A Preclinical Validation of Iron Oxide Nanoparticles for Treatment of Perianal Fistulizing Crohn’s Disease
Abstract
:1. Introduction
2. Results
2.1. Control Fistulas
2.2. Fistulas Treated with the Ultrasmall Particles of Iron Oxide
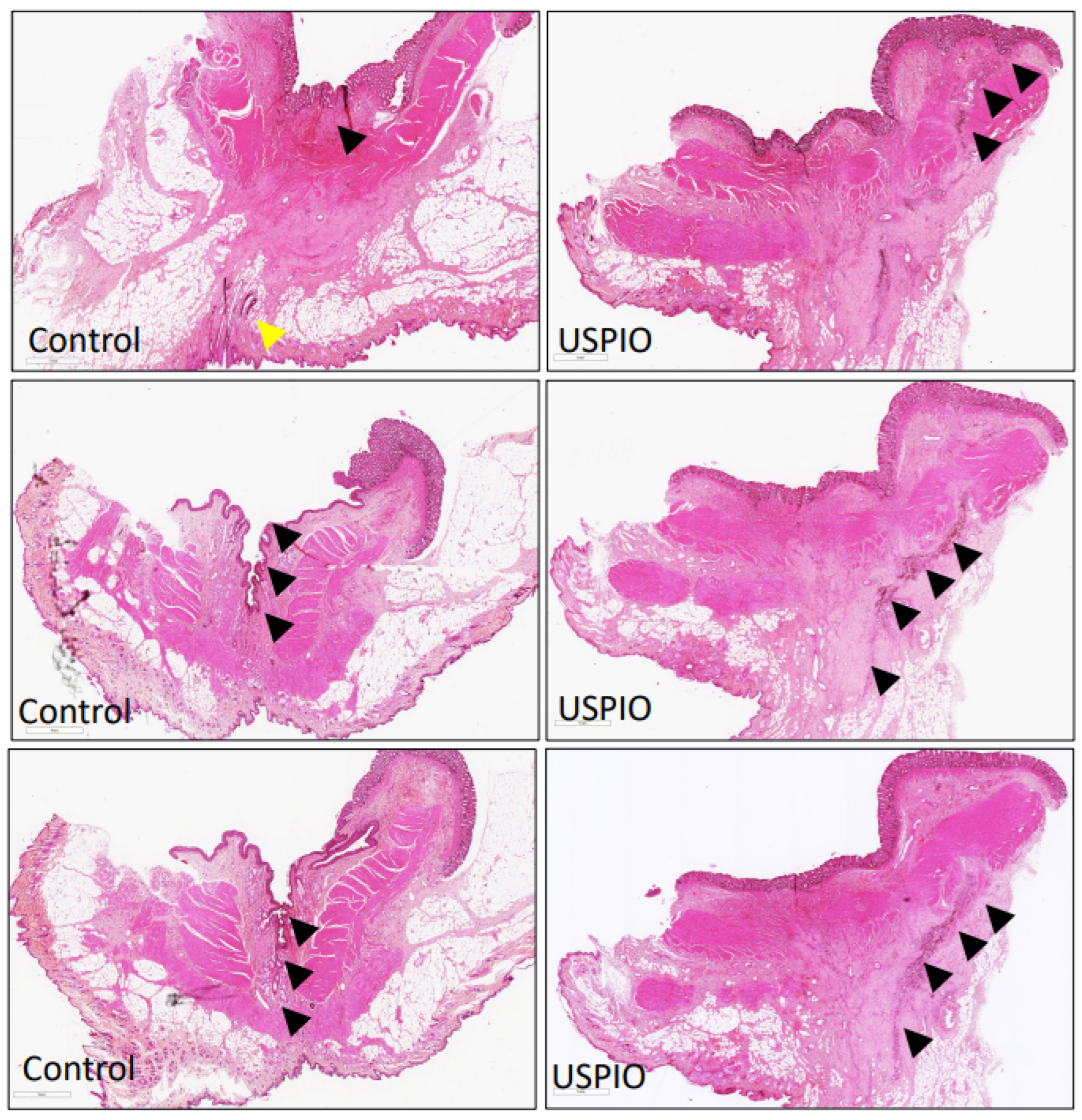
2.3. Pathological Examination
2.4. Safety
3. Discussion
4. Materials and Methods
4.1. Study Design and Animals
4.2. Preclinical Model of Perianal Fistulizing Crohn’s Disease
- -
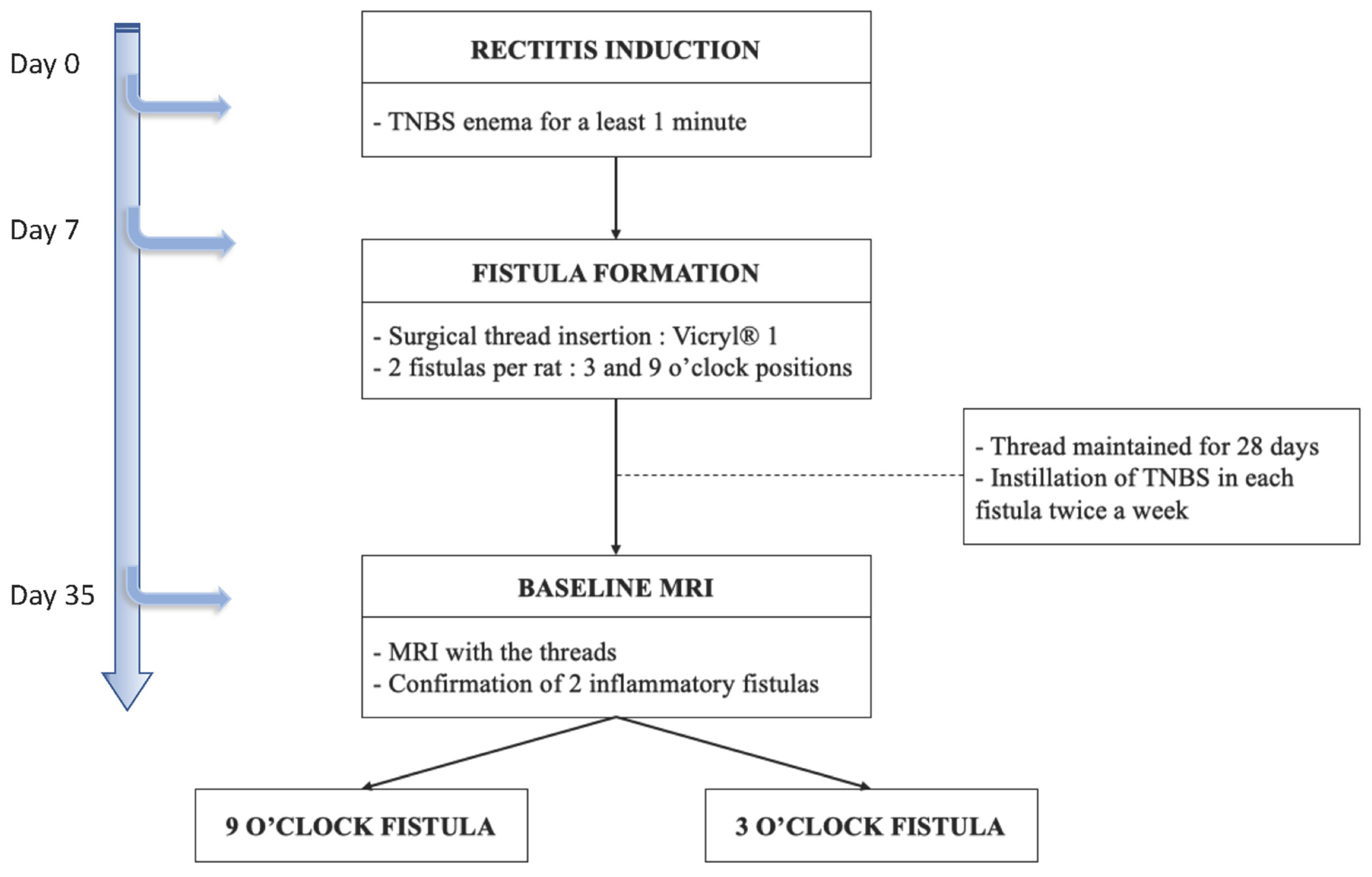
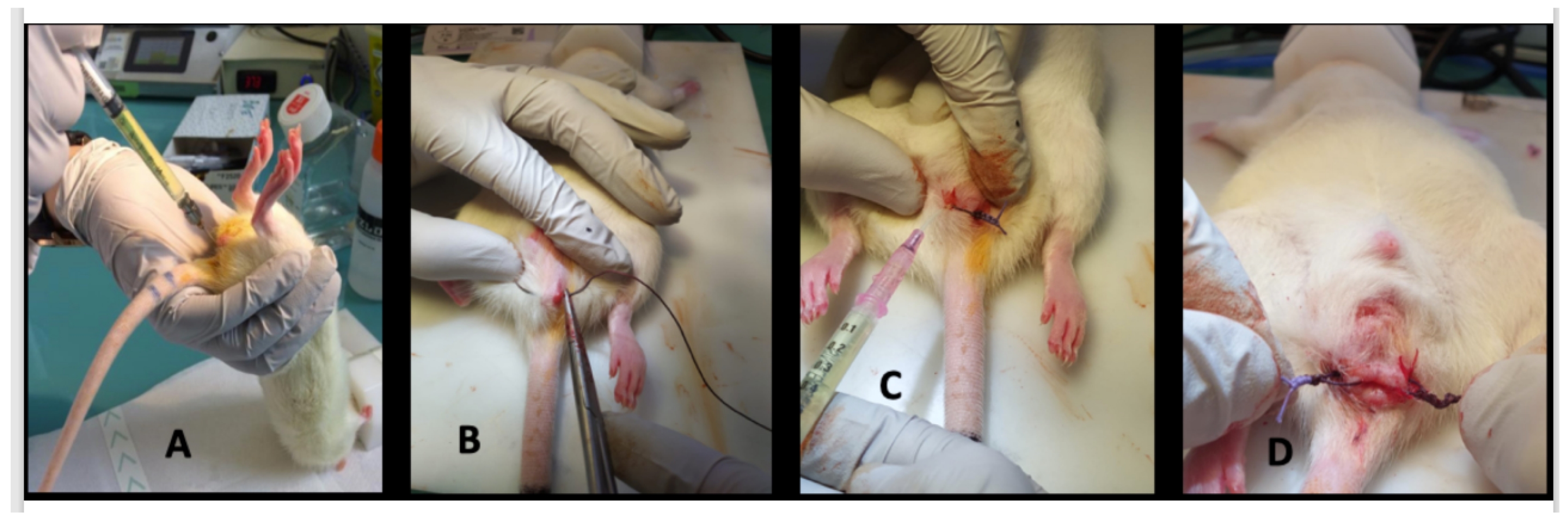
- day 0, proctitis induction: proctitis was induced with a 500 µL rectal enema containing a TNBS solution (2,4,6-trinitrobenzenesulfonic acid solution, picrylsulfonic acid, SIGMA laboratory). The TNBS solution contained 85.3 µL of TNBS (i.e., 25 mg), 250 µL of 100% ethanol, and 164.7 µL of saline solution. The rectal enema was maintained for at least 1 min (Figure 5A). The rectal inflammatory peak was reached between day 5 and day 7 after the enema.
- -
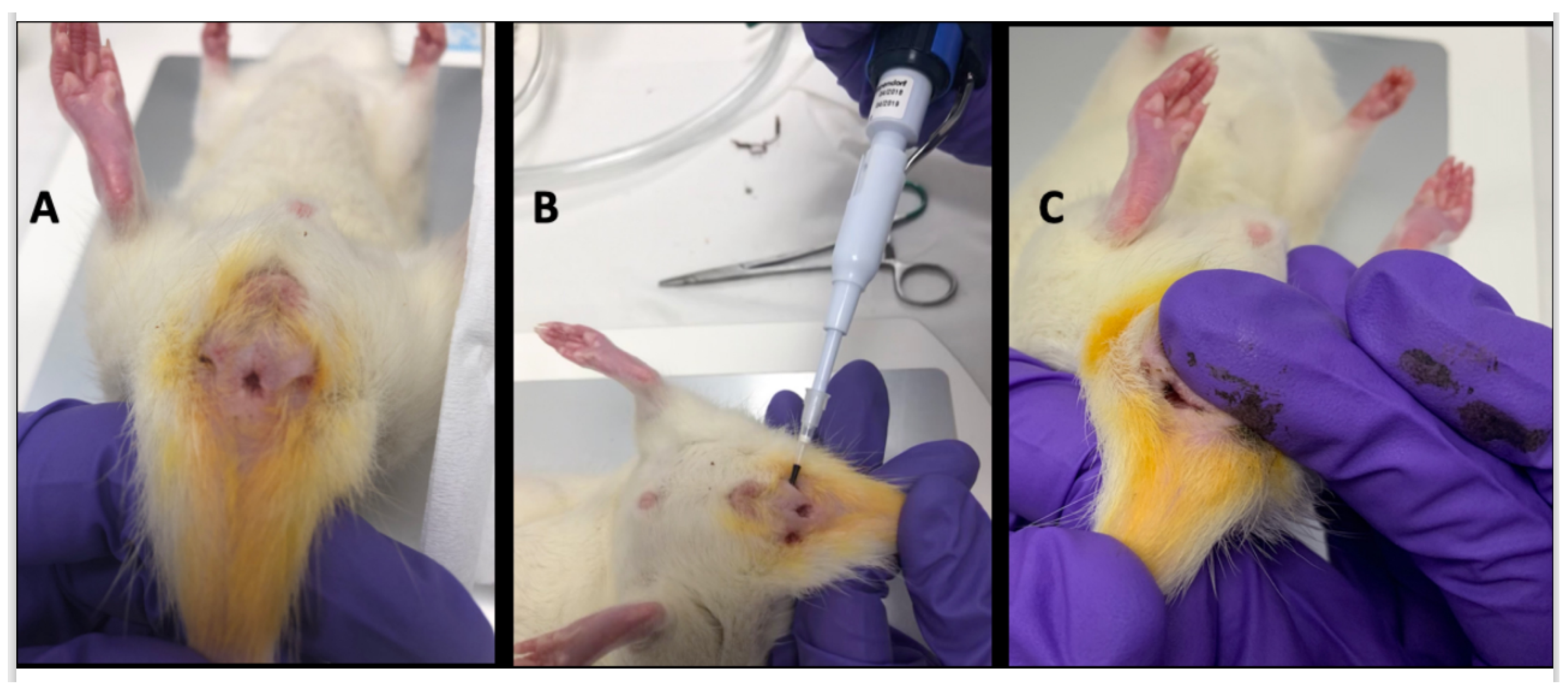
- day 7, fistula formation: at the peak of the rectal inflammation, 2 fistulas were created on each rat. The rats were placed in a supine position and this position was the reference to locate the fistulas (scrotum at 12 o’clock, tail at 6 o’clock). As shown in Figure 5B–D, the fistulas were created at 3 o’clock and 9 o’clock by inserting a surgical suture (Vicryl® 1, Ethicon Laboratory) through the rectum (internal orifice) and exiting at the perineum about 1 cm from the anal margin (external orifice). In order to obtain a good caliber, the fistula tract was sharpened with the surgical suture. An 18 G blunt fill needle was passed through the fistula as well as a 10 µL filter tip. We obtained internal and external orifices of approximately 2 mm in diameter. At the end of the surgical procedure, the thread was retained in the fistula tract and 100 µL of a TNBS solution was instilled within each tract (Figure 5C). The TNBS solution contained, for a 100 µL instillation, 17.06 µL of TNBS (i.e., 5 mg), 50 µL of pure ethanol, and 32.94 µL of saline solution. This concentration was identical to the rectal enema used for proctitis induction.
- -
- day 8 to day 34, monitoring threads in position: threads were maintained for 28 days. During this period, the rats were examined every 1 to 2 days to ensure that the fistulas were well-tolerated and that no threads fell out. If threads were lost, they were reinserted the same day during a short anesthesia. Twice a week, 100 µL of the same TNBS solution was instilled within each fistula tract.
- -
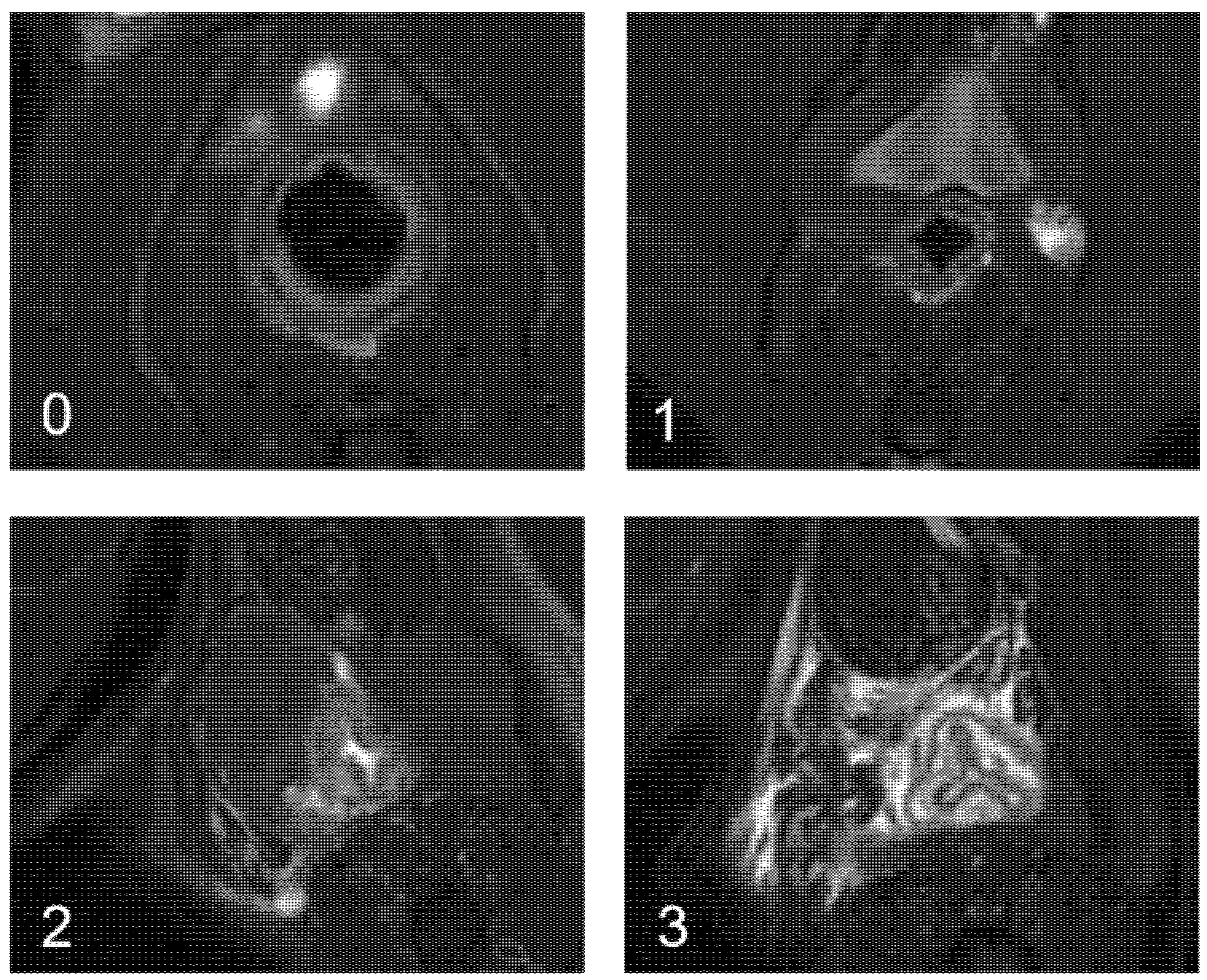
- day 35, baseline MRI: after maintaining the threads for 28 days, 2 inflammatory fistulas were obtained (Figure 6A) and a perineal MRI was performed to assess the pre-treatment tracts.
4.3. Treatment with the Ultrasmall Particles of Iron Oxide
4.4. Follow-Up and Fistula Assessment Methods
4.5. Outcomes
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sandborn, W.J.; Fazio, V.W.; Feagan, B.G.; Hanauer, S.B. AGA technical review on perianal Crohn’s disease. Gastroenterology 2003, 125, 1508–1530. [Google Scholar] [CrossRef] [PubMed]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; Overstraeten, A.D.B.V.; Burke, J.P.; Buskens, C.J.; Francesco, C.; et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohn’s Colitis 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Bouchard, D.; Abramowitz, L.; Bouguen, G.; Brochard, C.; Dabadie, A.; De Parades, V.; Eléouet-Kaplan, M.; Fathallah, N.; Faucheron, J.-L.; Maggiori, L.; et al. Anoperineal lesions in Crohn’s disease: French recommendations for clinical practice. Tech. Coloproctol. 2017, 21, 683–691. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342.e4. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.W.; Kim, I.; et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sus-tainable therapeutic effect for Crohn’s fistula. Stem. Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef]
- Lightner, A.L.; Wang, Z.; Zubair, A.C.; Dozois, E.J. A Systematic Review and Meta-analysis of Mesenchymal Stem Cell Injec-tions for the Treatment of Perianal Crohn’s Disease: Progress Made and Future Directions. Dis. Colon Rectum 2018, 61, 629–640. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2019, 14, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Flacs, M.; Collard, M.K.; Doblas, S.; Zappa, M.; Cazals-Hatem, D.; Maggiori, L.; Panis, Y.; Treton, X.; Ogier-Denis, E. Preclinical Model of Perianal Fistulizing Crohn’s Disease. Inflamm. Bowel Dis. 2019, 26, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2013, 505, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Laissy, J.P.; Mazighi, M.; Tchétché, D.; Louedec, L.; Adle-Biassette, H.; Chillon, S.; Henin, D.; Jacob, M.P.; Letourneur, D.; et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: Relationship between signal loss and macrophage infiltration. Arter. Thromb. Vasc. Biol. 2006, 26, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Smits, L.P.; Tiessens, F.; Zheng, K.H.; Stroes, E.S.; Nederveen, A.J.; Coolen, B.F. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017, 263, 211–218. [Google Scholar] [CrossRef]
- Zheng, K.H.; Schoormans, J.; Stiekema, L.C.; Calcagno, C.; Cicha, I.; Alexiou, C.; Strijkers, G.J.; Nederveen, A.J.; Stroes, E.S.; Coolen, B.F. Plaque Permeability Assessed With DCE-MRI Associates with USPIO Uptake in Patients With Peripheral Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Sangnier, A.P.; Van de Walle, A.B.; Curcio, A.; Le Borgne, R.; Motte, L.; Lalatonne, Y.; Wilhelm, C. Impact of magnetic nanoparticle surface coating on their long-term in-tracellular biodegradation in stem cells. Nanoscale 2019, 11, 16488–16498. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.E.; Veiseh, O.; Bhattarai, N.; Sun, C.; Hansen, S.J.; Ditzler, S.; Knoblaugh, S.; Lee, D.; Ellenbogen, R.; Zhang, M.; et al. Rapid pharmacokinetic and biodistribution studies using cholorotoxin-conjugated iron oxide nanoparticles: A novel non-radioactive method. PLoS ONE 2010, 5, e9536. [Google Scholar]
- Buchanan, G.N.; Sibbons, P.; Osborn, M.; Bartram, C.I.; Ansari, T.; Halligan, S.; Cohen, R.C.G. Experimental Model of Fistula-In-Ano. Dis. Colon Rectum 2005, 48, 353–358. [Google Scholar] [CrossRef]
- Aikawa, M.; Miyazawa, M.; Okada, K.; Akimoto, N.; Koyama, I.; Yamaguchi, S.; Ikada, Y. A Newly Designed Anal Fistula Plug: Clinicopathological Study in an Experimental Iatrogenic Fistula Model. Int. Surg. 2013, 98, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Arakaki, M.S.; dos Santos, C.H.M.; Falcão, G.R.; Cassino, P.C.; Nakamura, R.K.; Gomes, N.F.; dos Santos, R.G.C. Experimental model of anal fistula in rats. J. Coloproctol. 2013, 33, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Benlice, C.; Yildiz, M.; Baghaki, S.; Erguner, I.; Olgun, D.C.; Batur, S.; Erdamar, S.; Ambarcioglu, P.; Hamzaoglu, I.; Karahasanoglu, T.; et al. Fistula tract curettage and the use of biological dermal plugs improve high transsphincteric fistula healing in an animal model. Int. J. Color. Dis. 2015, 31, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ma-Mu-Ti-Jiang, A.B.; Chen, H.; Liu, X.; Wang, Y.H. Experimental porcine model of complex fistula-in-ano. World J. Gastroenterol. 2017, 23, 1828–1835. [Google Scholar] [CrossRef]
- Han, Y.M.; Kim, J.W.; Koh, S.J.; Kim, B.G.; Lee, K.L.; Im, J.P.; Kim, J.S.; Jung, H.C. Patients with perianal Crohn’s disease have poor disease outcomes after primary bowel re-section. J. Gastroenterol. Hepatol. 2016, 31, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Eder, V.; Caputo, G.; Journé, C.; Ou, P.; Bolley, J.; Louedec, L.; Guenin, E.; Motte, L.; Pinna, N.; et al. USPIO size control through microwave nonaqueous sol-gel method for neoangiogenesis T2MRI contrast agent. Nanomedicine 2016, 11, 2769–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]


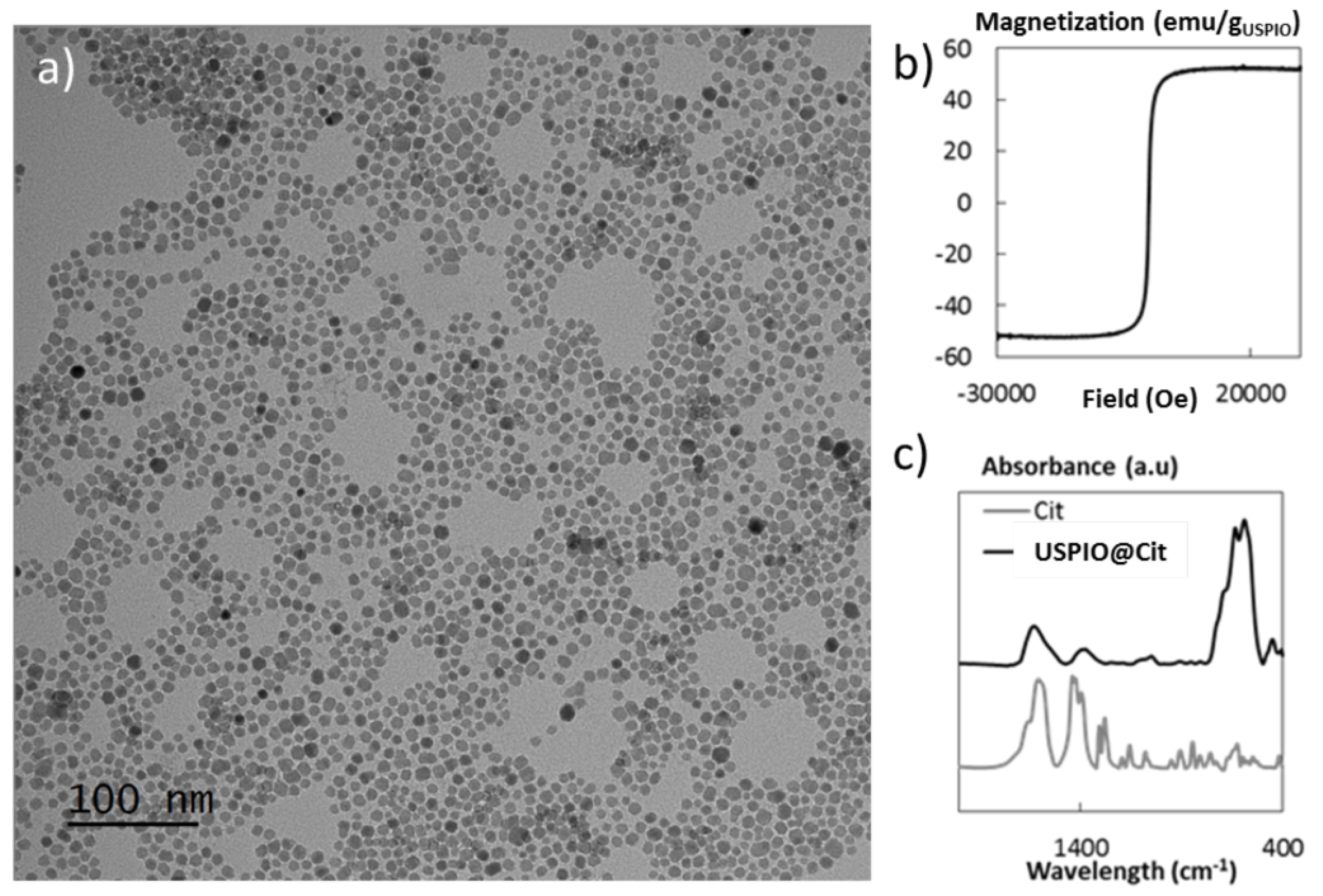

| Treated Fistula | Control Fistula | |
|---|---|---|
| (n = 17) | (n = 17) | |
| Clinical characteristics before treatment | ||
| Rectitis | ||
| - Soft stools | 17 (100) a | |
| - Perineal tumefaction | 17 (100) | |
| - Rectorrhagia | 10 (59) | |
| Weight (g) | 395 ± 51 [325–521] b | |
| External orifice presence | 17 (100) | 17 (100) |
| Internal orifice presence | 17 (100) | 17 (100) |
| MRI characteristics before treatment | ||
| Fistula tract c | 17 (100) | 17 (100) |
| External orifice presence | 17 (100) | 17 (100) |
| Internal orifice presence | 16 (94) | 17 (100) |
| Fistula tract diameter (mm) | 2.17 ± 0.6 [1.2–3.3] | 1.92 ± 0.3 [1.5–2.5] |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | 3.60 ± 0.56 | 3.50 ± 0.52 |
| [2.22–4.56] | [2.13–4.61] | |
| - ADC (mm2/s) | 1.42 ± 0.20 | 1.49 ± 0.23 |
| [1.058–1.709] | [1.09–1.92] | |
| USPIO Rats | Control Rat | |
|---|---|---|
| (n = 2) | (n = 1) | |
| MRI Characteristics before Treatment | ||
| Fistula tract a | 2 (100) b | 1 (100) |
| External orifice presence | 2 (100) | 1 (100) |
| Internal orifice presence | 2 (100) | 1 (100) |
| Fistula tract diameter (mm) | 1.3 [0.9–1.7] c | 1.9 |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | 4.96 [3.96–5.96] | 3.37 |
| - ADC (mm2/s) | 1.10 [0.91–1.28] | 1.40 |
| POD 7 | ||
| External orifice closure | 2 (100) | 0 |
| Internal orifice closure | 2 (100) | 0 |
| Filling/closing of fistula tract d | 2 (100) | 0 |
| Fistula tract diameter (mm) | 4.95 [4.7–5.2] | 2.3 |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | NM | 3.17 |
| - ADC (mm2/s) | NM | 0.997 |
| POD 14 | ||
| External orifice closure | 2 (100) | 0 |
| Internal orifice closure | 2 (100) | 0 |
| Filling/closing of fistula tract | 2 (100) | 0 |
| Fistula tract diameter (mm) | 4.15 [3.8–4.5] | 4 |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | NM | 2.66 |
| - ADC (mm2/s) | NM | 1.39 |
| Treated Fistula (n = 17) | Control Fistula (n = 17) | |
|---|---|---|
| External orifice closed | ||
| POD 1 | 17 (100) a | 3 (17) |
| POD 4 | 17 (100) | 15 (88) |
| POD 7 | 17 (100) | 15 (88) |
| Internal orifice closed | ||
| POD 1 | 17 (100) | 17 (100) |
| POD 4 | 17 (100) | 17 (100) |
| POD 7 | 17 (100) | 17 (100) |
| Weight (g) | ||
| POD 1 | 395 ± 51 [325–521] b | |
| POD 4 | 407 ± 53 [329–534] | |
| POD 7 | 414 ± 54 [337–538] | |
| Treated Fistula | Control Fistula | |
|---|---|---|
| (n = 17) | (n = 17) | |
| POD 1 | ||
| External orifice closure | 17 (100) a | 3 (17) |
| Internal orifice closure | 17 (100) | 0 |
| Filling/closing of fistula tract b | 17 (100) | 0 |
| Fistula tract diameter (mm) | 3.7 ± 0.9 [2.2–5.4] c | 1.8 ± 0.5 [1.2–2.9] |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | NM | 3.14 ± 0.5 [2.4–4.29] |
| - ADC (mm2/s) | NM | 1.54 ± 0.19 [1.20–1.94] |
| POD 4 | ||
| External orifice closure | 17 (100) | 7 (41) |
| Internal orifice closure | 17 (100) | 2 (11) |
| Filling/closing of fistula tract | 17 (100) | 3 (17) |
| Fistula tract diameter (mm) | 3.2 ± 0.8 [1.9–4.5] | 1.6 ± 0.4 [1.1–2.4] |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | NM | 2.49 ± 0.5 [1.79–3.46] |
| - ADC (mm2/s) | NM | 1.55 ± 0.29 [1.21–2.36] |
| POD 7 | ||
| External orifice closure | 17 (100) | 9 (53) |
| Internal orifice closure | 17 (100) | 4 (23) |
| Filling/closing of fistula tract | 17 (100) | 5 (29) |
| Fistula tract diameter (mm) | 3.2 ± 0.9 [1.2–4.8] | 1.4 ± 0.3 [0.9–2] |
| Peripheral tract inflammation | ||
| - T2 Signal (a.u) | NM | 2.25 ± 0.5 [1.64–3.35] |
| - ADC (mm2/s) | NM | 1.49 ± 0.27 [1.14–1.96] |
| T1-Weighted | T2-Weighted | UTE | DWI | |
|---|---|---|---|---|
| Echo time (ms) | 3.8 | 56 | 0.008 | 23 |
| Repetition time (ms) | 460 | 5300 | 4 | 2000 |
| Number of averages | 2 | 3 | 1 | 1 |
| Other specific parameters | FLASH sequence | RARE sequence | 3D acquisition | 20 segments; 3 directions; b values = 0, 150, 400, 800 s/mm2 |
| Field of view (mm) | 60 × 60 | 60 × 60 | 60 × 60 × 60 | 60 × 60 |
| Matrix | 256 × 256 | 256 × 256 | 128 × 128 × 128 | 128 × 128 |
| Slice thickness (mm) | 1 | 1 | - | 1 |
| Number of slices | 29 | 29 | - | 11 |
| Fat saturation | Yes | Yes | No | Yes |
| Acquisition time | 3 min 55 s | 8 min 29 s | 3 min 25 s | 6 min 40 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazelles, A.; Collard, M.K.; Lalatonne, Y.; Doblas, S.; Zappa, M.; Labiad, C.; Cazals-Hatem, D.; Maggiori, L.; Treton, X.; Panis, Y.; et al. A Preclinical Validation of Iron Oxide Nanoparticles for Treatment of Perianal Fistulizing Crohn’s Disease. Int. J. Mol. Sci. 2022, 23, 8324. https://doi.org/10.3390/ijms23158324
Cazelles A, Collard MK, Lalatonne Y, Doblas S, Zappa M, Labiad C, Cazals-Hatem D, Maggiori L, Treton X, Panis Y, et al. A Preclinical Validation of Iron Oxide Nanoparticles for Treatment of Perianal Fistulizing Crohn’s Disease. International Journal of Molecular Sciences. 2022; 23(15):8324. https://doi.org/10.3390/ijms23158324
Chicago/Turabian StyleCazelles, Antoine, Maxime K. Collard, Yoann Lalatonne, Sabrina Doblas, Magaly Zappa, Camélia Labiad, Dominique Cazals-Hatem, Léon Maggiori, Xavier Treton, Yves Panis, and et al. 2022. "A Preclinical Validation of Iron Oxide Nanoparticles for Treatment of Perianal Fistulizing Crohn’s Disease" International Journal of Molecular Sciences 23, no. 15: 8324. https://doi.org/10.3390/ijms23158324
APA StyleCazelles, A., Collard, M. K., Lalatonne, Y., Doblas, S., Zappa, M., Labiad, C., Cazals-Hatem, D., Maggiori, L., Treton, X., Panis, Y., Jarry, U., Desvallées, T., Eliat, P.-A., Pineau, R., Motte, L., Letourneur, D., Simon-Yarza, T., & Ogier-Denis, E. (2022). A Preclinical Validation of Iron Oxide Nanoparticles for Treatment of Perianal Fistulizing Crohn’s Disease. International Journal of Molecular Sciences, 23(15), 8324. https://doi.org/10.3390/ijms23158324








