High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype
Abstract
1. Introduction
2. Results
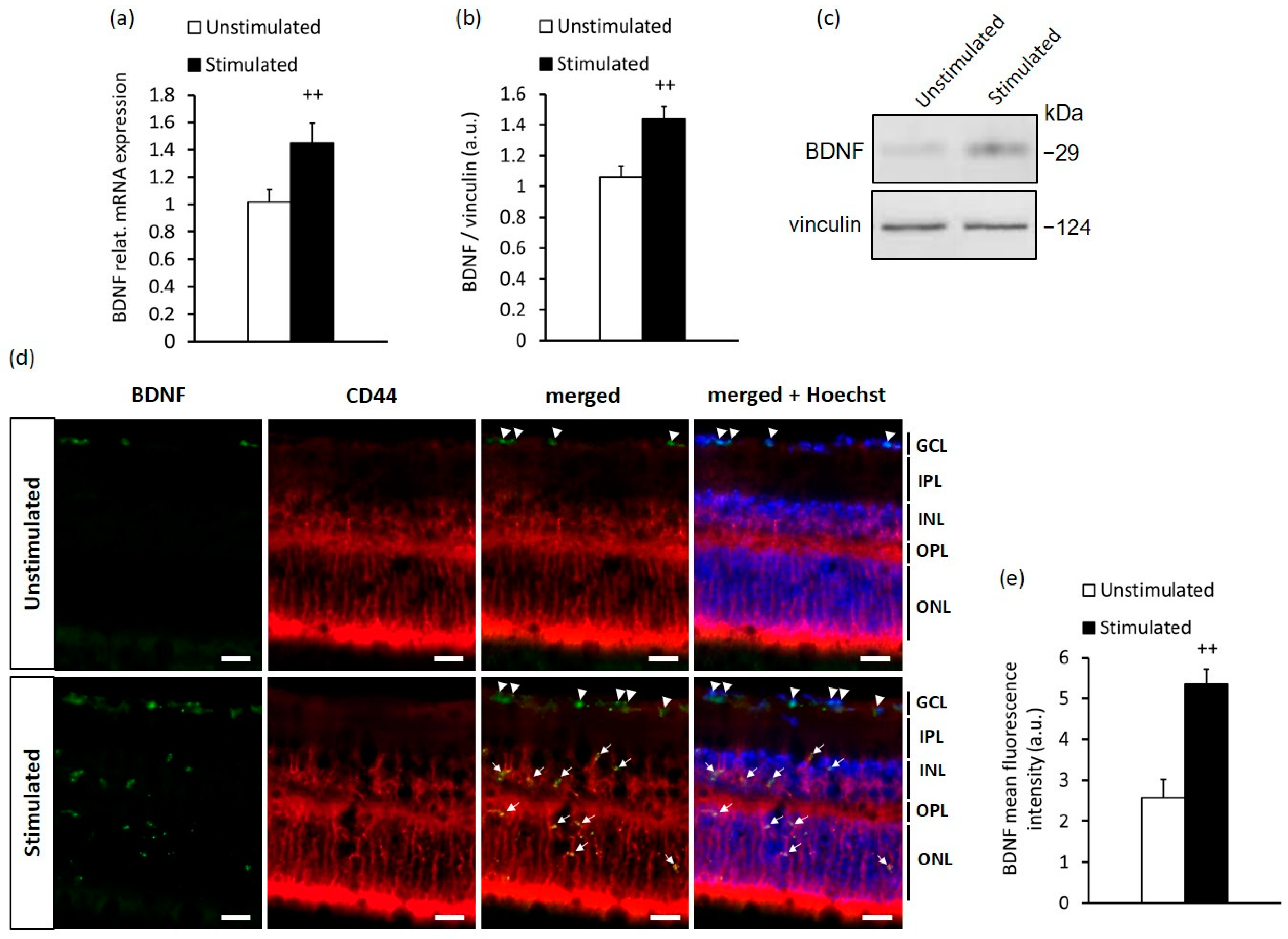
2.1. High-Contrast Stimulation of Adult Mice Induces BDNF Expression in MCs
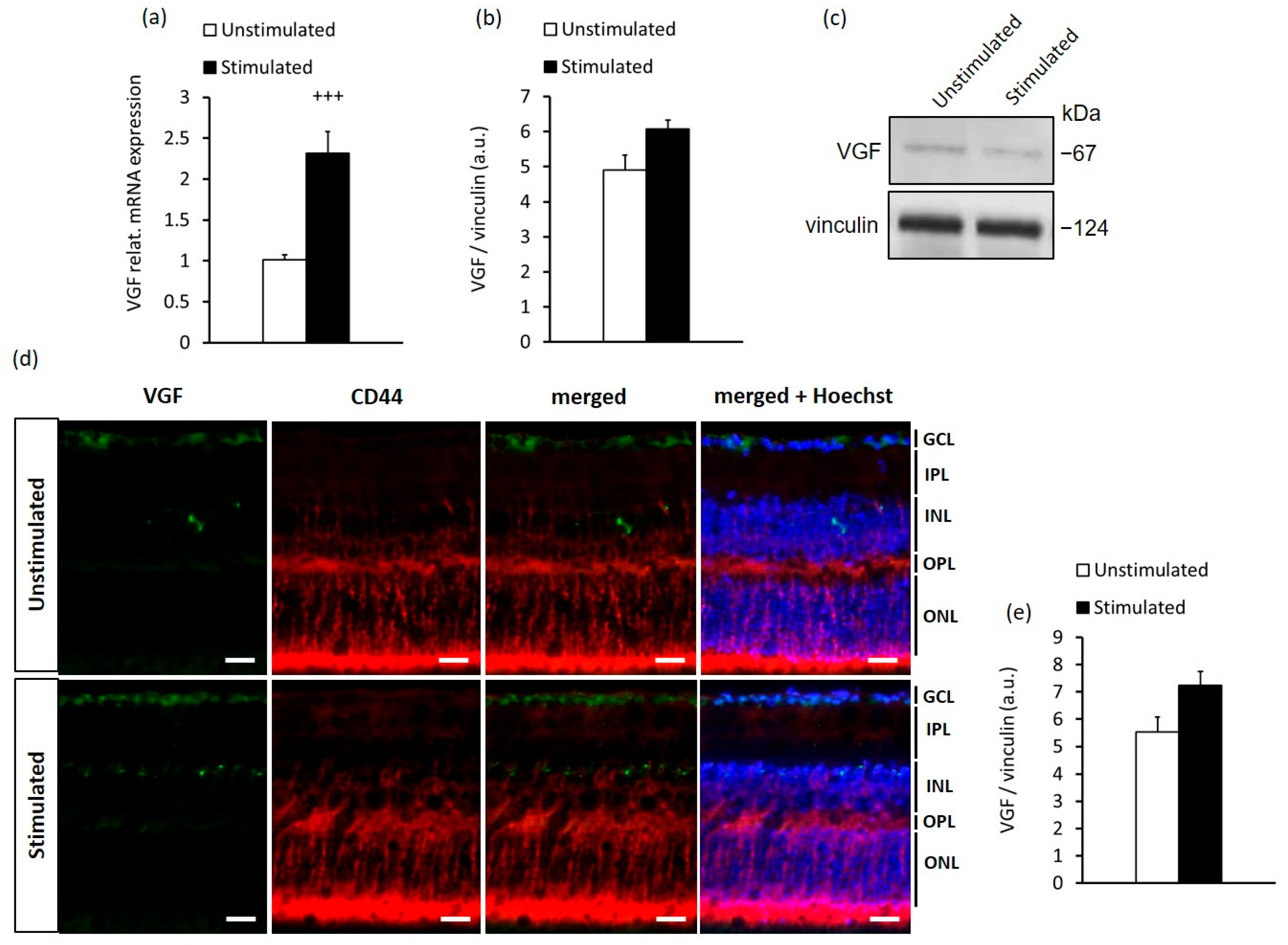
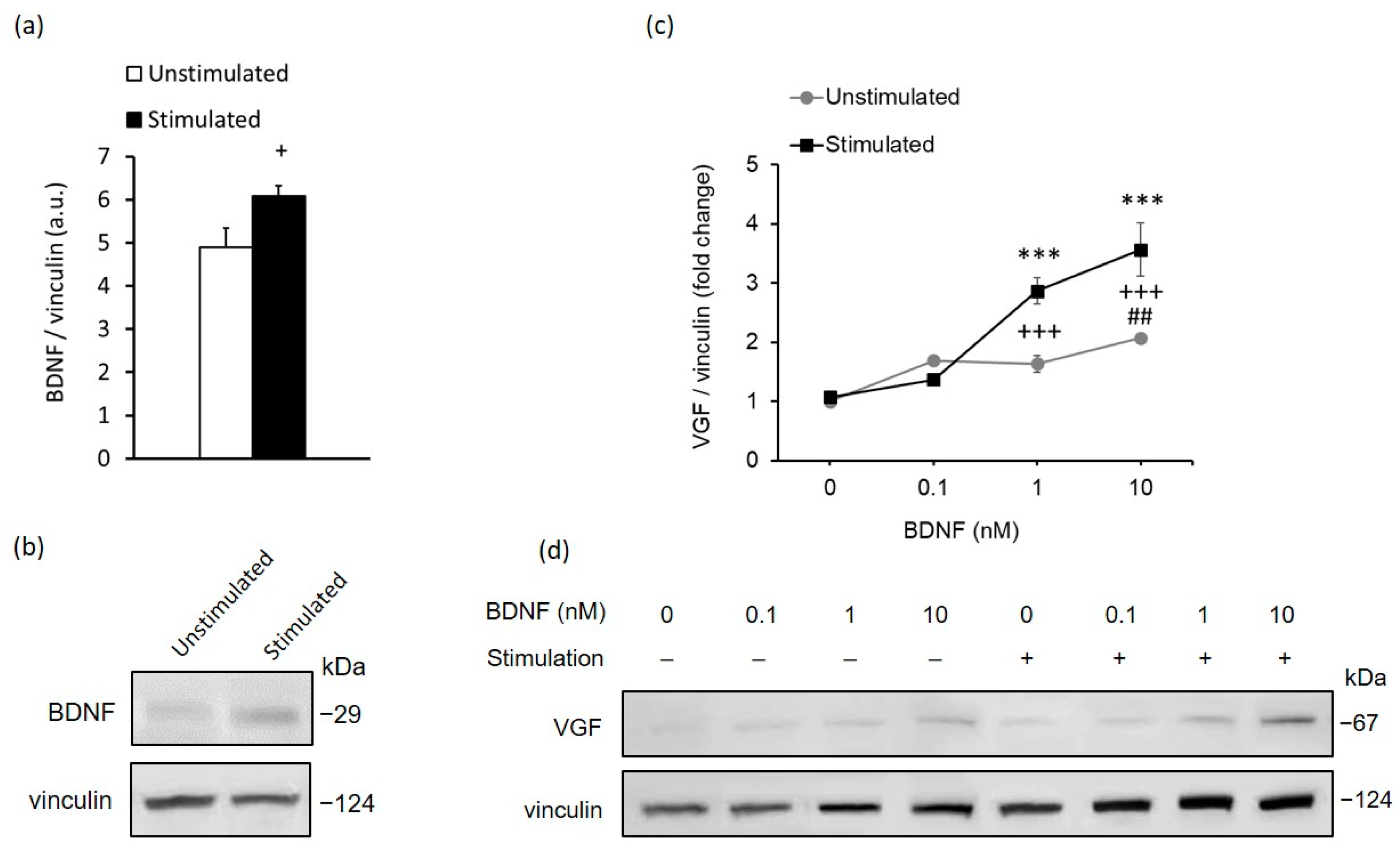
2.2. In Vitro Stimulation of MCs Recapitulates the In Vivo Response to High-Contrast Stimulation of BDNF and VGF Expression in the Retina
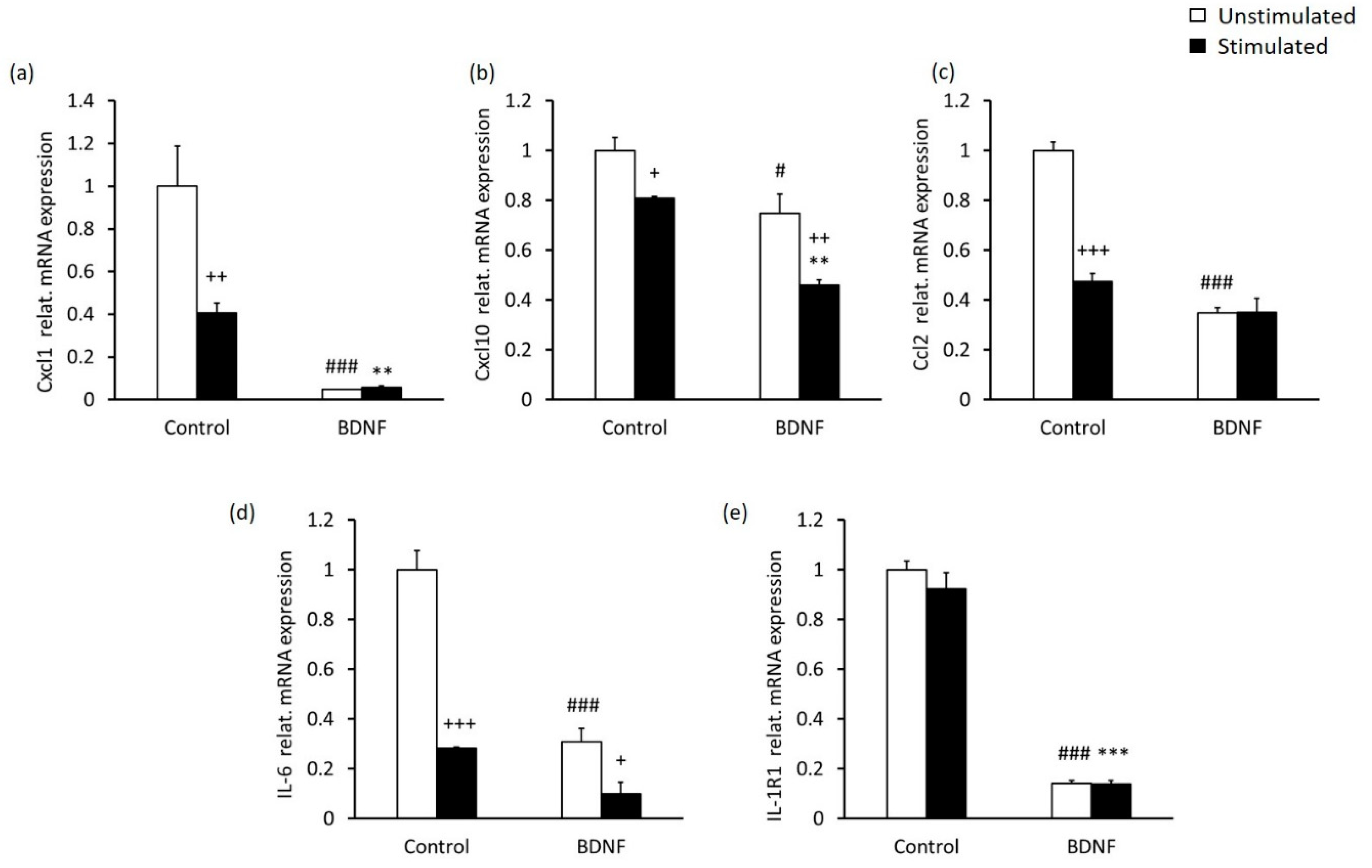
2.3. High-Contrast Stimulation and BDNF Treatment Promote Neurodifferentiation Potential and Suppress the Pro-Inflammatory Phenotype of MCs
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. High-Contrast Stimulation in Mice
4.4. High-Contrast Stimulation of Müller Cells
4.5. Protein Isolation and Western Blotting
4.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.7. Immunohistochemistry
4.8. Flow Cytometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izumi, Y.; Kirby, C.O.; Beny, A.M.; Olney, J.W.; Zorumski, C.F. Müller cell swelling, glutamate uptake, and excitotoxic neurodegeneration in the isolated rat retina. Glia 1999, 25, 379–389. [Google Scholar] [CrossRef]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front. Endocrinol. (Lousanne) 2013, 4, 48. [Google Scholar] [CrossRef]
- Poitry Yamate, C.L.; Poitry, S.; Tasacopoulos, M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [CrossRef] [PubMed]
- Perezleon, J.A.; Osorio-Paz, I.; Francois, L.; Salceda, R. Immunohistochemical localization of glycogen synthase and GSK3β: Control of glycogen content in retina. Neurochem. Res. 2013, 38, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.L.; Marc, R.E.; Kondo, M.; Terasaki, H.; Jones, B.W. Müller cell metabolic chaos during retinal degeneration. Exp. Eye Res. 2016, 150, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Morshedian, A.; Kaylor, J.J.; Ng, S.Y.; Tsan, A.; Frederiksen, R.; Xu, T.; Yuan, L.; Sampath, A.P.; Radu, R.A.; Fain, G.L.; et al. Light-Driven Regeneration of Cone Visual Pigments through a Mechanism Involving RGR Opsin in Müller Glial Cells. Neuron 2019, 102, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, T.; Fimbel, S.M.; Mallory, D.E.; Maaswinkel, H.; Spritzer, S.D.; Sand, J.A.; Li, L.; Hyde, D.R.; Stenkamp, D.L. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 2007, 68, 166–181. [Google Scholar] [CrossRef]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef]
- Lourenco, R.; Brandao, A.S.; Borbinha, J.; Gorgulho, R.; Jacino, A. Yap Regulates Müller Glia Reprogramming in Damaged Zebrafish Retinas. Front. Cell Dev. Biol. 2021, 9, 667796. [Google Scholar] [CrossRef]
- Parrilla, M.; Lillo, C.; Herrero-Turrion, M.J.; Arevalo, R.A. Pax2+ astrocytes in the fish optic nerve head after optic nerve crush. Brain Res. 2013, 1492, 18–32. [Google Scholar] [CrossRef]
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; van Houcke, J.; van Ham, T.J.; De Groef, L.; Andreis, L.; Moons, L. Müller glia-myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463. [Google Scholar] [CrossRef] [PubMed]
- Ooto, S.; Akagi, T.; Kageyama, R.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, J.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2015, 64, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Tian, C.Y.; Yin, Z.Q. Activation of Müller cells occurs during retinal degeneration in RCS rats. Adv. Exp. Med. Biol. 2010, 664, 575–583. [Google Scholar] [CrossRef]
- Cotinet, A.; Goureau, O.; Thilaye-Goldenberg, B.; Naud, M.C.; de Kozak, Y. Differential tumor necrosis factor and nitric oxide production in retinal Müller glial cells from C3H/HeN and C3H/HeJ mice. Ocul. Immunol. Inflamm. 1997, 5, 111–116. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 483–491. [Google Scholar] [CrossRef]
- Kirschenbaum, B.; Goldman, S.A. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. USA 1995, 92, 210–214. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Bennett, J.L.; Zeiler, S.R.; Jones, K.R. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2996–3005. [Google Scholar]
- Tworig, J.M.; Feller, M.B. Müller Glia in Retinal Development: From Specification to Circuit Integration. Front. Neural Circuits 2021, 15, 815923. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many singalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Seki, M.; Nawa, H.; Fukuchi, T.; Abe, H.; Takei, N. BDNF is Upregulated by Postnatal Development and Visual Experience: Quantitative and Immunohistochemical Analyses of BDNF in the Rat Retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3211–3218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oku, H.; Ikeda, T.; Honma, Y.; Sotozono, C.; Nishida, K.; Nakamura, Y.; Kida, Y.; Kinoshita, S. Gene Expression of Neurotrophins and Their High-Affinity Trk Receptors in Cultured Human Müller Cells. Ophthalmic Res. 2002, 34, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ghazi-Nouri, S.M.; Ellis, J.S.; Moss, S.; Limb, G.A.; Charteris, D.G. Expression and localisation of BDNF, NT4 and TrkB in proliferative vitreoretinopathy. Exp. Eye Res. 2008, 86, 819–827. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Bahr, M. The upregulation of GLAST-1 is an indirectantiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Differ. 2008, 15, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Lom, B.; Cohen-Cory, S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J. Neurosci. 1999, 19, 9928–9938. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Forster, V.; Hicks, D.; Vecino, E. Effects of Müller Glia on Cell Survival and Neuritogenesis in Adult Porcine Retina In Vitro. Investig. Ophthalmol. Vis. Sci. 2002, 48, 3735–3743. [Google Scholar]
- Hu, L.M.; Luo, Y.; Zhang, J.; Lei, X.; Shen, J.; Wu, Y.; Qin, M.; Unver, Y.B.; Zhong, Y.; Xu, G.T.; et al. EPO reduces reactive gliosis and stimulates neurotrophin expression in Muller Cells. Front. Biosci. 2011, 3, 1541–1555. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.; Li, N.; Wang, Y.; Gao, S.; Wang, J.; Shen, X. Regulation of inflammation by VEGF/BDNF signaling in mouse retinal Müller glial cells exposed to high glucose. Cell Tissue Res. 2022, 388, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Domenici, L.; Origlia, N.; Falsini, B.; Cerri, E.; Barloscio, D.; Fabiani, C.; Sanso, M.; Giovannini, L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS ONE 2014, 9, e115579. [Google Scholar] [CrossRef][Green Version]
- Cerri, E.; Origlia, N.; Falsini, B.; Barloscio, D.; Fabiani, C.; Sanso, M.; Ottino, S.; Giovannini, L.; Domenici, L. Conjunctivally Applied BDNF Protects Photoreceptors from Light-Induced Damage. Investig. Ophthalmol. Vis. Sci. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Tanaka, T.; Sakai, Y.; Fukuchi, T.; Abe, H.; Nawa, H.; Takei, N. Muller Cells as a source of brain-derived neurotrophic factor in the retina: Noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat. Muller Cells Neurochem. Res. 2005, 30, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Mui, A.M.; Yang, V.; Aung, M.H.; Fu, J.; Adekunle, A.N.; Prall, B.C.; Sidhu, C.S.; Park, H.N.; Boatright, J.H.; Iuvone, P.M.; et al. Daily visual stimulation in the critical period enhances multiple aspects of vision through BDNF-mediated pathways in the mouse retina. PLoS ONE 2018, 13, e0192435. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Fujikado, T.; Lee, T.S.; Tano, Y. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Ni, Y.Q.; Jin, Z.B.; Zhang, N.; Wu, J.H.; Zhu, Y.; Xu, G.Z.; Gan, D.K. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller Cells. Exp. Neurol. 2012, 238, 192–208. [Google Scholar] [CrossRef]
- Shinoe, T.; Kuribayashi, H.; Saya, H.; Seiki, M.; Aburatani, H.; Watanabe, S. Identification of CD44 as a cell surface marker for Müller glia precursor Cells. J. Neurochem. 2010, 115, 1633–1642. [Google Scholar] [CrossRef]
- Too, L.K.; Gracie, G.; Hasic, E.; Iwakura, J.H.; Cherepanoff, S. Adult human retinal Müller glia display distinct peripheral and macular expression of CD117 and CD44 stem cell-associated proteins. Acta Histochem. 2017, 119, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Ru, J.; Ma, W.; Gao, Y.; Liang, Z.; Liu, J.; Guo, J.H.; Li, L.Y. BDNF promotes the growth of human neurons through crosstalk with the Wnt/β-catenin signaling pathway via GSK-3β. Neuropeptides 2015, 54, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Thakker-Varia, S.; Bangasser, D.A.; Kuroiwa, M.; Plummer, M.R.; Shors, T.J.; Black, I.B. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J. Neurosci. 2003, 23, 10800–10808. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Rich, E.; Tronel, S.; Sadahiro, M.; Patterson, K.; Shapiro, M.L.; Alberini, C.M.; Huntley, G.W.; Salton, S.R. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci. 2008, 28, 9857–9869. [Google Scholar] [CrossRef]
- Takeuchi, H.; Inagaki, S.; Morozumi, W.; Nakano, Y.; Inoue, Y.; Kuse, Y.; Mizoguchi, T.; Nakamura, S.; Funato, M.; Kaneko, H.; et al. VGF nerve growth factor inducible is involved in retinal ganglion cells death induced by optic nerve crush. Sci. Rep. 2018, 8, 16443. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.N.; Marchese, N.A.; Guido, M.E. Expression of Non-visual Opsins Opn3 and Opn5 in the Developing Inner Retinal Cells of Birds. Light Responses in Muller Glial Cells. Front. Cell Neurosci. 2019, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M.; Hyde, D.R.; Masai, I. TNFα Induces Müller Glia to Transition from Non-proliferative Gliosis to a Regenerative Response in Mutant Zebrafish Presenting Chronic Photoreceptor Degeneration. Front. Cell Dev. Biol. 2019, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Shimozaki, K. Sox2 transcription network acts as a molecular switch to regulate properties of neural stem Cells. World J. Stem Cells 2014, 6, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Xiang, A.P.; Mao, F.F.; Zhang, L.; Di, C.G.; Liu, X.M.; Shao, Y.; Ma, B.F.; Lee, J.H.; Ha, K.S.; et al. Nestin is required for the proper self-renewal of neural stem Cells. Stem Cells 2010, 28, 2162–2171. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K.W. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2016, 38, 364–374. [Google Scholar] [CrossRef]
- Rothaeusler, K.; Baumgarth, N. Assessment of cell proliferation by 5-bromodeoxyuridine (BrdU) labeling for multicolor flow cytometry. Curr. Protoc. Cytom. 2007, 7, 31. [Google Scholar] [CrossRef]
- Brambilla, R.; Bracchi-Ricard, V.; Hu, W.H.; Frydel, B.; Bramwell, A.; Karmally, S.; Green, E.J.; Bethea, J.R. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005, 202, 145–156. [Google Scholar] [CrossRef]
- Sticozzi, C.; Belmonte, G.; Meini, A.; Carbotti, P.; Grasso, G.; Palmi, M. IL-1β induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFκB/Ca²⁺-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience 2013, 252, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Trindade, P.; Loiola, E.C.; Gasparotto, J.; Ribeiro, C.T.; Cardozo, P.L.; Devalle, S.; Salerno, J.A.; Ornelas, I.M.; Ledur, P.F.; Ribeiro, F.M.; et al. Short and long TNF-alpha exposure recapitulates canonical astrogliosis events in human-induced pluripotent stem cells-derived astrocytes. Glia 2020, 68, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Palazzp, I.; Suarez, L.; Liu, X.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 2019, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Stafford, B.K.; Nguyen, P.L.; Lien, B.V.; Wang, C.; Zukor, K.; He, Z.; Huberman, A.D. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 2016, 19, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Marchese, N.A.; Rios, M.N.; Guido, M.E. The Intrinsic Blue Light Responses of Avian Muller Glial Cells Imply Calcium Release from Internal Stores. ASN Neuro. 2022, 14, 17590914221076698. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Hallbook, F. Kainic acid, tetrodotoxin and light modulate expression of brain-derived neurotrophic factor in developing avian retinal ganglion cells and their tectal target. Neuroscience 1998, 83, 137–150. [Google Scholar] [CrossRef]
- Pollock, G.S.; Vernon, E.; Forbes, M.E.; Yan, Q.; Ma, Y.T.; Hsieh, T.; Robichon, R.; Frost, D.O.; Johnson, J.E. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J. Neurosci. 2001, 21, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, H.; Sasaki, H. Expression of brain-derived neurotrophic factor in cholinergic and dopaminergic amacrine cells in the rat retina and the effects of constant light rearing. Exp. Eye Res. 2008, 86, 335–343. [Google Scholar] [CrossRef]
- Enayati, S.; Chang, K.; Achour, H.; Cho, K.S.; Xu, F.; Guo, S.; Enayati, Z.K.; Xie, J.; Zhao, E.; Turunen, T.; et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells 2020, 9, 781. [Google Scholar] [CrossRef]
- Levi, A.; Ferri, G.L.; Watson, E.; Possenti, R.; Salton, S.R.J. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol. Neurobiol. 2004, 24, 517–533. [Google Scholar] [CrossRef]
- Lin, W.J.; Zhao, Y.Z.; Zheng, S.; Zou, J.J.; Warren, N.A.; Bali, P.; Wu, J.; Xing, M.; Jiang, C.; Tang, Y.; et al. An increase in VGF expression through a rapid, transcription-independent, autofeedback mechanism improves cognitive function. Transl. Psychiatry 2021, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Ventimiglia, R.; Anderson, K.; Lindsay, R.M.; Rudge, J.S. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur. J. Neurosci. 1996, 8, 1220–1230. [Google Scholar] [CrossRef]
- Thakker-Varia, S.; Jernstedt Krol, J.; Nettleton, J.L.; Bilimoria, P.M.; Bangasser, D.A.; Shors, T.J.; Black, R.B.; Alder, J. The Neuropeptide VGF Produces Antidepressant-Like Behavioral Effects and Enhances Proliferation in the Hippocampus. J. Neurosci. 2007, 27, 12156–12167. [Google Scholar] [CrossRef] [PubMed]
- Graca, A.B.; Hippert, C.; Pearson, R.A. Müller Glia Reactivity and Development of Gliosis in Response to Pathological Conditions. Adv. Exp. Med. Biol. 2018, 1074, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Linberg, K.A.; Geller, S.F.; Guérin, C.J.; Fisher, S.K. Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest. Ophthalmol Vis. Sci. 1999, 40, 153015–153044. [Google Scholar]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Stofkova, A.; Zloh, M.; Andreanska, D.; Fiserova, I.; Kubovciak, J.; Hejda, J.; Kutilek, P.; Murakami, M. Depletion of retinal dopaminergic activity in a mouse model of rod dysfunction exacerbates experimental autoimmune uveoretinitis: A role for the gateway reflex. Int. J. Mol. Sci. 2021, 23, 453. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zloh, M.; Kutilek, P.; Stofkova, A. High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype. Int. J. Mol. Sci. 2022, 23, 8615. https://doi.org/10.3390/ijms23158615
Zloh M, Kutilek P, Stofkova A. High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype. International Journal of Molecular Sciences. 2022; 23(15):8615. https://doi.org/10.3390/ijms23158615
Chicago/Turabian StyleZloh, Miloslav, Patrik Kutilek, and Andrea Stofkova. 2022. "High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype" International Journal of Molecular Sciences 23, no. 15: 8615. https://doi.org/10.3390/ijms23158615
APA StyleZloh, M., Kutilek, P., & Stofkova, A. (2022). High-Contrast Stimulation Potentiates the Neurotrophic Properties of Müller Cells and Suppresses Their Pro-Inflammatory Phenotype. International Journal of Molecular Sciences, 23(15), 8615. https://doi.org/10.3390/ijms23158615





