J. 2008, 137, 72–83. [Google Scholar] [CrossRef]
- Wang, F.; Liao, Q.; Chen, K.; Pan, S.; Lu, M. The crystallization and ftir spectra of zro2-doped 36fe2o3–10b2o3–54p2o5 glasses and crystalline compounds. J. Alloy. Compd. 2014, 611, 278–283. [Google Scholar] [CrossRef]
- Chen, K.; Chen, G.; Wei, S.; Yang, X.; Zhang, D.; Xu, L. Preparation and property of high strength and low friction pva-ha/paa composite hydrogel using annealing treatment. Mater. Sci. Eng. C 2018, 91, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, A.R. Effect of ultrasonication duration of polyvinyl alcohol (pva) gel on characterizations of pva film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Jiang, P.; Lin, P.; Yang, C.; Qin, H.; Wang, X.; Zhou, F. 3D printing of dual-physical cross-linking hydrogel with ultrahigh strength and toughness. Chem. Mater. 2020, 32, 9983–9995. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, Q.; Liang, K.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinked methacrylated poly(vinyl alcohol)/hydroxyapatite nanocomposite hydrogels with enhanced mechanical strength and cell adhesion. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1882–1889. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W. Development of magnetic nanocomposite hydrogel with potential cartilage tissue engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef]
- Arroyo, M.; Lopez-Manchado, M.A.; Avalos, F. Crystallization kinetics of polypropylene: Ii. Effect of the addition of short glass fibres. Polymer 1997, 38, 5587–5593. [Google Scholar] [CrossRef]
- Manchado, M.A.L.; Torre, J.B.L.; Kenny, J.M. Effects of reinforcing fibers on the crystallization of polypropylene. Polym. Eng. Sci. 2000, 40, 2194. [Google Scholar] [CrossRef]
- Roth, V.; Mow, C.V. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. J. Bone Jt. Surg. 1980, 62, 1102–1117. [Google Scholar] [CrossRef]
- Almarza, A.J.; Athanasiou, A.K. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 2004, 32, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Tissakht, M.; Ahmed, A. Tensile stress-strain characteristics of the human meniscal material. J. Biomech. 1995, 28, 411–422. [Google Scholar] [CrossRef]
- Sweigart, M.; Zhu, C.; Burt, D.; DeHoll, P.; Agrawal, C.; Clanton, T.; Athanasiou, K.A. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann. Biomed. Eng. 2004, 32, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, J.; Zhang, Y.; Zu, Q.; Rudd, C.; Liu, X. Time-dependent degradation behaviour of phosphate glass fibre reinforced composites with different fibre architecture. Mech. Time-Depend. Mater. 2020, 25, 663–678. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.M.; Ahmed, I.; Hossain, Z.K.M. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 204173141771917. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Ahmed, I.; Parsons, A.; Hossain, K.Z.; Rudd, C.; Liu, J.; Liu, X. Structural, thermal, in vitro degradation and cytocompatibility properties of p2o5-b2o3-cao-mgo-na2o-fe2o3 glasses. J. Non-Cryst. Solids 2017, 457, 77–85. [Google Scholar] [CrossRef]
- Zhu, C.; Ahmed, I.; Parsons, A.; Wang, Y.; Tan, C.; Liu, J.; Rudd, C.; Liu, X. Novel bioresorbable phosphate glass fiber textile composites for medical applications. Polym. Compos. 2018, 39, 140–151. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Differential scanning calorimetry of crystallized pva hydrogels. J. Appl. Polym. Sci. 1976, 20, 1457–1465. [Google Scholar] [CrossRef]
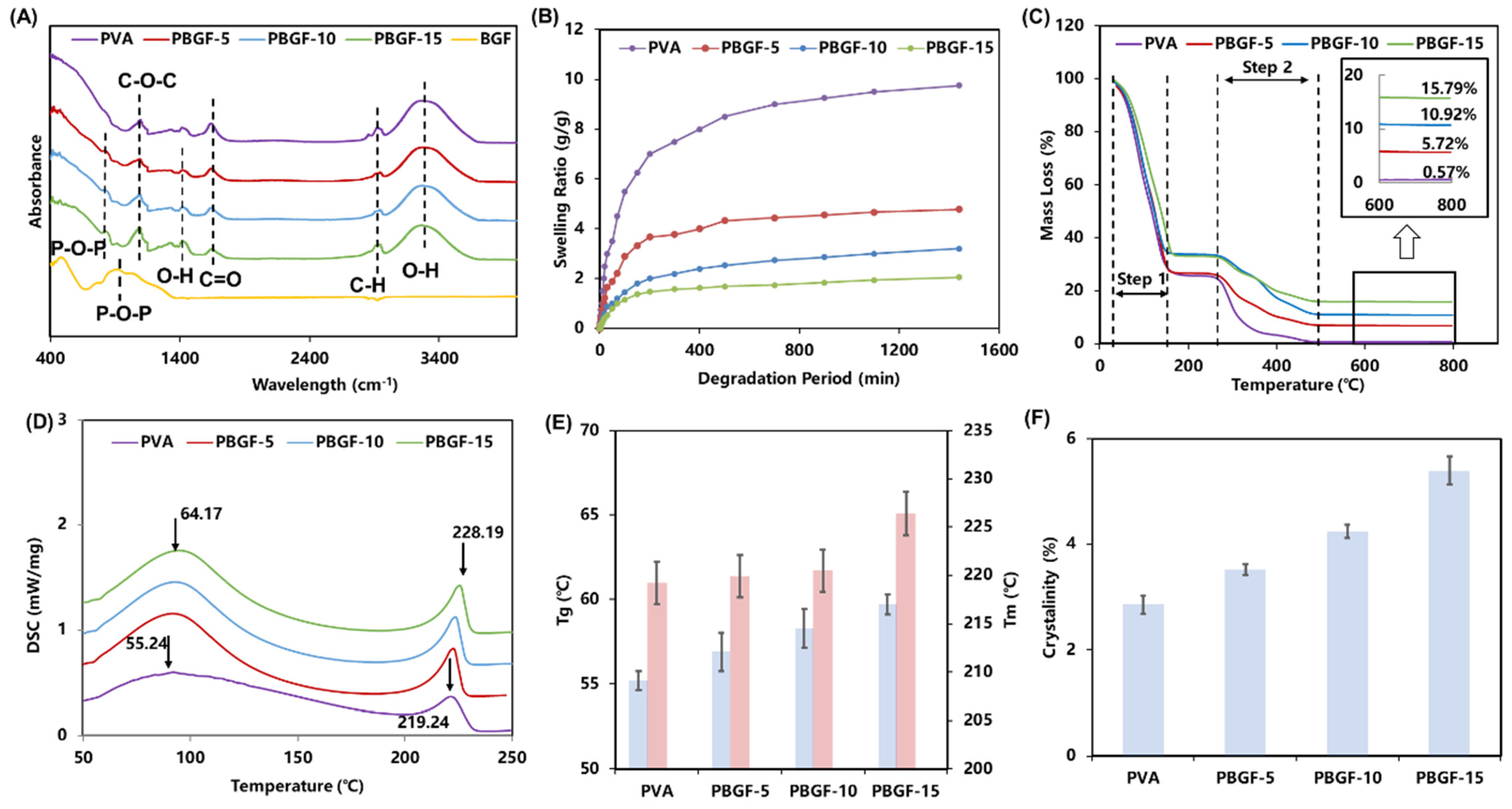
| Functional Group | Wavenumber (cm−1) | Note | Ref |
|---|---|---|---|
| P-O-P | 750–880 | The symmetric mode of P-O-P in biodegradable glass fiber | [23] |
| C-O-C | 1143 | The C-O-C stretching vibration of PVA | [19,20] |
| O-H | 1300–1380 | The stretching vibration of the H-bond for interface | [24,25] |
| C=O | 1750 | The C=O stretching vibration of PVA matrix | [22] |
| C-H | 2920 | The C-H stretching vibration of PVA matrix | [21] |
| O-H | 3100–3600 | The stretching vibration of the H-bond for PVA matrix | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Huang, C.; Zhang, W.; Ding, X.; Yang, Y. Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 8717. https://doi.org/10.3390/ijms23158717
Zhu C, Huang C, Zhang W, Ding X, Yang Y. Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair. International Journal of Molecular Sciences. 2022; 23(15):8717. https://doi.org/10.3390/ijms23158717
Chicago/Turabian StyleZhu, Chenkai, Changyong Huang, Wuxiang Zhang, Xilun Ding, and Yang Yang. 2022. "Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair" International Journal of Molecular Sciences 23, no. 15: 8717. https://doi.org/10.3390/ijms23158717
APA StyleZhu, C., Huang, C., Zhang, W., Ding, X., & Yang, Y. (2022). Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair. International Journal of Molecular Sciences, 23(15), 8717. https://doi.org/10.3390/ijms23158717






