High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice
Abstract
1. Introduction
2. Results
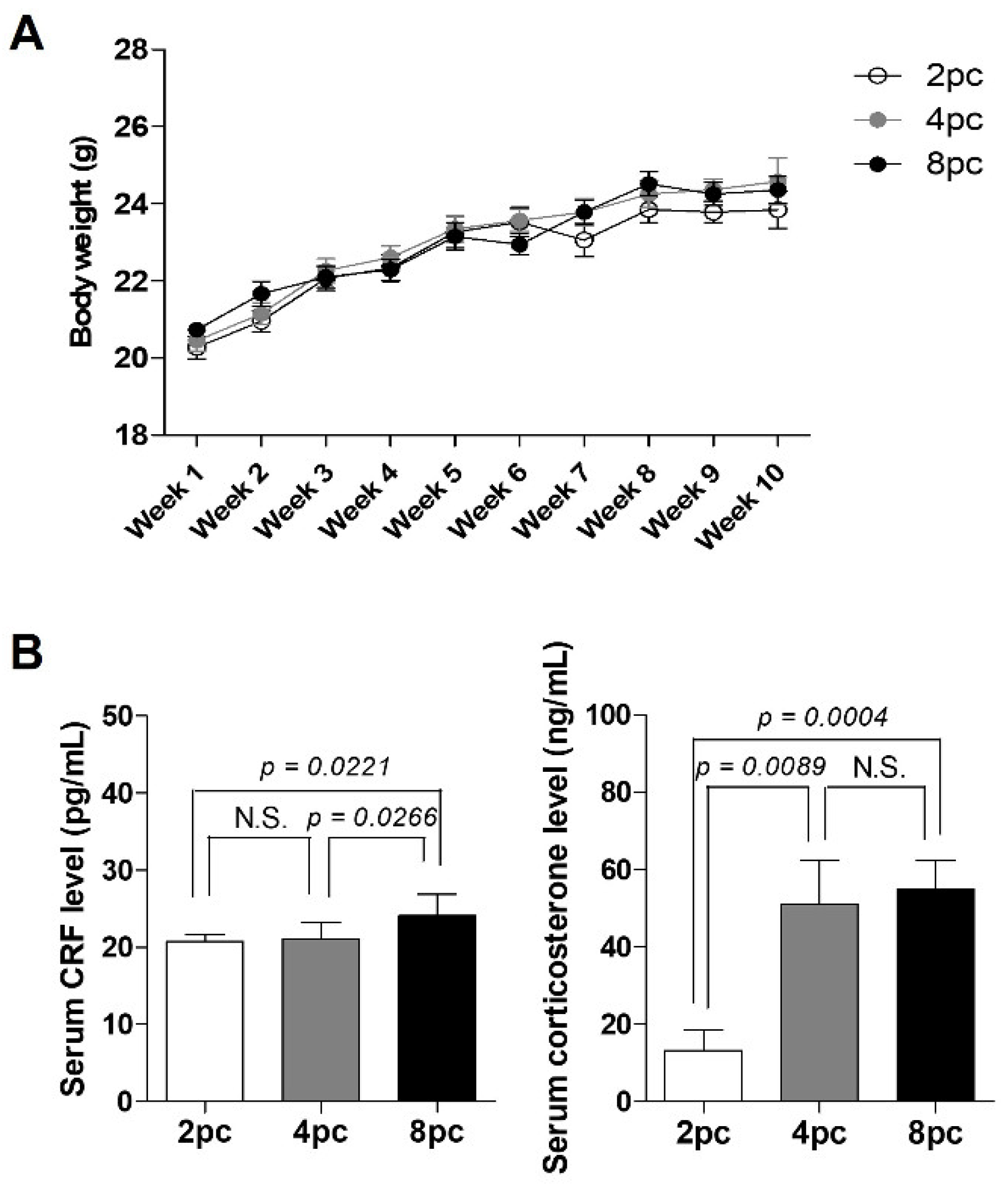
2.1. High Housing Density Induces Physiological Stress without Weight Loss
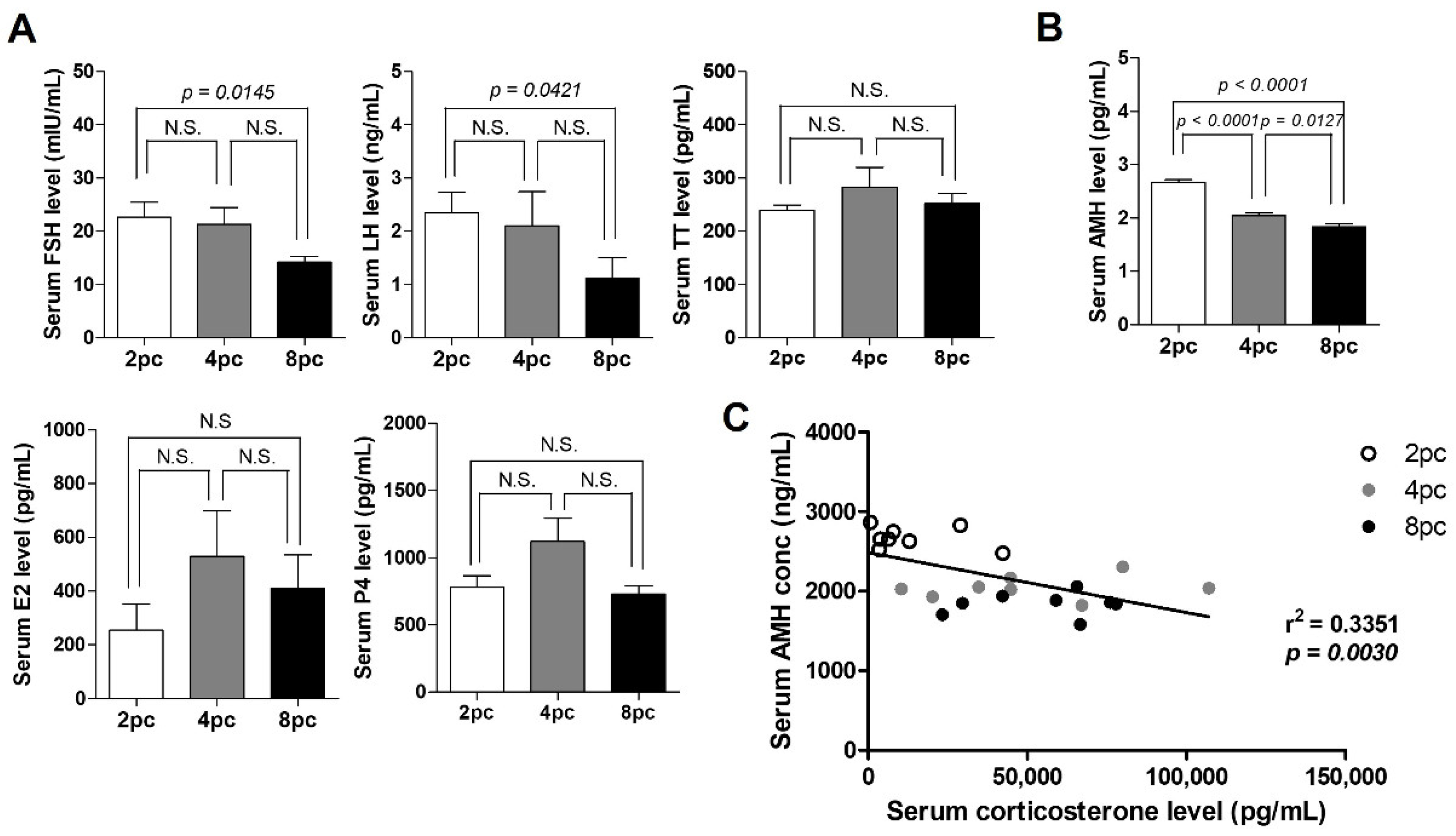
2.2. High Housing Density Induces Hormonal Imbalance
2.3. High Housing Density Induces Follicle Loss
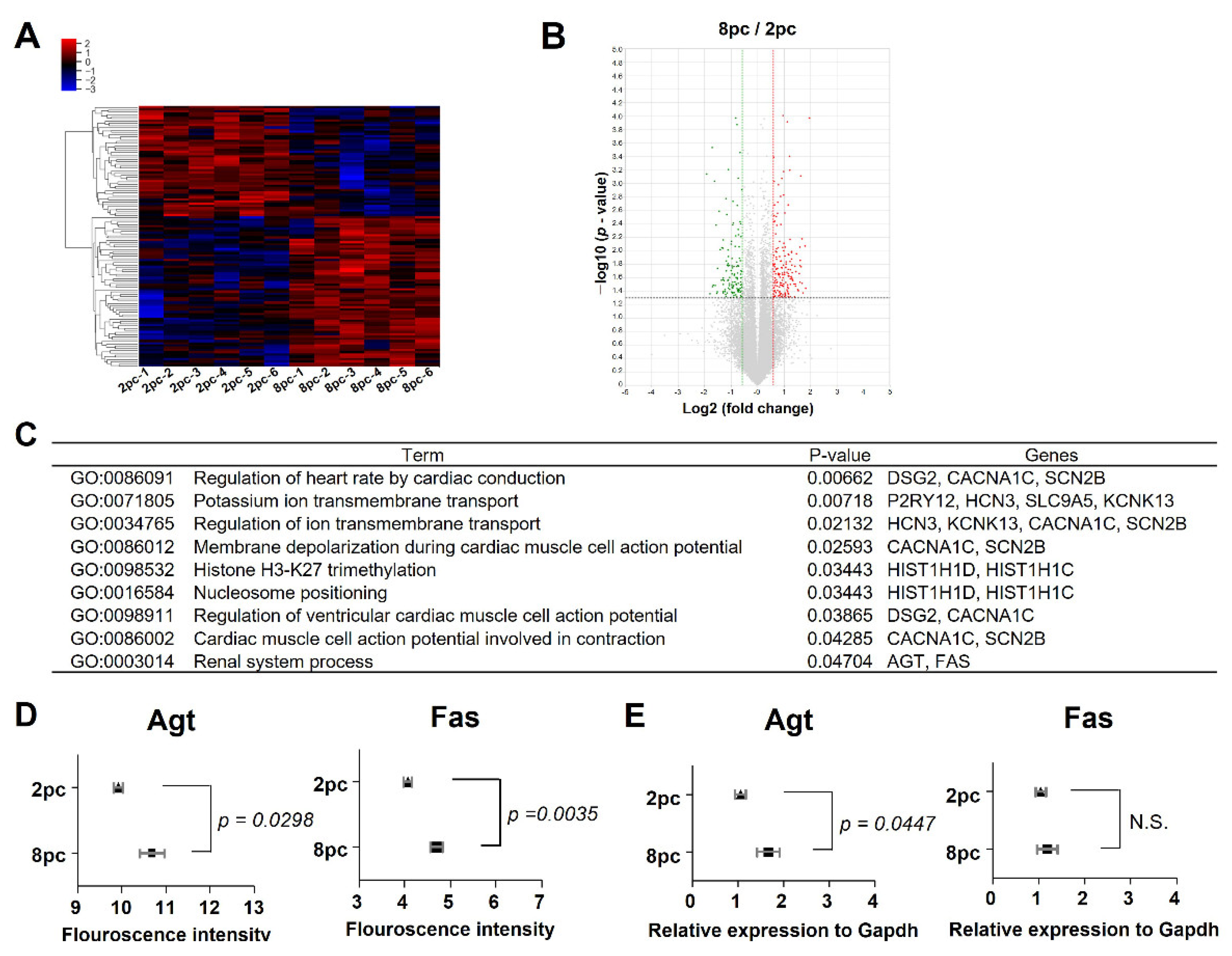
2.4. High Housing Density Induces Changes in Ovarian mRNA Expression
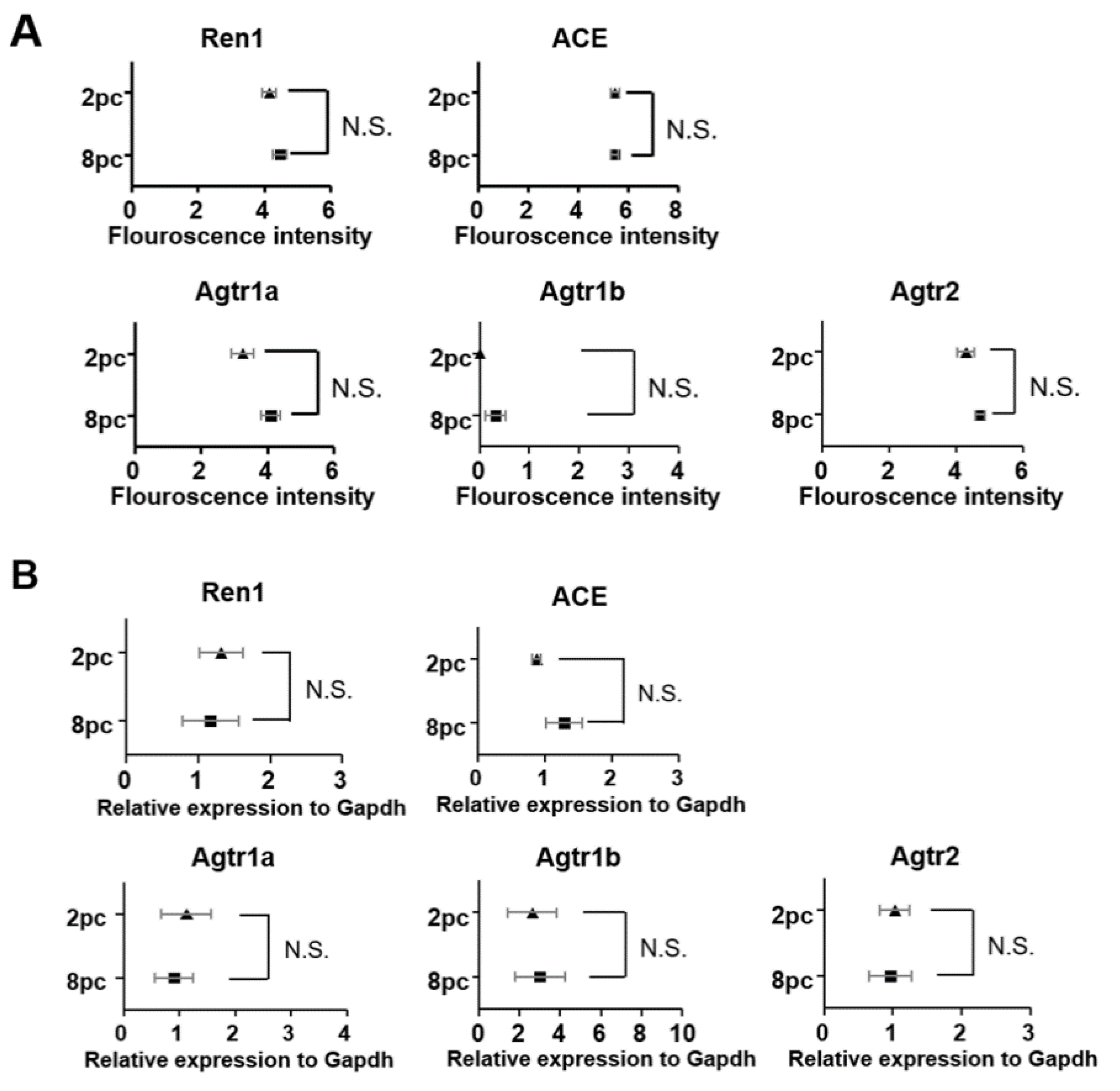
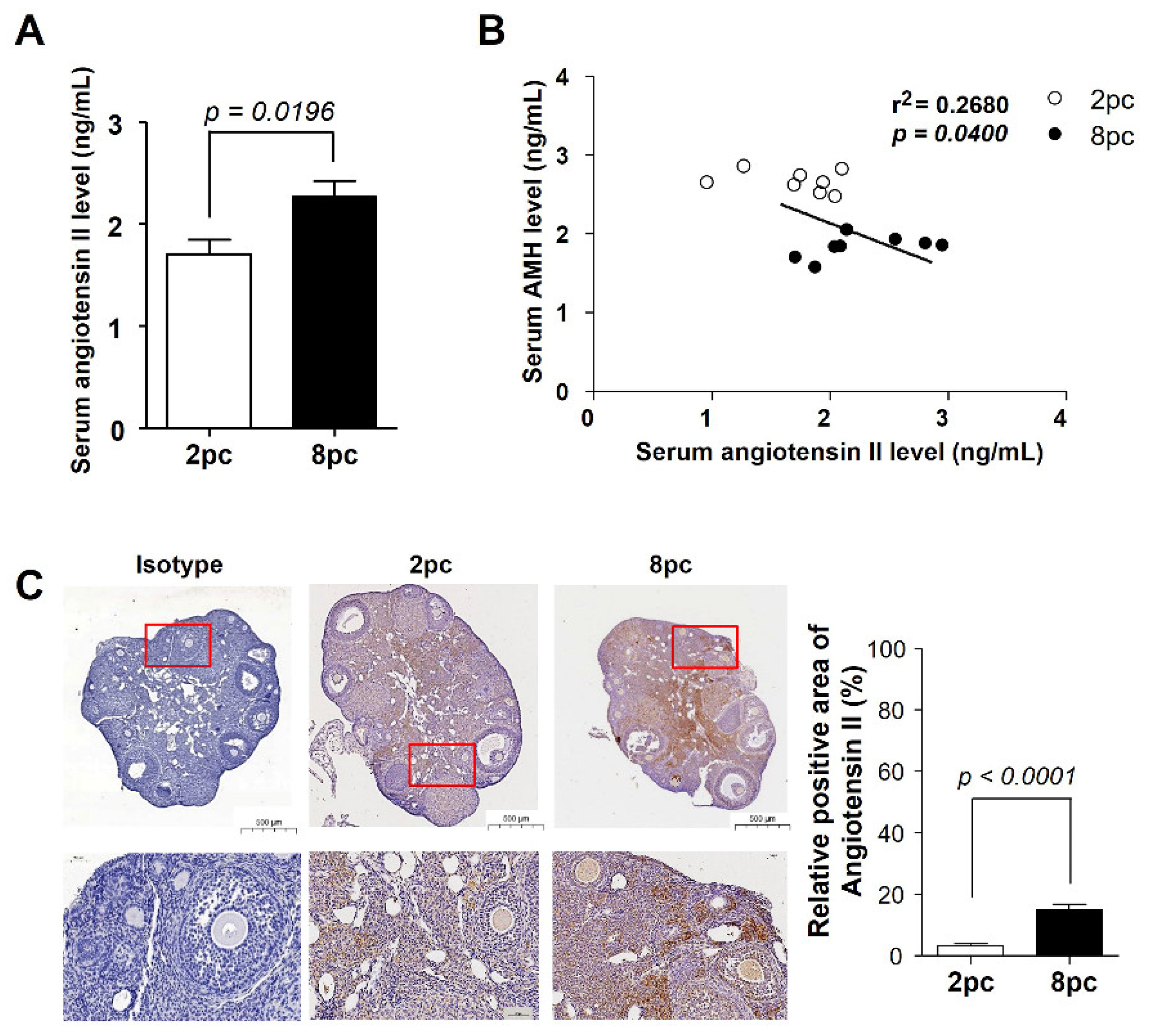
2.5. Housing Density Induces Changes in the Ovarian Renin-Angiotensin System
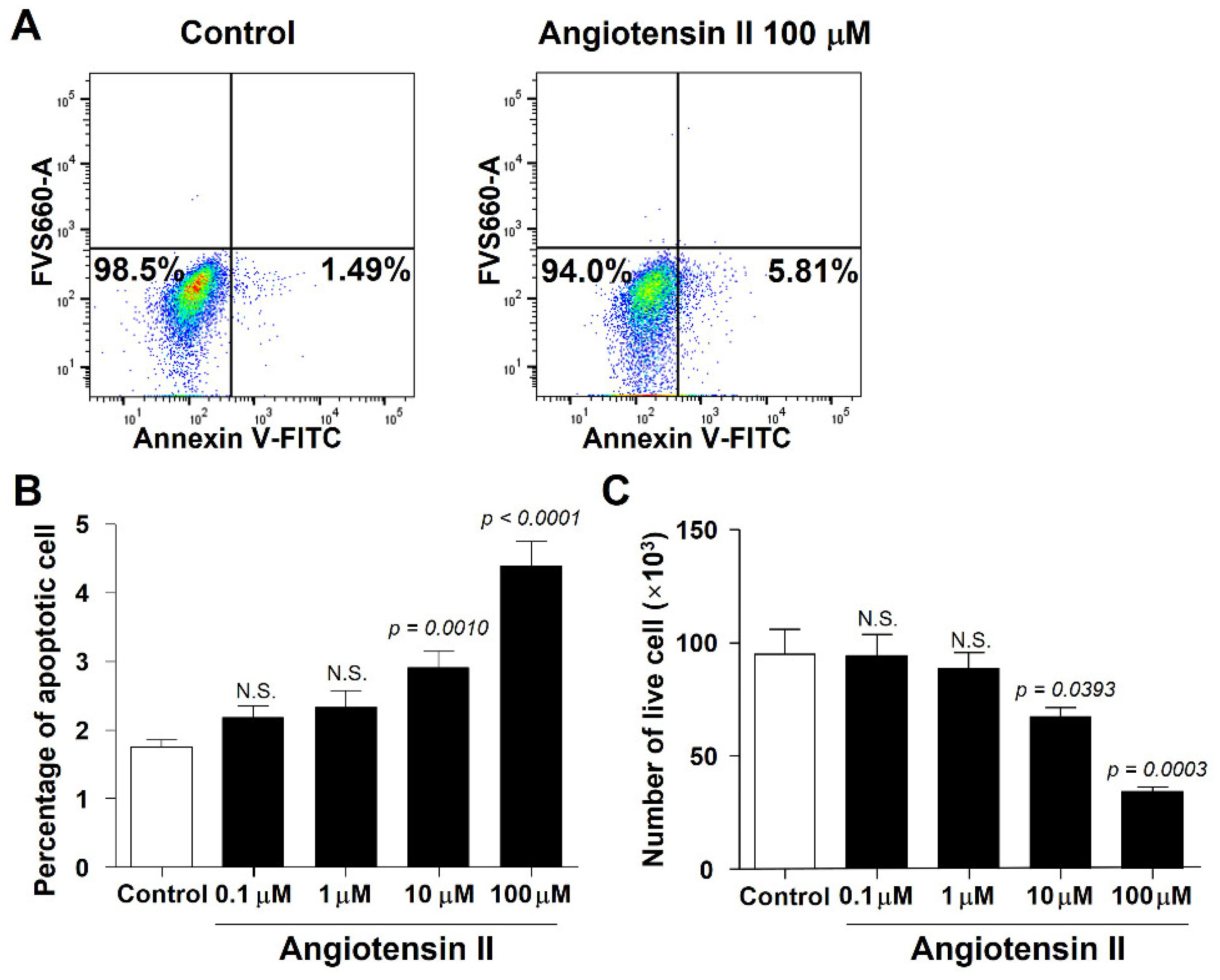
2.6. Angiotensin II Induces Granulosa Cell Apoptosis In Vitro
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Enzyme-Linked Immunosorbent Assay for Hormone Assessment
4.3. Histological Assessment of Ovarian Follicles
4.4. RNA-Seq for mRNA Expression and Functional Annotation Analysis
4.5. Validation of Selected DEGs in the Ovaries
4.6. Immunohistochemical Observation of the Ovaries
4.7. Culture of Human Granulosa Cell Line
4.8. Flow Cytometry of the Human Granulosa Cell Line
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, K.; Sheps, S.; Arck, P.C. Stress and reproductive failure: Past notions, present insights and future directions. J. Assist. Reprod. Genet. 2008, 25, 47–62. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef] [PubMed]
- Karin, O.; Raz, M.; Tendler, A.; Bar, A.; Kohanim, Y.K.; Milo, T.; Alon, U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol. Syst. Biol. 2020, 16, e9510. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.-Y.; Wang, J.-J.; Tian, Y.; Liu, X.; Song, Z. Review of psychological stress on oocyte and early embryonic development in female mice. Reprod. Biol. Endocrinol. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Ann. d’Endocrinol. 2010, 71, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-Z.; Zhou, F.-J.; Sun, Y.-P. Psychological stress is related to a decrease of serum anti-müllerian hormone level in infertile women. Reprod. Biol. Endocrinol. 2017, 15, 51. [Google Scholar] [CrossRef]
- Pankhurst, M.W. A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J. Endocrinol. 2017, 233, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva. Endocrinol. 2010, 35, 109–126. [Google Scholar] [PubMed]
- Whirledge, S.; Cidlowski, J.A. A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology 2013, 154, 4450–4468. [Google Scholar] [CrossRef]
- Kala, M.; Nivsarkar, M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen. Comp. Endocrinol. 2016, 225, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, W.; De Kloet, E.R. The use of various animal models in the study of stress and stress-related phenomena. Lab. Anim. 1994, 28, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, J.; Wang, Q.; Zhang, Q.; Wei, C.; Lai, D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS ONE 2018, 13, e0194894. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-A.; Obora, T.; Bondonny, L.; Toniolo, A.; Mivielle, J.; Yamaguchi, Y.; Kato, A.; Takita, M.; Goto, Y. The effects of housing density on social interactions and their correlations with serotonin in rodents and primates. Sci. Rep. 2018, 8, 3497. [Google Scholar] [CrossRef] [PubMed]
- Fullwood, S.; Hicks, T.A.; Brown, J.C.; Norman, R.L.; McGlone, J.J. Floor Space needs for laboratory mice: C57BL/6 males in solid-bottom cages with bedding. ILAR J. 1998, 39, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.J.; Grunberg, N.E. Effects of housing on male and female rats: Crowding stresses male but calm females. Physiol. Behav. 1995, 58, 1085–1089. [Google Scholar] [CrossRef]
- Yoshimura, Y. The ovarian renin–angiotensin system in reproductive physiology. Front. Neuroendocr. 1997, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Do results obtained with RNA-sequencing require independent verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef]
- Evans, G.W.; Palsane, M.N.; Lepore, S.J.; Martin, J. Residential density and psychological health: The mediating effects of social support. J. Pers. Soc. Psychol. 1989, 57, 994–999. [Google Scholar] [CrossRef]
- Schweitzer, L.; Su, W.H. Population density and the rate of mental illness. Am. J. Public Health 1977, 67, 1165–1172. [Google Scholar] [CrossRef][Green Version]
- Whittaker, A.L.; Howarth, G.S.; Hickman, D.L. Effects of space allocation and housing density on measures of wellbeing in la-boratory mice: A review. Lab. Anim. 2012, 46, 3–13. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metab. 2013, 28, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.A.; Johnston, N.A.; Verhulst, S.; Trammell, R.A.; Toth, L.A. Identification of markers for imminent death in mice used in longevity and aging research. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 282–288. [Google Scholar]
- Sakai, S.; Wakasugi, T.; Yagi, K.; Ohnishi, A.; Ito, N.; Takeda, Y.; Yamagishi, M. Successful pregnancy and delivery in a patient with adult GH deficiency: Role of GH replacement therapy. Endocr. J. 2011, 58, 65–68. [Google Scholar] [CrossRef]
- Themmen, A.P.N. Anti-mullerian hormone: Its role in follicular growth initiation and survival and as an ovarian reserve marker. J. Natl. Cancer Inst. Monogr. 2005, 2005, 18–21. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Féral, C.; Le Gall, S.; Leymarie, P. Angiotensin II modulates steroidogenesis in granulosa and theca in the rabbit ovary: Its possible involvement in atresia. Eur. J. Endocrinol. 1995, 133, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Kotani, E.; Sugimoto, M.; Kamata, H.; Fujii, N.; Saitoh, M.; Usuki, S.; Kubo, T.; Song, K.; Miyazaki, M.; Murakami, K.; et al. Biological roles of angiotensin II via its type 2 receptor during rat follicle atresia. Am. J. Physiol. Metab. 1999, 276, E25–E33. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ohnishi, J.; Ozawa, Y.; Sugimoto, M.; Usuki, S.; Naruse, M.; Murakami, K.; Miyazaki, H. Characterization of an-giotensin II receptor type 2 during differentiation and apoptosis of rat ovarian cultured granulosa cells. Biochem. Biophys. Res. Commun. 1995, 207, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.B.; Ferreira, R.; Gasperin, B.; Oliveira, J.F. Role of angiotensin in ovarian follicular development and ovulation in mammals: A review of recent advances. Reproduction 2012, 143, 11–20. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Liu, X.-M.; Nin, L.-F.; Shi, L.; Chen, S.-R. Serine protease and ovarian paracrine factors in regulation of ovulation. Front. Biosci.-Landmark 2013, 18, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lv, H.; Wei, W.; Zhang, D.; Guan, Y. Angiotensin-converting enzyme D/I and plasminogen activator inhibitor-1 4G/5G gene polymorphisms are associated with increased risk of spontaneous abortions in polycystic ovarian syndrome. J. Endocrinol. Investig. 2009, 33, 77–82. [Google Scholar] [CrossRef]
- Peña, O.; Palumbo, A.; González-Fernández, R.; Hernández, J.; Naftolin, F.; Ávila, J. Expression of angiotensin II type 1 (AT1) and angiotensin II type 2 (AT2) receptors in human granulosa-lutein (GL) cells: Correlation with infertility diagnoses. Fertil. Steril. 2010, 93, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Ávila, J.; Naftolin, F. The ovarian renin-angiotensin system (OVRAS): A major factor in ovarian function and disease. Reprod. Sci. 2016, 23, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. Reproduction 1968, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using imageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; You, S. After cyclophosphamide exposure, granulosa cells recover their anti-Müllerian hormone-producing ability but not their numbers. Cytom. A 2020, 99, 807–813. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; You, S. High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice. Int. J. Mol. Sci. 2022, 23, 8614. https://doi.org/10.3390/ijms23158614
Kim J, You S. High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice. International Journal of Molecular Sciences. 2022; 23(15):8614. https://doi.org/10.3390/ijms23158614
Chicago/Turabian StyleKim, Jihyun, and Sooseong You. 2022. "High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice" International Journal of Molecular Sciences 23, no. 15: 8614. https://doi.org/10.3390/ijms23158614
APA StyleKim, J., & You, S. (2022). High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice. International Journal of Molecular Sciences, 23(15), 8614. https://doi.org/10.3390/ijms23158614






