Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers
Abstract
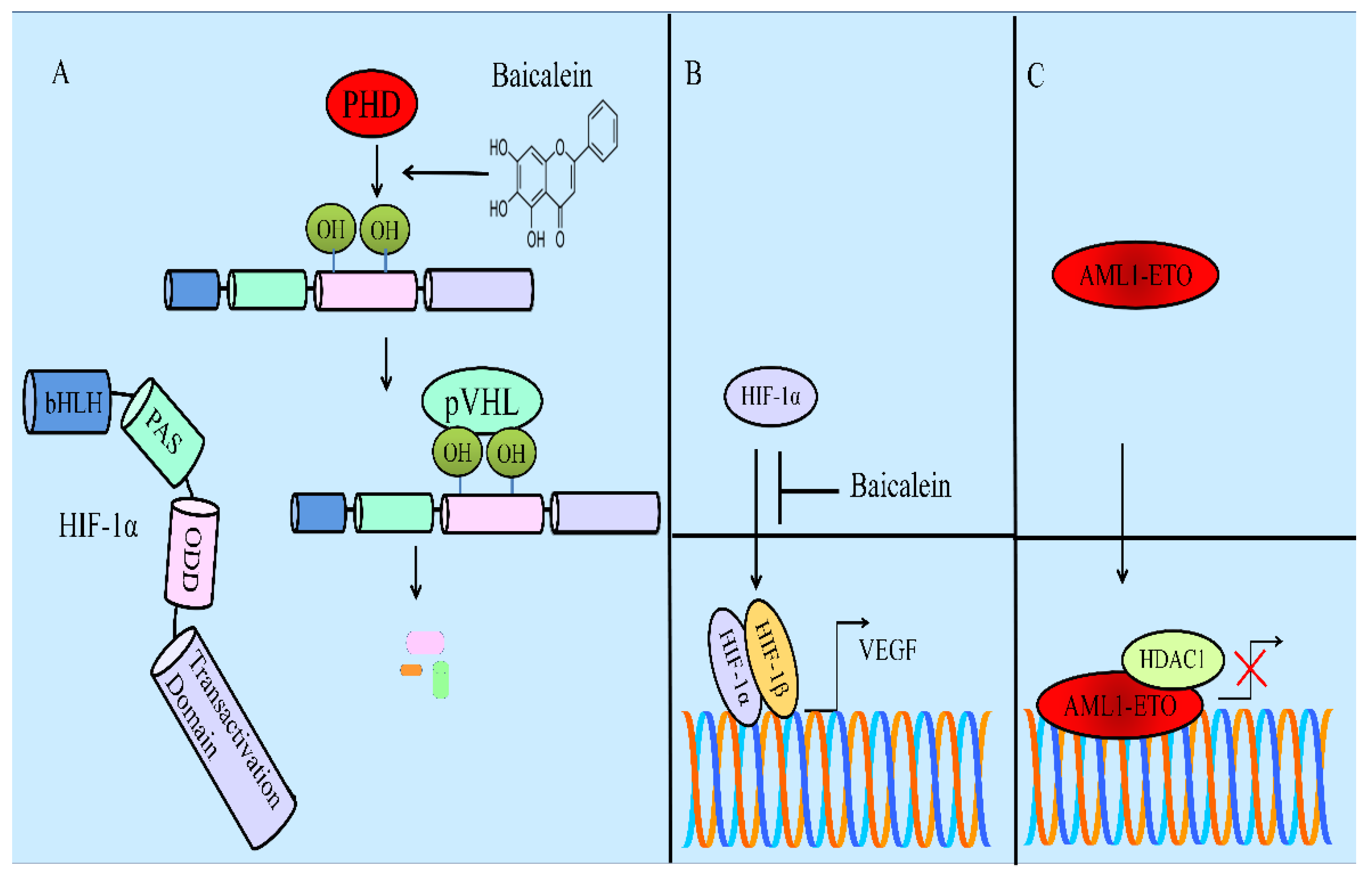
:1. Introduction
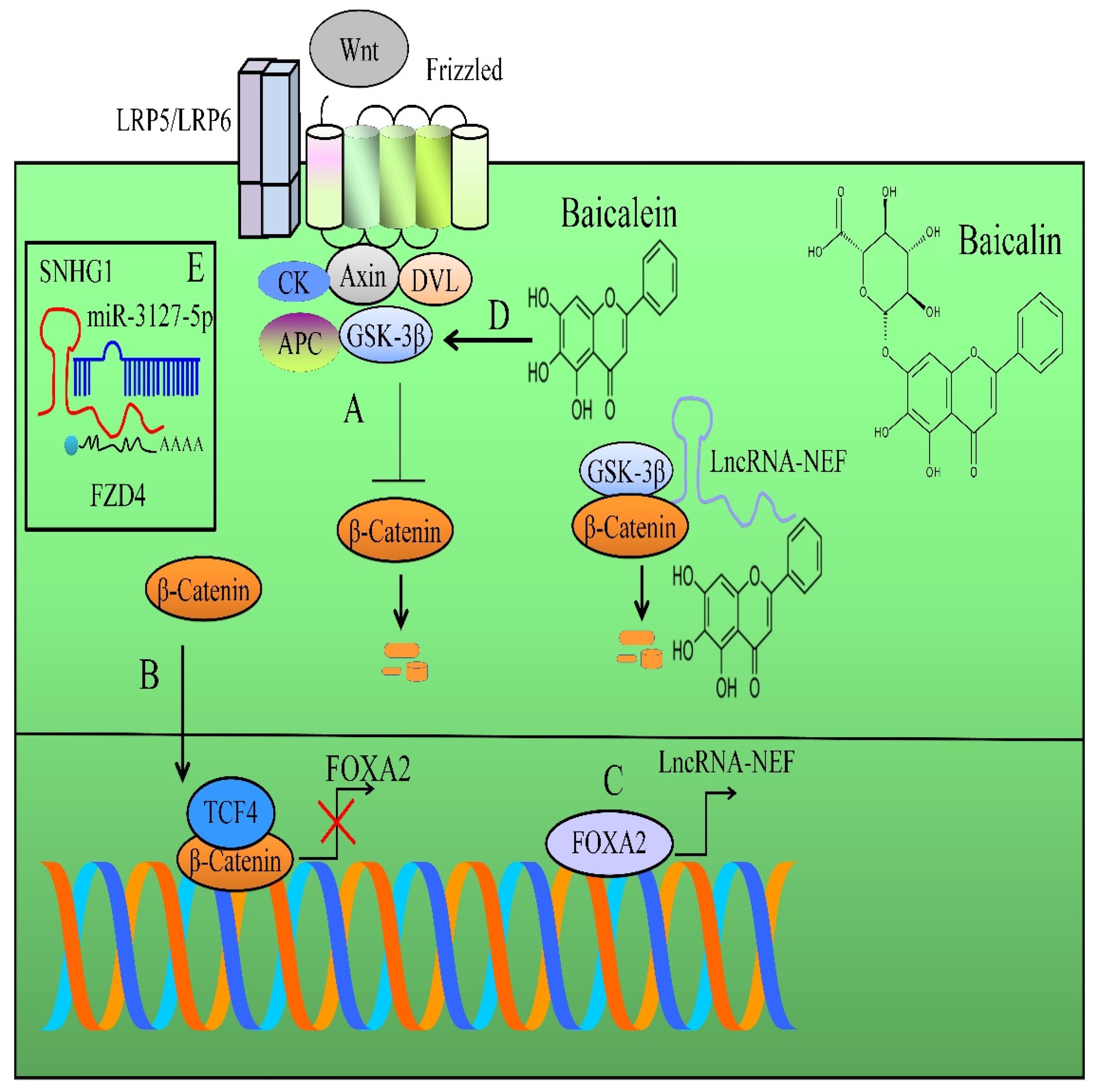
2. Regulation of WNT/β-Catenin Pathway
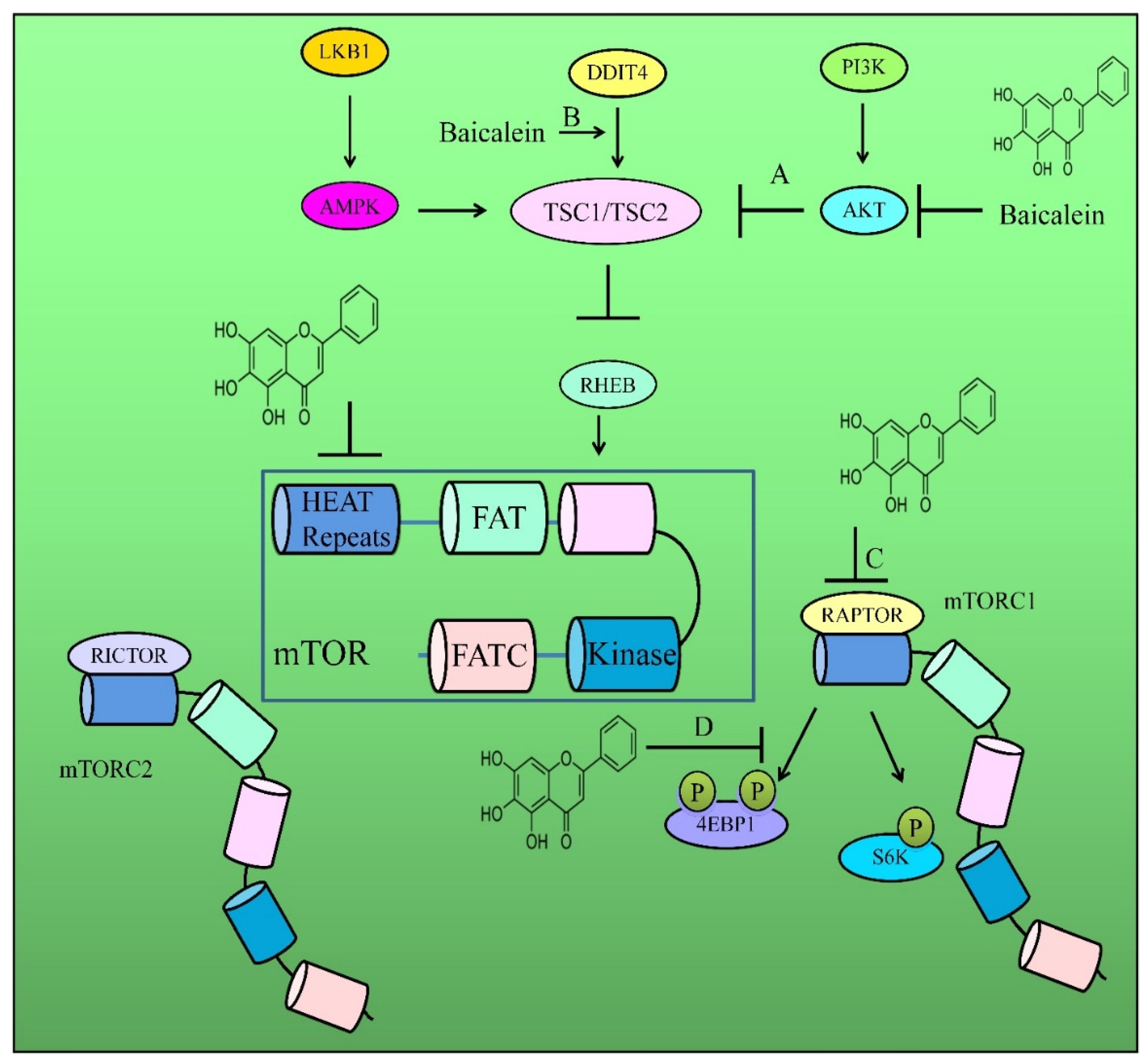
3. Regulation of AKT/mTOR Pathway
4. Regulation of Matrix Metalloproteinases
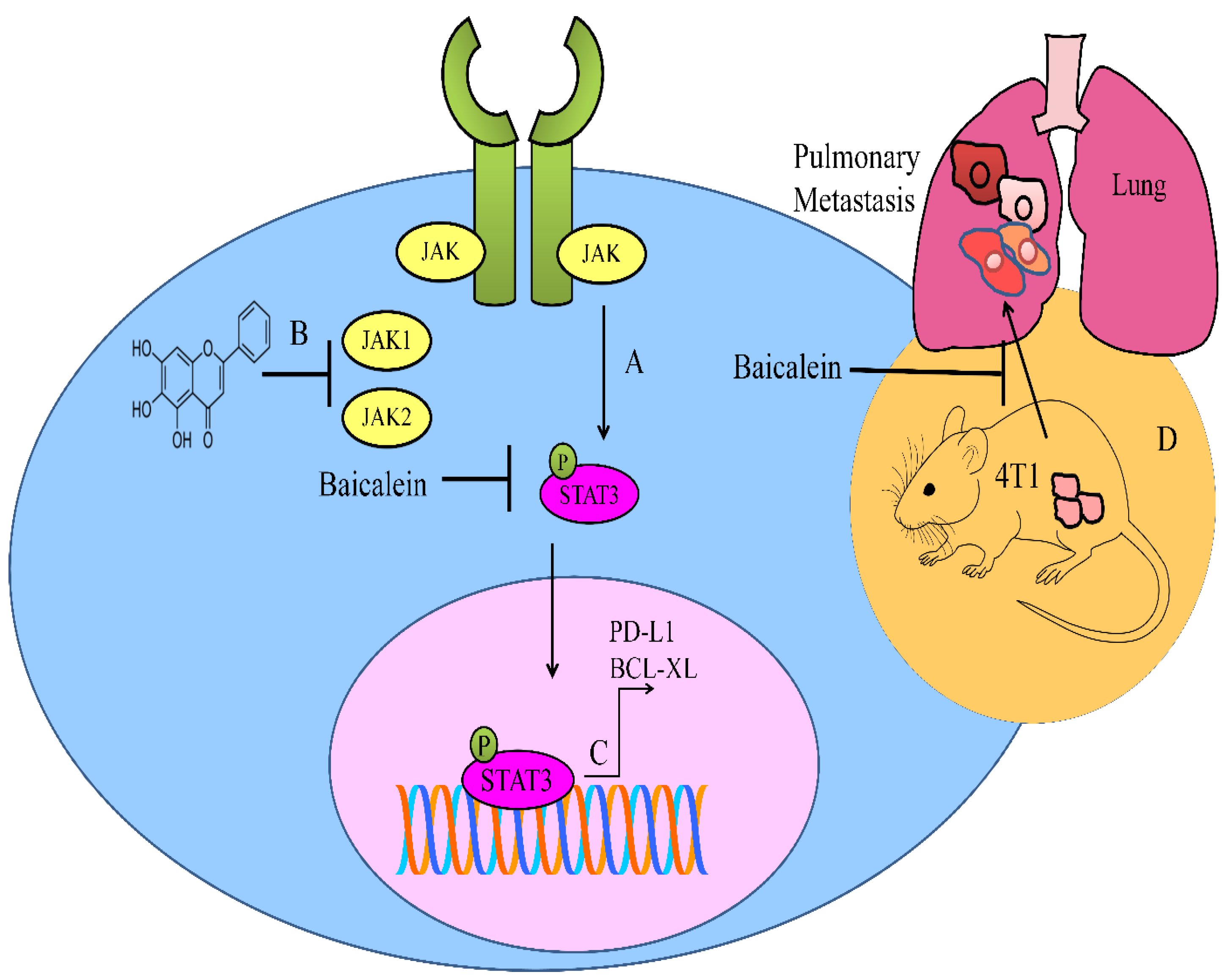
5. Regulation of JAK/STAT Pathway
6. Regulation of MAPKs
7. Regulation of NOTCH Pathway
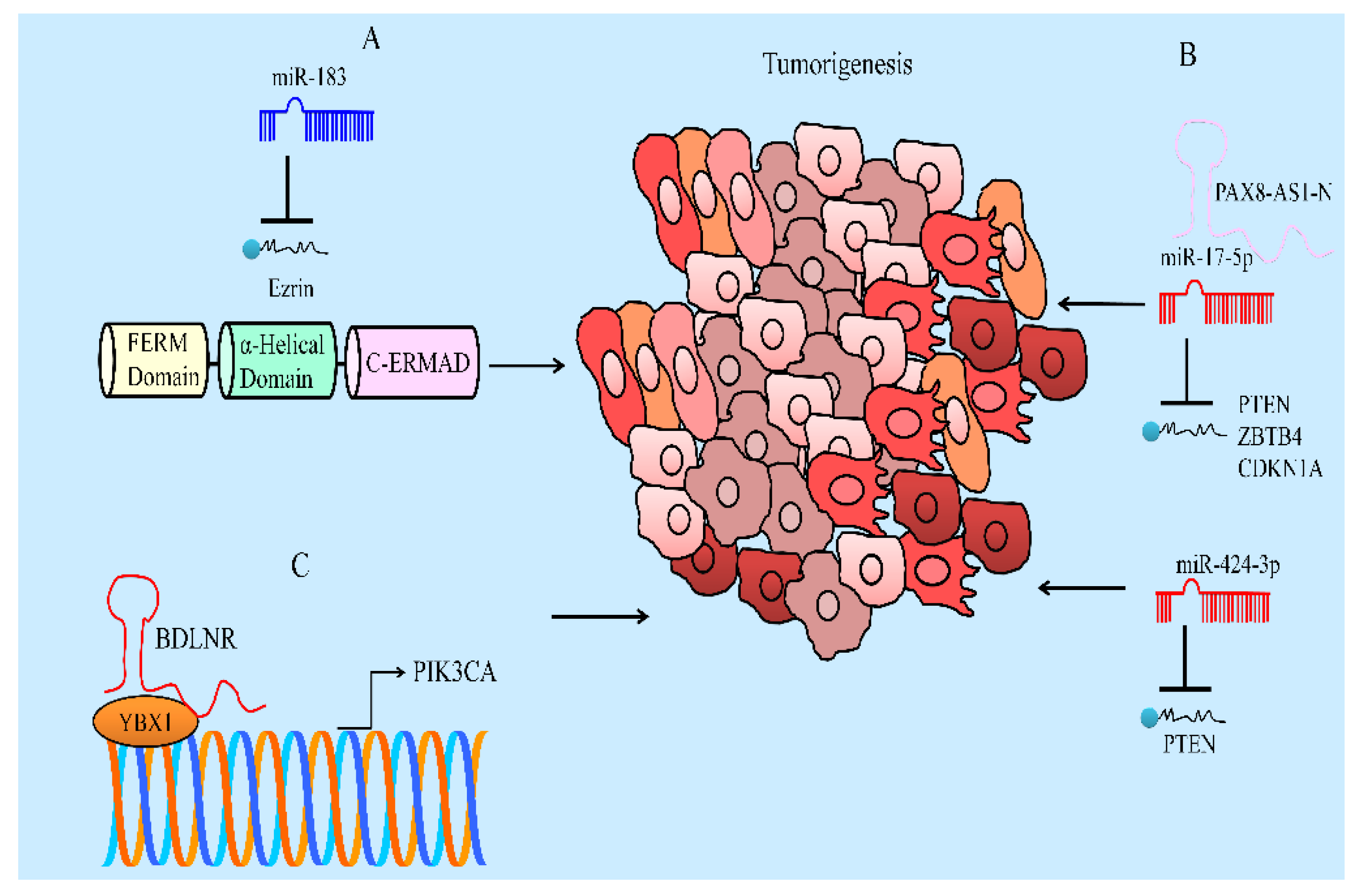
8. Regulation of Non-Coding RNAs
9. Animal Models
10. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Min, W.; Wu, M.; Fang, P.; Yu, M.; Shi, M.; Zhang, Z.; Bo, P. Effect of Baicalein on GLUT4 Translocation in Adipocytes of Diet-Induced Obese Mice. Cell Physiol. Biochem. 2018, 50, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell Longev. 2021, 29, 8377362. [Google Scholar] [CrossRef]
- Dong, Y.; Xing, Y.; Sun, J.; Sun, W.; Xu, Y.; Quan, C. Baicalein Alleviates Liver Oxidative Stress and Apoptosis Induced by High-Level Glucose through the Activation of the PERK/Nrf2 Signaling Pathway. Molecules 2020, 30, 599. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, X.P.; Ji, H.S.; Liu, Y.F.; Li, E.Z. Baicalein Protects Rats with Diabetic Cardiomyopathy Against Oxidative Stress and Inflammation Injury via Phosphatidylinositol 3-Kinase (PI3K)/AKT Pathway. Med. Sci. Monit. 2018, 2, 5368–5375. [Google Scholar] [CrossRef]
- Yin, H.; Huang, L.; Ouyang, T.; Chen, L. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2018, 55, 55–62. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wu, T.; Li, S.; Sun, Y.N.; Liu, Z.H. Baicalein suppresses high glucose-induced inflammation and apoptosis in trophoblasts by targeting the miRNA-17-5p-Mfn1/2-NF-κB pathway. Placenta 2022, 121, 126–136. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef] [Green Version]
- Awadalla, A.; Mahdi, M.R.; Zahran, M.H.; Abdelbaset-Ismail, A.; El-Dosoky, M.; Negm, A. Baicalein and Alpha-Tocopherol Inhibit Toll-like Receptor Pathways in Cisplatin-Induced Nephrotoxicity. Molecules 2022, 28, 2179. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein Inhibits Staphylococcus aureus Biofilm Formation and the Quorum Sensing System In Vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Kong, J.L.; Dong, B.Y.; Huang, H.; Wang, K.; Wu, L.H.; Hou, C.C.; Liang, Y.; Li, B.; Chen, Y.Q. Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016, 10, 183–203. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Fu, Y.; Zhang, W.; Chen, X.; Zhao, J.; Song, W.; Li, Y.; Huang, Y.; Wu, Z.; Sun, R.; et al. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016, 16, 181. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Li, X.; Wang, G.; Wang, C.; Feng, J.; Lu, W.; Wang, X.; Chen, H.; Liu, M.; Tan, C. Baicalein Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 5829. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, H.; Lan, T.; Chen, Y.; Chen, X.; Feng, Y.; Luo, Y.; Yao, Y.; Li, Y.; Pan, X.; et al. Co-delivery of antibiotic and baicalein by using different polymeric nanoparticle cargos with enhanced synergistic antibacterial activity. Int. J. Pharm. 2021, 599, 120419. [Google Scholar] [CrossRef]
- Liu, T.; Luo, J.; Bi, G.; Du, Z.; Kong, J.; Chen, Y. Antibacterial synergy between linezolid and baicalein against methicillin-resistant Staphylococcus aureus biofilm in vivo. Microb. Pathog. 2020, 147, 104411. [Google Scholar] [CrossRef]
- Hengphasatporn, K.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Chavasiri, W.; Visitchanakun, P.; Leelahavanichkul, A.; Paunrat, W.; Boonyasuppayakorn, S.; Rungrotmongkol, T.; et al. Halogenated Baicalein as a Promising Antiviral Agent toward SARS-CoV-2 Main Protease. J. Chem. Inf. Model 2022, 62, 1498–1509. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Zhang, P.; Ren, S.; Bao, J.; Gao, D.; Sun, R.; Wang, M.; Lin, J.; et al. Both Baicalein and Gallocatechin Gallate Effectively Inhibit SARS-CoV-2 Replication by Targeting Mpro and Sepsis in Mice. Inflammation 2022, 45, 1076–1088. [Google Scholar] [CrossRef]
- Zandi, K.; Musall, K.; Oo, A.; Cao, D.; Liang, B.; Hassandarvish, P.; Lan, S.; Slack, R.L.; Kirby, K.A.; Bassit, L.; et al. Baicalein and Baicalin Inhibit SARS-CoV-2 RNA-Dependent-RNA Polymerase. Microorganisms 2021, 9, 893. [Google Scholar] [CrossRef]
- Tang, J.; Yan, B.; Tang, Y.; Zhou, X.; Ji, Z.; Xu, F. Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int. J. Neurosci. 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Choi, E.O.; Jeong, J.W.; Park, C.; Hong, S.H.; Kim, G.Y.; Hwang, H.J.; Cho, E.J.; Choi, Y.H. Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int. J. Mol. Med. 2016, 37, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.H.; Ma, K.H.; Liu, P.S.; Kuo, J.K.; Chueh, S.H. Baicalein Decreases Hydrogen Peroxide-Induced Damage to NG108-15 Cells via Upregulation of Nrf2. J. Cell Physiol. 2015, 230, 1840–1851. [Google Scholar] [CrossRef]
- Cui, G.; Luk, S.C.; Li, R.A.; Chan, K.K.; Lei, S.W.; Wang, L.; Shen, H.; Leung, G.P.; Lee, S.M. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. J. Cardiovasc. Pharmacol. 2015, 65, 39–46. [Google Scholar] [CrossRef]
- Kuo, C.P.; Wen, L.L.; Chen, C.M.; Huh, B.; Cherng, C.H.; Wong, C.S.; Liaw, W.J.; Yeh, C.C.; Lin, B.F.; Wu, C.T. Attenuation of neurological injury with early baicalein treatment following subarachnoid hemorrhage in rats. J. Neurosurg. 2013, 119, 1028–1037. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Wang, H.; Ma, D.; Wang, H.; Chu, L.; Zhang, Y.; Gao, Y. Baicalein Ameliorates Myocardial Ischemia Through Reduction of Oxidative Stress, Inflammation and Apoptosis via TLR4/MyD88/MAPKS/NF-κB Pathway and Regulation of Ca2+ Homeostasis by L-type Ca2+ Channels. Front. Pharmacol. 2022, 13, 842723. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Wang, S.; Wang, W. Mechanism of Baicalein in Brain Injury After Intracerebral Hemorrhage by Inhibiting the ROS/NLRP3 Inflammasome Pathway. Inflammation 2022, 45, 590–602. [Google Scholar] [CrossRef]
- Wang, X.; Cai, H.; Chen, Z.; Zhang, Y.; Wu, M.; Xu, X.; Yang, L. Baicalein alleviates pyroptosis and inflammation in hyperlipidemic pancreatitis by inhibiting NLRP3/Caspase-1 pathway through the miR-192-5p/TXNIP axis. Int. Immunopharmacol. 2021, 101 Pt B, 108315. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J.; Xie, H.; Guan, H.; Li, R.; Chen, C.; Dong, H.; Zhou, Y.; Zhang, W. Baicalein suppresses lipopolysaccharide-induced acute lung injury by regulating Drp1-dependent mitochondrial fission of macrophages. Biomed. Pharmacother. 2022, 145, 112408. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Ruan, W.; Wan, X.; Lv, C.; He, L.; Lu, L.; Guo, X. Targeting inflammation-associated AMPK//Mfn-2/MAPKs signaling pathways by baicalein exerts anti-atherosclerotic action. Phytother. Res. 2021, 35, 4442–4455. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Pandi, A. Baicalein: A review on its anti-cancer effects and mechanisms in lung carcinoma. J. Food Biochem. 2022, 11, e14230. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K.; et al. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef]
- Gong, W.Y.; Zhao, Z.X.; Liu, B.J.; Lu, L.W.; Dong, J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 27, 844–852. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar]
- Suzuki, H.; Watkins, D.N.; Jair, K.W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar]
- Behrens, J.; Jerchow, B.A.; Würtele, M.; Grimm, J.; Asbrand, C.; Wirtz, R.; Kühl, M.; Wedlich, D.; Birchmeier, W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC.; and GSK3beta. Science 1998, 280, 596–599. [Google Scholar]
- Liang, W.C.; Ren, J.L.; Wong, C.W.; Chan, S.O.; Waye, M.M.; Fu, W.M.; Zhang, J.F. LncRNA-NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis-regulating FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene 2018, 37, 1445–1456. [Google Scholar] [CrossRef]
- Zhang, F.W.; Peng, L.Y.; Shi, C.J.; Li, J.C.; Pang, F.X.; Fu, W.M.; Pan, X.H.; Zhang, J.F. Baicalein mediates the anti-tumor activity in Osteosarcoma through lncRNA-NEF driven Wnt/β-catenin signaling regulatory axis. J. Orthop. Translat. 2022, 33, 132–141. [Google Scholar] [CrossRef]
- Yu, X.; Xia, J.; Cao, Y.; Tang, L.; Tang, X.; Li, Z. SNHG1 represses the anti-cancer roles of baicalein in cervical cancer through regulating miR-3127-5p/FZD4/Wnt/β-catenin signaling. Exp. Biol. Med. 2021, 246, 20–30. [Google Scholar] [CrossRef]
- Örenlili Yaylagül, E.; Ülger, C. The effect of baicalein on Wnt/β-catenin pathway and miR-25 expression in Saos-2 osteosarcoma cell line. Turk J. Med. Sci. 2020, 50, 1168–1179. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, M.; Wang, X.; Zhang, Y.; Chai, Z.; Ying, M.; Guan, J.; Gong, W.; Zhao, Z.; Liu, L.; et al. Baicalein enhances the antitumor efficacy of docetaxel on nonsmall cell lung cancer in a β-catenin-dependent manner. Phytother. Res. 2020, 34, 104–117. [Google Scholar] [CrossRef]
- Xia, X.; Xia, J.; Yang, H.; Li, Y.; Liu, S.; Cao, Y.; Tang, L.; Yu, X. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, L.; Chakraborty, T.; Ghosh, T.; Chanda, P.; Roy, S. Construing the Biochemical and Molecular Mechanism Underlying the In Vivo and In Vitro Chemotherapeutic Efficacy of Ruthenium-Baicalein Complex in Colon Cancer. Int. J. Biol. Sci. 2019, 15, 1052–1071. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Zheng, D.; Wang, Q.; Yang, J.; Liu, G.; Song, Q.; Sun, X.; Tao, C.; Hu, Q.; Gao, T.; et al. Baicalein inhibits progression of osteosarcoma cells through inactivation of the Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 86098–86116. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, S.; Chen, J.; He, L.; Meng, X.; Liu, S. Baicalein suppresses the proliferation of acute T-lymphoblastic leukemia Jurkat cells by inhibiting the Wnt/β-catenin signaling. Ann. Hematol. 2016, 95, 1787–1793. [Google Scholar] [CrossRef]
- He, N.; Zhang, Z. Baicalein suppresses the viability of MG-63 osteosarcoma cells through inhibiting c-MYC expression via Wnt signaling pathway. Mol. Cell Biochem. 2015, 405, 187–196. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Gremke, N.; Polo, P.; Dort, A.; Schneikert, J.; Elmshäuser, S.; Brehm, C.; Klingmüller, U.; Schmitt, A.; Reinhardt, H.C.; Timofeev, O.; et al. mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability. Nat. Commun. 2020, 11, 4684. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, X.; Xing, Z.; Xing, R.; Lv, R.; Cheng, X.; Su, J.; Zhou, Z.; Xu, Z.; Nilsson, S.; et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol. Cell Biochem. 2015, 406, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Han, S.E.; Nam-Goong, I.S.; Kim, Y.I.; Kim, E.S. Combined Effects of Baicalein and Docetaxel on Apoptosis in 8505c Anaplastic Thyroid Cancer Cells via Downregulation of the ERK and Akt/mTOR Pathways. Endocrinol. Metab. 2018, 33, 121–132. [Google Scholar] [CrossRef]
- Wu, R.; Murali, R.; Kabe, Y.; French, S.W.; Chiang, Y.M.; Liu, S.; Sher, L.; Wang, C.C.; Louie, S.; Tsukamoto, H. Baicalein Targets GTPase-Mediated Autophagy to Eliminate Liver Tumor-Initiating Stem Cell-Like Cells Resistant to mTORC1 Inhibition. Hepatology 2018, 68, 1726–1740. [Google Scholar] [CrossRef]
- Cao, H.J.; Zhou, W.; Xian, X.L.; Sun, S.J.; Ding, P.J.; Tian, C.Y.; Tian, F.L.; Jiang, C.H.; Fu, T.T.; Zhao, S.; et al. A Mixture of Baicalein, Wogonin, and Oroxylin-A Inhibits EMT in the A549 Cell Line via the PI3K/AKT-TWIST1-Glycolysis Pathway. Front Pharmacol. 2022, 9, 821485. [Google Scholar] [CrossRef]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Dev. Ther. 2018, 16, 3961–3972. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys. Res. Commun. 2020, 20, 320–327. [Google Scholar] [CrossRef]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis. Oncol. Targets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef]
- Yu, G.; Chen, L.; Hu, Y.; Yuan, Z.; Luo, Y.; Xiong, Y. Antitumor Effects of Baicalein and Its Mechanism via TGFβ Pathway in Cervical Cancer HeLa Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 5527190. [Google Scholar] [CrossRef]
- Wang, Y.; Han, E.; Xing, Q.; Yan, J.; Arrington, A.; Wang, C.; Tully, D.; Kowolik, C.M.; Lu, D.M.; Frankel, P.H.; et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 2015, 358, 170–179. [Google Scholar] [CrossRef]
- Aryal, P.; Kim, K.; Park, P.H.; Ham, S.; Cho, J.; Song, K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef]
- Choi, E.O.; Cho, E.J.; Jeong, J.W.; Park, C.; Hong, S.H.; Hwang, H.J.; Moon, S.K.; Son, C.G.; Kim, W.J.; Choi, Y.H. Baicalein Inhibits the Migration and Invasion of B16F10 Mouse Melanoma Cells through Inactivation of the PI3K/Akt Signaling Pathway. Biomol. Ther. 2017, 25, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Xin, S.; Wang, H.; Ma, J.; Zhang, H.; Wei, H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-κB signaling pathway. Anticancer Drugs 2015, 26, 649–656. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Wang, K.S.; Mi, C.; Wang, Z.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol. Rep. 2016, 36, 2771–2776. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.Y.; Wu, J.F.; Liu, B.J.; Zhang, H.Y.; Cao, Y.X.; Sun, J.; Lv, Y.B.; Wu, X.; Dong, J.C. Flavonoid components in Scutellaria baicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int. J. Oncol. 2014, 44, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, S.; Ji, Y.; Li, J.; An, P.; Ren, H.; Liang, R.; Yang, J.; Li, Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS ONE 2013, 8, e72927. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, Y.; Nie, Q.; Tian, R.; Huang, J.; Mao, G. Baicalein inhibits invasion and promotes apoptosis in glioma cells through the PI3K/Akt pathway. J. BUON 2021, 26, 395–401. [Google Scholar]
- Wu, J.Y.; Tsai, K.W.; Li, Y.Z.; Chang, Y.S.; Lai, Y.C.; Laio, Y.H.; Wu, J.D.; Liu, Y.W. Anti-Bladder-Tumor Effect of Baicalein from Scutellaria baicalensis Georgi and Its Application In Vivo. Evid. Based Complement. Altern. Med. 2013, 2013, 579751. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.W.; Lin, T.H.; Huang, W.S.; Teng, C.Y.; Liou, Y.S.; Kuo, W.H.; Lin, W.L.; Huang, H.I.; Tung, J.N.; Huang, C.Y.; et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol. Appl. Pharmacol. 2011, 255, 316–326. [Google Scholar] [CrossRef]
- Rui, X.; Yan, X.I.; Zhang, K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol. Lett. 2016, 11, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, N.; Selvamani, A.; Subramanian, R.; Pandi, A.; Thiruvengadam, D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol. Appl. Pharmacol. 2012, 261, 10–21. [Google Scholar] [CrossRef]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Recio, C.; Guerra, B.; Guerra-Rodríguez, M.; Aranda-Tavío, H.; Martín-Rodríguez, P.; de Mirecki-Garrido, M.; Brito-Casillas, Y.; García-Castellano, J.M.; Estévez-Braun, A.; Fernández-Pérez, L. Signal transducer and activator of transcription (STAT)-5: An opportunity for drug development in oncohematology. Oncogene 2019, 38, 4657–4668. [Google Scholar] [CrossRef]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef]
- Susmitha, G.D.; Miyazato, K.; Ogura, K.; Yokoyama, S.; Hayakawa, Y. Anti-metastatic Effects of Baicalein by Targeting STAT3 Activity in Breast Cancer Cells. Biol. Pharm. Bull 2020, 43, 1899–1905. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Z.; Cai, H.; Li, Q.; Rong, W.; Kawano, M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur. J. Haematol. 2010, 84, 137–144. [Google Scholar] [CrossRef]
- Lei, H.; Shi, J.; Teng, Y.; Song, C.; Zou, L.; Ye, F.; Zhang, H. Baicalein modulates the radiosensitivity of cervical cancer cells in vitro via miR-183 and the JAK2/STAT3 signaling pathway. Adv. Clin. Exp. Med. 2021, 30, 727–736. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, J.; Zhao, S.; Luo, Q.; Tang, Q.; Yang, L.; Li, L.; Wu, W.; Hann, S.S. Baicalein increases the expression and reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKα and MEK/ERK1/2 signaling pathways in human non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2015, 34, 41. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma. J. Recept. Signal Transduct. Res. 2020, 40, 49–57. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, Y.J.; Baik, J.S.; Han, J.Y.; Lee, C.G.; Heo, K.; Park, Y.S.; Kim, J.S.; Ji, H.D.; Park, S.I.; et al. Baicalein inhibits agonist- and tumor cell-induced platelet aggregation while suppressing pulmonary tumor metastasis via cAMP-mediated VASP phosphorylation along with impaired MAPKs and PI3K-Akt activation. Biochem. Pharmacol. 2014, 92, 251–265. [Google Scholar] [CrossRef]
- Lian, H.; Hui, Y.; Xiaoping, T.; Wei, T.; Jiyi, X.; Xiaolan, Y. Baicalein suppresses the proliferation of human cervical cancer cells via Notch 1/Hes signaling pathway. J. Cancer Res. Ther. 2019, 15, 1216–1220. [Google Scholar] [CrossRef]
- Su, G.; Chen, H.; Sun, X. Baicalein suppresses non small cell lung cancer cell proliferation, invasion and Notch signaling pathway. Cancer Biomark 2018, 22, 13–18. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar]
- Khraiwesh, B.; Arif, M.A.; Seumel, G.I.; Ossowski, S.; Weigel, D.; Reski, R.; Frank, W. Transcriptional control of gene expression by microRNAs. Cell 2010, 140, 111–122. [Google Scholar]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar]
- Chiu, H.S.; Somvanshi, S.; Patel, E.; Chen, T.W.; Singh, V.P.; Zorman, B.; Patil, S.L.; Pan, Y.; Chatterjee, S.S.; The Cancer Genome Atlas Research Network; et al. Pan-Cancer Analysis of lncRNA Regulation Supports Their Targeting of Cancer Genes in Each Tumor Context. Cell Rep. 2018, 23, 297–312.e12. [Google Scholar]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, W.; Zhou, Y.B.; Xiang, Y.X.; Wang, L.S.; Hu, W.K.; Wang, W.J. Baicalein inhibits osteosarcoma cell proliferation and invasion through the miR-183/Ezrin pathway. Mol. Med. Rep. 2018, 18, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Li, J.; Bie, B.; Sun, J.; Mu, Y.; Shi, M.; Zhang, S.; Kong, G.; Li, Z.; Guo, Y. MiR-3663-3p participates in the anti-hepatocellular carcinoma proliferation activity of baicalein by targeting SH3GL1 and negatively regulating EGFR/ERK/NF-κB signaling. Toxicol. Appl. Pharmacol. 2021, 420, 115522. [Google Scholar] [CrossRef]
- Yu, X.; Cao, Y.; Tang, L.; Yang, Y.; Chen, F.; Xia, J. Baicalein inhibits breast cancer growth via activating a novel isoform of the long noncoding RNA PAX8-AS1-N. J. Cell Biochem. 2018, 119, 6842–6856. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3K/Akt pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, K.; Tan, J.; Meng, M.; Liu, C.M.; Chen, B.; Huang, C.; Wong, H.L.X.; Bian, Z.; Su, T.; et al. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clin. Transl. Med. 2021, 11, e564. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Luo, J.; Tong, L.; Gao, Y.; Feng, W.; Wang, F.; Cui, W.; Li, S.; Sun, Z. Baicalein suppresses growth of non-small cell lung carcinoma by targeting MAP4K3. Biomed. Pharmacother. 2021, 133, 110965. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, Q.; An, Y.; Fan, L.; Wang, J.; Li, H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m6A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine 2022, 94, 153823. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Wang, P.; Gao, S.; Qu, C.; Liu, L. Effects of baicalein on pancreatic cancer stem cells via modulation of sonic Hedgehog pathway. Acta Biochim. Biophys. Sin. 2018, 50, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, H.; Hu, P.; Qing, Y.; Wang, X.; Zhu, M.; Wang, H.; Wang, Z.; Xu, J.; Guo, Q.; et al. Natural HDAC-1/8 inhibitor baicalein exerts therapeutic effect in CBF-AML. Clin. Transl. Med. 2020, 10, e154. [Google Scholar] [CrossRef]
- Wang, F.R.; Jiang, Y.S. Effect of treatment with baicalein on the intracerebral tumor growth and survival of orthotopic glioma models. J. Neurooncol. 2015, 124, 5–11. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Zhang, M.; Song, Y.; Zhang, Y.; Fan, S.; Ren, S.; Fu, L.; Zhang, N.; Hui, H.; et al. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin. Transl. Med. 2021, 11, e577. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Sun, J.; Lu, L.; Liu, L.; Qiu, J.; Li, Q.; Yan, C.; Jiang, S.; Mohammadtursun, N.; et al. Baicalein Inhibits Orthotopic Human Non-Small Cell Lung Cancer Xenografts via Src/Id1 Pathway. Evid. Based Complement. Altern. Med. 2019, 2019, 9806062. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Xu, F.; Zhang, X.; Gu, Z. Baicalein Inhibits the SMYD2/RPS7 Signaling Pathway to Inhibit the Occurrence and Metastasis of Lung. Cancer J. Oncol. 2022, 2022, 3796218. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Liu, X.; Wang, J.; Ma, X.; Zhao, X.; Yang, Q.; Yan, W.; Zhao, Z.; Hui, Y.; et al. Baicalein suppress EMT of breast cancer by mediating tumor-associated macrophages polarization. Am. J. Cancer Res. 2018, 8, 1528–1540. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, A.A.; Kapanova, G.; Kalmakhanov, S.; Tanbayeva, G.; Zhakipbekov, K.S.; Rakhmetova, V.S.; Syzdykbayev, M.K. Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers. Int. J. Mol. Sci. 2022, 23, 8377. https://doi.org/10.3390/ijms23158377
Farooqi AA, Kapanova G, Kalmakhanov S, Tanbayeva G, Zhakipbekov KS, Rakhmetova VS, Syzdykbayev MK. Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers. International Journal of Molecular Sciences. 2022; 23(15):8377. https://doi.org/10.3390/ijms23158377
Chicago/Turabian StyleFarooqi, Ammad Ahmad, Gulnara Kapanova, Sundetgali Kalmakhanov, Gulnur Tanbayeva, Kairat S. Zhakipbekov, Venera S. Rakhmetova, and Marat K. Syzdykbayev. 2022. "Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers" International Journal of Molecular Sciences 23, no. 15: 8377. https://doi.org/10.3390/ijms23158377
APA StyleFarooqi, A. A., Kapanova, G., Kalmakhanov, S., Tanbayeva, G., Zhakipbekov, K. S., Rakhmetova, V. S., & Syzdykbayev, M. K. (2022). Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers. International Journal of Molecular Sciences, 23(15), 8377. https://doi.org/10.3390/ijms23158377






