Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies
Abstract
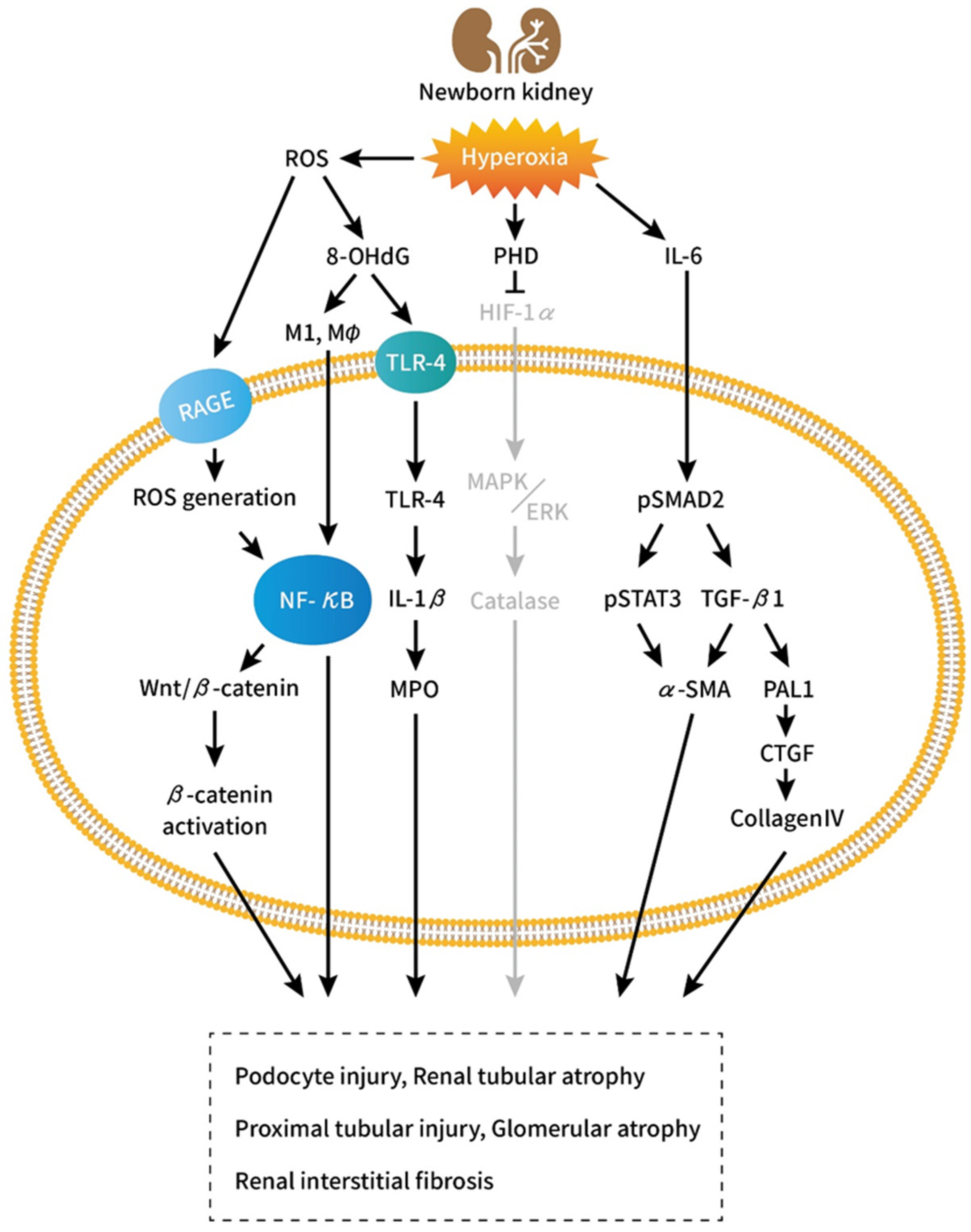
:1. Introduction
2. Experimental Oxygen Studies and Kidney Injury
3. Predisposition to CKD Due to Hyperoxia-Induced Kidney Injuries
3.1. Proximal Tubular Injury and Interstitial Fibrosis
3.2. Nephron Number Loss and Increase in Glomeruli Diameter
3.3. Glomerular and Podocyte Injury
3.4. Similarities and Differences between Hyperoxia- and Other Factors-Induced Pathomechanisms in CKD
4. Cellular and Molecular Aspects
4.1. Influence of Hyperoxia-Inducible Factor-1a (HIF-1α) on Tubular Development
4.2. Role of the Proinflammatory Cytokine Interleukin-6
4.3. Influence of Hyperoxia on Nephrogenesis and Renal Fibrosis through Wnt/β-Catenin Signaling
5. Therapeutic Approach According to Molecular Markers
6. Future Studies on Hyperoxia-Induced Kidney Injuries
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into midadulthood: National cohort study. BMJ 2019, 365, l1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, H.A.; Klebanoff, M.A. The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Update on oxygen radical disease in neonatology. Curr. Opin. Obstet. Gynecol. 2001, 2, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.F.; Bueters, R.R.; Huigen, M.C.; Russel, F.G.; Masereeuw, R.; van den Heuvel, L.P. Effect of drugs on renal development. Clin. J. Am. Soc. Nephrol. 2011, 1, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O’Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A. Extremely Low Gestational Age Newborns Study Investigators. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics 2009, 2, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Luyckx, V.A.; Brenner, B.M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 2010, 21, 898–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquette, K.; Fernandes, R.O.; Xie, L.F.; Cloutier, A.; Fallaha, C.; Girard-Bock, C.; Mian, M.O.R.; Lukaszewski, M.A.; Mâsse, B.; El-Jalbout, R.; et al. Kidney size, renal function, ang (angiotensin) peptides, and blood pressure in young adults born preterm. Hypertension 2018, 72, 918–928. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Monga, G.; Zaffanello, M.; Van Eyken, P.; Fanos, V. Morphogenesis and molecular mechanisms involved in human kidney development. J. Cell. Physiol. 2012, 227, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, S.A.; Sargent, P.H.; Howard, C.V.; Chan, Y.F.; van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Investig. 1991, 64, 777–784. [Google Scholar] [PubMed]
- Georgeson, G.D.; Szony, B.J.; Streitman, K.; Varga, I.S.; Kovács, A.; Kovács, L.; László, A. Antioxidant enzyme activities are decreased in preterm infants and in neonates born via caesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 136–139. [Google Scholar] [CrossRef]
- Saugstad, O. Mechanisms of tissue injury by oxygen radicals: Implications for neonatal disease. Acta Paediatr. 1996, 85, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J.; Arduini, A.; Escobar, J.; Sastre, J.; et al. Preterm Resuscitation with Low Oxygen Causes Less Oxidative Stress, Inflammation, and Chronic Lung Disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Perrone, S.; Longini, M.; Vezzosi, P.; Marzocchi, B.; Paffetti, P.; Bracci, R. Oxidative Stress in Preterm Neonates at Birth and on the Seventh Day of Life. Pediatr. Res. 2002, 52, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Salvi, G.; Bellieni, C.V.; Buonocore, G. Oxidative Stress and Nutrition in the Preterm Newborn. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S178–S182. [Google Scholar] [CrossRef]
- Hirano, K.; Morinobu, T.; Kim, H.; Hiroi, M.; Ban, R.; Ogawa, S.; Ogihara, H.; Tamai, H.; Ogihara, T. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 84, F188–F193. [Google Scholar] [CrossRef] [Green Version]
- Andresen, J.H.; Saugstad, O.D. Oxygen metabolism and oxygenation of the newborn. Semin. Fetal Neonatal Med. 2020, 2, 101078. [Google Scholar] [CrossRef]
- Ma, D.; Gao, W.; Liu, J.; Kong, D.; Zhang, Y.; Qian, M. Mechanism of oxidative stress and Keap-1/Nrf2 signaling pathway in bronchopulmonary dysplasia. Medicine 2020, 26, e20433. [Google Scholar] [CrossRef] [PubMed]
- Torbati, D.; Tan, G.H.; Smith, S.; Frazier, K.S.; Gelvez, J.; Fakioglu, H.; Totapally, B.R. Multiple-organ effect of normobaric hyperoxia in neonatal rats. J. Crit. Care 2006, 21, 85–94. [Google Scholar] [CrossRef]
- Vento, M.; Sastre, J.; Asensi, M.A.; Viña, J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am. J. Respir. Crit. Care Med. 2005, 172, 1393–1398. [Google Scholar] [CrossRef]
- Perrone, S.; Mussap, M.; Longini, M.; Fanos, V.; Bellieni, C.V.; Proietti, F.; Cataldi, L.; Buonocore, G. Oxidative kidney damage in preterm newborns during perinatal period. Clin. Biochem. 2007, 40, 656–660. [Google Scholar] [CrossRef]
- Sutherland, M.R.; O’Reilly, M.; Kenna, K.; Ong, K.; Harding, R.; Sozo, F.; Black, M.J. Neonatal hyperoxia: Effects on nephrogenesis and long term glomerular structure. Am. J. Physiol. Ren. Physiol. 2013, 304, F1308–F1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.S.; Chou, H.C.; Yeh, T.F.; Chen, C.M. Neonatal hy.yperoxia exposure induces kidney fibrosis in rats. Pediatr. Neonatol. 2015, 56, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Zhao, Y.; Zhang, B.; Xu, B.; Yang, Y.; Wang, Y.; Liu, C. Resveratrol attenuates hyperoxia-induced oxidative stress, inflammation and fibrosis and suppresses Wnt/β-catenin signalling in lungs of neonatal rats. Clin. Exp. Pharmacol. Physiol. 2015, 10, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Zoetis, T.; Hurtt, M.E. Species comparison of anatomical and functional renal development. Birth Defects Res. B Dev. Reprod. Toxicol. 2003, 68, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Seely, J.C. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: Juvenile animal relevancy. J. Toxicol. Pathol. 2017, 30, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, R.S.; Tarloff, J.B.; Hook, J.B. Age-related nephropathy in laboratory rats. FASEB J. 1988, 2, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Age-related changes in renal hemodynamics in female rats: Role of multiple pregnancy and NO. Am. J. Physiol. 1997, 272, R1985–R1989. [Google Scholar] [CrossRef] [PubMed]
- Black, M.J.; Lim, K.; Zimanyi, M.A.; Sampson, A.K.; Bubb, K.J.; Flower, R.L.; Parkington, H.C.; Tare, M.; Denton, K.M. Accelerated age-related decline in renal and vascular function in female rats following early-life growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1153–R1161. [Google Scholar] [CrossRef] [Green Version]
- Bacchetta, J.; Harambat, J.; Dubourg, L.; Guy, B.; Liutkus, A.; Canterino, I.; Kassaï, B.; Putet, G.; Cochat, P. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009, 76, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, N.; Satija, Y.K.; Das, S. PGC-1a, a key modulator of p53 promotes cell survival upon metabolic stress. Mol. Cell 2011, 44, 621–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keijzer-Veen, M.G.; Schrevel, M.; Finken, M.J.J.; Dekker, F.W.; Nauta, J.; Hille, E.T.M.; Fr¨olich, M.; van der Heijden, B.J.; Dutch POPS-19 Collaborative Study Group. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J. Am. Soc. Nephrol. 2005, 16, 2762–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalziel, S.R.; Parag, V.; Rodgers, A.; Harding, J.E. Cardiovascular risk factors at age 30 following pre-term birth. Int. J. Epidemiol. 2007, 36, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Atherton, K.; Power, C. Gestational age and risk factors for cardiovascular disease: Evidence fromthe 1958 British birth cohort followed to mid-life. Int. J. Epidemiol. 2009, 38, 235–244. [Google Scholar] [CrossRef] [PubMed]
- De Jong, F.; Monuteaux, M.C.; van Elburg, R.M.; Gillman, M.W.; Belfort, M.B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012, 59, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Popescu, C.R.; Sutherland, M.R.; Cloutier, A.; Benoît, G.; Bertagnolli, M.; Yzydorczyk, C.; Germain, N.; Phan, V.; Lelièvre-Pegorier, M.; Sartelet, H.; et al. Hyperoxia exposure impairs nephrogenesis in the neonatal rat: Role of HIF-1α. PLoS ONE 2013, 8, e82421. [Google Scholar] [CrossRef] [Green Version]
- Mohr, J.; Voggel, J.; Vohlen, C.; Dinger, K.; Dafinger, C.; Fink, G.; Göbel, H.; Liebau, M.C.; Dötsch, J.; Alejandre Alcazar, M.A. IL-6/Smad2 signaling mediates acute kidney injury and regeneration in a murine model of neonatal hyperoxia. FASEB J. 2019, 33, 5887–5902. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Comte, B.; Cambonie, G.; Lavoie, J.C.; Germain, N.; Ting Shun, Y.; Wolff, J.; Deschepper, C.; Touyz, R.M.; Lelièvre-Pegorier, M.; et al. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension 2008, 5, 889–895. [Google Scholar] [CrossRef]
- Chen, C.M.; Chou, H.C. Maternal inflammation exacerbates neonatal hyperoxia-induced kidney injury in rat offspring. Pediatr. Res. 2019, 86, 174–180. [Google Scholar] [CrossRef]
- Chou, H.C.; Chen, C.M. Cathelicidin attenuates hyperoxia-induced kidney injury in newborn rats. Ren. Fail. 2019, 41, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; You, K.; Bu, R. Proximal Tubular Development Is Impaired with Downregulation of MAPK/ERK Signaling, HIF-1α, and Catalase by Hyperoxia Exposure in Neonatal Rats. Oxidative Med. Cell Longev. 2019, 2019, 9219847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, X.; Gao, L.; Liu, C.; You, K. Neonatal Hyperoxia Downregulates Claudin-4, Occludin, and ZO-1 Expression in Rat Kidney Accompanied by Impaired Proximal Tubular Development. Oxidative Med. Cell Longev. 2020, 2020, 2641461. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.R.; Béland, C.; Lukaszewski, M.A.; Cloutier, A.; Bertagnolli, M.; Nuyt, A.M. Age- and sex-related changes in rat renal function and pathology following neonatal hyperoxia exposure. Physiol. Rep. 2016, 15, e12887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.H.S.; Wang, H.; Kishkurno, S.; Paturi, B.S.; Nielsen, L.; Ryan, R.M. Long-Term Effects of Neonatal Hyperoxia in Adult Mice. Anat. Rec. 2018, 301, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease–A Systematic Review and Meta-Analysis. PLoS ONE 2016, 7, e0158765. [Google Scholar] [CrossRef] [Green Version]
- Fogo, A.B. Mechanisms of progression of chronic kidney disease. Pediatr. Nephrol. 2007, 22, 2011–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gewin, L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018, 68–69, 248–262. [Google Scholar] [CrossRef]
- Gewin, L.; Zent, R.; Pozzi, A. Progression of chronic kidney disease: Too much cellular talk causes damage. Kidney Int. 2017, 3, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.A.; Mak, R.H. Progression of chronic kidney disease in children-role of glomerular hemodynamics and interstitial fibrosis. Curr. Opin. Pediatr. 2018, 2, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, S.; Cantley, L.G. Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr. Opin. Nephrol. Hypertens 2008, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of tubulointerstitial fibrosis and the chronic kidney disease phenotype-role of risk factors and epigenetics. Front. Pharmacol. 2017, 8, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetter, T.H.; Olson, J.L.; Rennke, H.G.; Venkatachalam, M.A.; Brenner, B.M. Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am. J. Physiol. 1981, 1, F85–F93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.R.; Brenner, B.M.; Hebert, S.C. Uninephrectomy and dietary protein affect fluid absorption in rabbit proximal straight tubules. Am. J. Physiol. 1987, 2 Pt 2, F222–F233. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Chan, L.; Schrier, R.W. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Phys. 1988, 2 Pt 2, F267–F276. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016, 1, F145–F161. [Google Scholar] [CrossRef]
- Nath, K.A.; Croatt, A.J.; Hostetter, T.H. Oxygen consumption and oxidant stress in surviving nephrons. Am. J. Physiol. 1990, 5 Pt 2, F1354–F1362. [Google Scholar] [CrossRef]
- Kriz, W.; Lemley, K.V. Mechanical challenges to the glomerular filtration barrier: Adaptations and pathway to sclerosis. Pediatr. Nephrol. 2017, 32, 405–417. [Google Scholar] [CrossRef]
- Lamana, G.L.; Ferrari, A.L.L.; Gontijo, J.A.R.; Boer, P.A. Gestational and Breastfeeding Low-Protein Intake on Blood Pressure, Kidney Structure, and Renal Function in Male Rat Offspring in Adulthood. Front. Physiol. 2021, 12, 658431. [Google Scholar] [CrossRef]
- Srivastava, T.; Thiagarajan, G.; Alon, U.S.; Sharma, R.; El-Meanawy, A.; McCarthy, E.T.; Savin, V.J.; Sharma, M. Role of biomechanical forces in hyperfiltration-mediated glomerular injury in congenital anomalies of the kidney and urinary tract. Nephrol. Dial. Transplant. 2017, 32, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, C.S.; Jeansson, M.; Quaggin, S.E. Vascular growth factors and glomerular disease. Annu. Rev. Physiol. 2016, 78, 437–461. [Google Scholar] [CrossRef]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H. Podocyte biology in 2015: New insights into the mechanisms of podocyte health. Nat. Rev. Nephrol. 2016, 12, 63–64. [Google Scholar] [CrossRef]
- Kriz, W.; LeHir, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005, 2, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahammer, F.; Wanner, N.; Huber, T.B. Podocyte regeneration: Who can become a podocyte? Am. J. Pathol. 2013, 183, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic. Kidney Dis. 2017, 2, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Peired, A.; Angelotti, M.L.; Ronconi, E.; la Marca, G.; Mazzinghi, B.; Sisti, A.; Lombardi, D.; Giocaliere, E.; Della Bona, M.; Villanelli, F.; et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 2013, 11, 1756–1768. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, P.; Perico, N.; Gagliardini, E.; Novelli, R.; Alison, M.R.; Remuzzi, G.; Benigni, A. Nature and mediators of parietal epithelial cell activation in glomerulonephritides of human and rat. Am. J. Pathol. 2013, 6, 1769–1778. [Google Scholar] [CrossRef]
- Clark, W.F.; Macnab, J.J.; Sontrop, J.M.; Jain, A.K.; Moist, L.; Salvadori, M.; Suri, R.; Garg, A.X. Dipstick proteinuria as a screening strategy to identify rapid renal decline. J. Am. Soc. Nephrol. 2011, 9, 1729–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Dai, C.; Wu, C.; Liu, Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Investig. 2003, 112, 503–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399.e408. [Google Scholar] [CrossRef] [Green Version]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compernolle, V.; Brusselman, K.; Franco, D.; Moorman, A.; Dewerchin, M.; Collen, D.; Carmeliet, P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc. Res. 2003, 60, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Ryan, H.E.; Lo, J.; Johnson, R.S. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998, 17, 3005–3015. [Google Scholar] [CrossRef] [Green Version]
- Nazıroğlu, M. Molecular role of catalase on oxidative stress-induced Ca (2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012, 3, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Wang, B.; Zhang, M.; Gu, Y. Tempol, a Superoxide Dismutase-Mimetic Drug, Ameliorates Progression of Renal Disease in CKD Mice. Cell Physiol. Biochem. 2015, 6, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Hafstad, A.D.; Nabeebaccus, A.A.; Shah, A.M. Novel aspects of ROS signalling in heart failure. Basic Res. Cardiol. 2013, 108, 359. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 2001, 29, S99–S106. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.W.; Kim, B.I.; Kim, H.-S.; Park, J.D.; Choi, J.-H.; Son, D.W. Increase of interleukin-6 in tracheal aspirate at birth: A predictor of subsequent bronchopulmonary dysplasia in preterm infants. Acta Paediatr. 2006, 95, 38–43. [Google Scholar] [CrossRef]
- Lecart, C.; Cayabyab, R.; Buckley, S.; Morrison, J.; Kwong, K.Y.; Warburton, D.; Ramanathan, R.; Jones, C.A.; Minoo, P. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol. Neonate 2000, 77, 217–223. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Chang, J.C.; Tsao, L.Y.; Yang, R.C.; Chen, H.N.; Lee, C.H.; Lin, C.Y.; Tsai, Y.G. Correlates of Elevated Interleukin-6 and 8-Hydroxy-2′-Deoxyguanosine Levels in Tracheal Aspirates from Very Low Birth Weight Infants Who Develop Bronchopulmonary Dysplasia. Pediatr. Neonatol. 2017, 1, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Johnston, C.J.; Wright, T.W.; Reed, C.K.; Finkelstein, J.N. Comparison of adult and newborn pulmonary cytokine mRNA expression after hyperoxia. Exp. Lung Res. 1997, 23, 537–552. [Google Scholar] [CrossRef]
- Jiang, J.S.; Chou, H.C.; Chen, C.M. Cathelicidin attenuates hyperoxia-induced lung injury by inhibiting oxidative stress in newborn rats. Free Radic. Biol. Med. 2020, 150, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. Oxidative stress and nuclear factor-kappa B activation: A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Gewin, L. The many talents of transforming growth factor-β in the kidney. Curr. Opin. Nephrol. Hypertens 2019, 3, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Koesters, R.; Kaissling, B.; Lehir, M.; Picard, N.; Theilig, F.; Gebhardt, R.; Glick, A.B.; Hähnel, B.; Hosser, H.; Gröne, H.J.; et al. Tubular overexpression of transforming growth factorbeta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 2010, 2, 632–643. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, P.J. The Cytoskeletal Network Regulates Expression of the Profibrotic Genes PAI-1 and CTGF in Vascular Smooth Muscle Cells. Adv. Pharmacol. 2018, 81, 79–94. [Google Scholar]
- Chen, J.T.; Wang, C.Y.; Chen, M.H. Curcumin inhibits TGF-β1-induced connective tissue growth factor expression through the interruption of Smad2 signaling in human gingival fibroblasts. J. Formos. Med. Assoc. 2018, 12, 1115–1123. [Google Scholar] [CrossRef]
- Sand, J.M.; Leeming, D.J.; Byrjalsen, I.; Bihlet, A.R.; Lange, P.; Tal-Singer, R.; Miller, B.E.; Karsdal, M.A.; Vestbo, J. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD-results from the ECLIPSE study. Respir. Res. 2016, 1, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, J.P.; Hirani, D.; Thielen, F.; Klein, F.; Vohlen, C.; Dinger, K.; Dötsch, J.; Alejandre Alcázar, M.A. Strain-dependent effects on lung structure, matrix remodeling, and Stat3/Smad2 signaling in C57BL/6N and C57BL/6J mice after neonatal hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 1, R169–R181. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.; Sakurai, R.; Wang, Y.; Guo, P.; Ambalavanan, N.; Torday, J.S.; Rehan, V.K. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 6, L1031–L1041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Lu, M.; Wu, Q.; Yuan, Q.; Hu, C.; Miao, J.; Zhang, Y.; Li, H.; Hou, F.F.; et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 2019, 4, 830–845. [Google Scholar] [CrossRef]
- Angers, S.; Moon, R.T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell. Biol. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Tan, R.J.; Fu, H.; Liu, Y. Wnt/beta-catenin signaling in kidney injury and repair: A double-edged sword. Lab. Investig. 2016, 96, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-β-catenin signalling-A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Hao, S.; Zhou, D.; Tan, R.J.; Nie, J.; Hou, F.F.; Kahn, M.; Liu, Y. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 2015, 26, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Zhou, D.; Tan, R.J.; Fu, H.; Zhou, L.; Hou, F.F.; Liu, Y. Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 2016, 27, 1727–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Mo, H.; Miao, J.; Zhou, D.; Tan, R.J.; Hou, F.F.; Liu, Y. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am. J. Pathol. 2015, 185, 3211–3223. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Stolz, D.B.; Kiss, L.P.; Monga, S.P.; Holzman, L.B.; Liu, Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 2009, 20, 1997–2008. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.N.; Sallustio, F.; Serino, G.; Pontrelli, P.; Verrienti, R.; Pesce, F.; Torres, D.D.; Ancona, N.; Stifanelli, P.; Zaza, G.; et al. Altered modulation of WNT-beta catenin and PI3K/Akt pathways in IgA nephropathy. Kidney Int. 2010, 78, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Huang, X.F.; Yan, Q.R.; Bao, C.D. Aberrant activation of the WNT/beta catenin signaling pathway in lupus nephritis. PLoS ONE 2014, 9, e84852. [Google Scholar]
- Li, C.; Siragy, H.M. High glucose induces podocyte injury via enhanced (pro) renin receptor-Wnt-catenin-snail signaling pathway. PLoS ONE 2014, 9, e89233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Tao, J.; Chen, Y.; Zhang, Y.; Li, F.; Zhang, Y.; Han, X.; Zhao, Z.; Liu, G.; Li, H. Selenium Deficiency Leads to Changes in Renal Fibrosis Marker Proteins and Wnt/β-Catenin Signaling Pathway Components. Biol. Trace Elem. Res. 2022, 3, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- DiRocco, D.P.; Kobayashi, A.; Taketo, M.M.; McMahon, A.P.; Humphreys, B.D. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 2013, 9, 1399–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Fu, H.; Zhang, L.; Zhang, K.; Min, Y.; Xiao, L.; Lin, L.; Bastacky, S.I.; Liu, Y. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 2017, 8, 2322–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nlandu-Khodo, S.; Neelisetty, S.; Phillips, M.; Manolopoulou, M.; Bhave, G.; May, L.; Clark, P.E.; Yang, H.; Fogo, A.B.; Harris, R.C.; et al. Blocking TGF-beta and beta-catenin epithelial crosstalk exacerbates CKD. J. Am. Soc. Nephrol. 2017, 12, 3490–3503. [Google Scholar] [CrossRef] [Green Version]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Neusser, M.A.; Lindenmeyer, M.T.; Moll, A.G.; Segerer, S.; Edenhofer, I.; Sen, K.; Stiehl, D.P.; Kretzler, M.; Grone, H.J.; Schlondorff, D.; et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am. J. Pathol. 2010, 176, 594–607. [Google Scholar] [CrossRef] [Green Version]
| Model | Species | Primary Target Lesion | Molecules | Ref |
|---|---|---|---|---|
| 85% O2, P1 to P28, 21% O2 till P70 (Mohr et al.) | mouse | Glomerular filtration rate Kidney cortex area Glomerular number Glomerular diameter Proximal tubular proliferation | IL-6 Collagen IV PAI-1 CTGF Smad2 | [37] |
| 80% O2 P3 to P10 (Popeseu et al.) | rat | Nephrogenic zone Glomerular diameter Glomerular apoptotic cells | HIF-1α | [36] |
| 95% O2, P1 to P7, 60% O2 till P21 (Jiang et al.) | rat | Tubular injury score Glomerular size | Total collagen 8-oHdG CTGF | [22] |
| 80% O2, P1 to P14 (Chen et al.) | rat | Kidney injury score Glomerular number Glomerular injury score | 8-OHdG MPO activity TLR4 IL-1β | [39] |
| 85% O2, P1 to P7 (Chou et al.) | rat | Tubular injury score | M1 macrophage 8-OHdG Collagen NF-κB | [40] |
| 85% O2, P0 to P14; 21% O2 till P60 (Xu et al.) | rat | Nephrogenic zone Epithelial cells of mature proximal tubules Tubular cell apoptosis | MAPK/ERK HIF-1α Catalase IL-6 TNF-α Claudin-4 Occludin Zonula occluden-1 (ZO-1) | [41,42] |
| 65% O2, P1 to P7; 21% O2 till P56 and P10m (Sutherland et al.) | mouse | Nephron number Renal corpuscles | - | [21] |
| 80% O2, P3 to P10; 21% O2 till P11ms (Sutherland et al.) | rat | Glomerular injury Creatinine clearance | - | [43] |
| 85% O2, P3 to P15; 21% O2 till P9ms (Kumar et al.) | mouse | Glomerular diameter Glomerular volume Nephron number | - | [44] |
| 80% O2, P3 to P10; 21% O2 till P15wks (Yzydorczyk et al.) | rat | Blood pressure Microvascular rarefaction Nephron number | Superoxide dismutase analogue | [38] |
| >98% O2 P0 to P4; 21% O2 till P5, P8 (Torbati et al.) | rat | Tubular necrosis, dilation, and degeneration, Interstitial inflammation | - | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-T.; Chen, C.-M. Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies. Int. J. Mol. Sci. 2022, 23, 8492. https://doi.org/10.3390/ijms23158492
Huang L-T, Chen C-M. Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies. International Journal of Molecular Sciences. 2022; 23(15):8492. https://doi.org/10.3390/ijms23158492
Chicago/Turabian StyleHuang, Liang-Ti, and Chung-Ming Chen. 2022. "Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies" International Journal of Molecular Sciences 23, no. 15: 8492. https://doi.org/10.3390/ijms23158492
APA StyleHuang, L.-T., & Chen, C.-M. (2022). Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies. International Journal of Molecular Sciences, 23(15), 8492. https://doi.org/10.3390/ijms23158492






