Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges
Abstract
1. Introduction
2. Stem Cell-Based Therapies in IBD
2.1. Haematopoietic Stem Cells
2.2. Mesenchymal (Stem) Stromal Cells
2.2.1. MSC and Intestinal Homeostasis
2.2.2. MSC and Gut Microbiota
3. Therapeutic Potential of MSC in IBD
| First Author/Year | Experimental | Cell Surge | Administration Route | Dose of Product | Time | More Relevant Results |
|---|---|---|---|---|---|---|
| Barnhoorn (2020) [97] | DSS-induced colitis | BM-MSC | IP | 2 × 106 cells | 11 days | MSC after in vivo aggregation show a favorable RNA expression profile for the treatment of colitis. MSC spheroids showed high expression of Ki-67 and low levels of apoptotic marker cleaved caspase-3. Locally applied MSC and MSC spheroids are both able to ameliorate DSS-induced colitis and show similar clinical effects, including improvement in the macro and microscopic IBD score. |
| Barnhoorn (2020) [97] | DSS-induced colitis | BM-MSC | Endoscopic | 2 × 106 cells | 4–6 days | Endoscopic injection can be a feasible and effective novel application route for MSC therapy in patients with luminal IBD. |
| Chao (2016) [71] | TNBS-induced colitis | UC-MSC | IP | 10 × 106 cells | 14 days | The mortality in UC-MSC-treated TNBS mice was 20% (55% in colitis model); the treatment reduced the inflammation of the transmural area, depletion of epithelial cells and focal loss of crypts. IL-20 and TGF-Beta were significantly higher in UC-MSC-treated mice (p = 0.04 and 0.02 respectively). |
| Cheng (2017) [82] | DSS-induced colitis | BM-MSC/IL-25-BM-MSC | IV | 5 × 106 cells | 8 days | IL-25-MSC treatment significantly attenuate the colon shortening (12 ± 0.62 cm); IL-25 could enhance immunomodulatory ability of MSC via inhibiting Th17 immune response and promoting the regulation of Tregs cells. However, the study failed to confirm that IL-25 affected the migratory and regenerative capacities of MSC in vivo. |
| de Aguiar (2018) [84] | DSS-induced colitis | AT-MSC | IP | 10 × 106 cells | 7 days | ADMSC-treated mice did not present severe reduction in colon length, and presented a reduced tissue damage score index (3); less detachment of mucosa and submucosa layers, low villous blunting and partial preservation of crypt and epithelial integrity. The level of ZO-1 expression in the colon was re-established in ADMSC-treated mice. Significant reduction of IFN-gamma and TNF-Alpha, and reduction of IL-6 and MCP-1 protein levels. ADMSC treatment reduced DCs and macrophages presence in the colon. |
| de Aguiar (2018) [84] | DSS-induced colitis | AT-MSC | IP | 2 × 106 cells | 7 days | DMSC ameliorated the severity of DSS-induced colitis, reducing colitis pathological score and preventing colon shortening. |
| de la Portilla (2013) [98] | TNBS-induced colitis | AT-MSC | Local | 60 × 106 cells | 24 weeks | First study which shows the homing migration of MSC to areas of experimentally-induced colitis following rectal installation. |
| de la Portilla (2018) [90] | TNBS-induced colitis | AT-MSC | Local | 2 × 106 cells | 10 days | There were no differences in component rectal wall thicknesses with a higher Hunter score in the treated group compared with the controls. |
| Fu (2017) [78] | TNBS-induced colitis | AT-MSC | Mesenteric injection | 2 × 106 cells | 6 days | Dcreased the weight loss and DAI score, MPO activity; moreover relieved colitis, decreased colonic shortening, inflammatory cell infiltration and mucosal ulceration. Reduced levels of ROR(lamda)t and IL-17A; inhibited STAT3 phosphorylation, but increased STAT5 phosphorylation. |
| González-Rey (2009) [77] | DSS-induced chronic colitis | AT-MSC | IP | 10 × 106 cells | 27 days | AT-MSC treatment protects against DSS-induced acute colitis as well as chronic severe colitis (p 0.01 and p 0.001 respectively); reduces colonic inflammatory responses in DSS-induced chronic colitis (p 0.001). |
| Gregoire (2018) [99] | Fistulising Crohn’s disease | AT-MSC | Single intrafistular injection | 3 × 106−30 × 106 cells | 8 weeks | 6/8 fistulas healed, 2/8 improved. |
| Heidari (2021) [100] | DSS-induced colitis | AT-MSC/MSC-CM | IP | 10 × 106 cells | 34 days | There was no significant difference in the survival rate among the study groups; however, there was a significant increase in terms of the colon length (p 0.005). In the treated mice the level of mucosal damage was significantly lower (p 0.005), and the structure of the crypts also showed improvement of tissue healing. |
| Heidari (2021) [100] | DSS-induced colitis | AT-MSC | IP | 2 × 106 cells | 34 day | The regulatory effects of AT-MSC and their CM in inflammatory conditions because of colitis |
| In Kap (2010) [93] | DSS-induced colitis | MSC | IV | 1 × 106 cells | 7 days | Anti-addressin Ab coating on MSC increased cell delivery to inflamed colon and increased the efficacy of MSC treatment of IBD. |
| Jianxia Hu (2016) [101] | Luminal Crohn’s disease | UCB-MSC | IV | 0.5 × 106 cells | 3 month | 30/36 patients showed good response and diffuse and deep ulcer formation and severe inflammatory mucosa were improved markedly. |
| Lee (2016) [70] | DSS-induced colitis | BM-MSC | IV | 10 × 106 cells | 33 days | IL-10 production was upregulated by about 10-fold in BM-MSC-treated mice and showed a preventive effect on weight loss. |
| Lee (2016) [70] | DSS-induced colitis | BM-MSC | IV | 30 × 106 cells | 33 days | Infusion of BM-MSC at the onset of disease exerted preventive and rapid recovery effects. |
| Lee (2018) [79] | DSS-induced colitis | UCB-MSC | IP | 2 × 106 cells | 12 days | The survival rate was further increased by co-treatment compared to UCB-MSC or MIS416 single treatments; colon lengths were significantly increased in co-treatment; colonic inflammation was more effectively resolved by co-treatment with MSI416 and UCB-MSC, and only co-treatment markedly decreased fibrosis and enhanced tissue regeneration. Exposure to MIS416 increases the number of immune cells via activation of CD14+ macrophages. |
| Legaki (2016) [83] | DSS-induced colitis | Amniotic fluid-MSC | IP | 1.5 × 106 cells, 200 μL/dose | 7 days | CM treatment significantly decreased the extension and severity of the inflammation in comparison to the DSS-treated mice; the relative expression levels of IL-10 mRNA were significantly increased, similarly TNF-a and IL-1B levels were decreased at mRNA level. Additionally, TGFb1 was significantly higher (p 0.0001). |
| Mao (2017) [102] | DSS-induced colitis | UCB-MSC | IV | 400 μg exosomes/1.3 × 106 cells | 11 days | Exosomes from MSC have profound effects on alleviating DSS-induced IBD and may exert their impact through the modulation of IL-7 expression in macrophages. |
| Martín (2018) [103] | TNBS-induced colitis | AT-MSC | Local | 10 × 106 cells | 11 days | Submucosal injection of human ASCs ameliorates the course of TNBS colitis in immunocompetent rats. |
| Martin Arranz (2018) [81] | TNBS-induced colitis | AT-MSC | Endoscopic | 10 × 106 cells | 11 days | The endoscopic score improved in the ASC group by 47.1% ± 5.3% vs. 21.8% ± 6.6% in the vehicle group. |
| Miyamoto (2017) [87] | TNBS-induced colitis | AT-MSC | IV and Local | 1 × 106 cells IV and 400 μL Local | 7 days | hAMSC transplantation significantly decreased the number of neutrophils, attenuated acute inflammation, suppressed the expression levels of inflammatory mediators in the colons; in the TNBS-CM gel group ulcers were shallow and bleeding was not detected, therefore improved endoscopic score. In the gel group mRNA expression levels of TNF-Alpha, CXCL1, CCL2 and IL-6 were increased. |
| Molendijk (2015) [104] | Fistulising Crohn’s disease | BM-MSC | Single intrafistular injection | 10, 30, 90 × 106 cells | 6, 12, 24 weeks | At week twelve, 3 of 9 individual fistulas had healed in group 1 (33.3%), 6 of 7 had healed in group 2 (85.7%), 2 of 7 had healed in group 3 (28.6%), and 3 of 9 had healed in the placebo group (33.3%). |
| Pak (2018) [88] | DSS-induced colitis | BM-MSC/AT-MSC | Endoscopic | 8 × 105 cells/1.1 × 106 cells | 1–3 days | The success rate was 37.60% for AT-MSC group and 35.20% for BM-MSC group. |
| Panés (2016) [105] | Fistulising Crohn’s disease | AT-MSC | Single intrafistular injection | 120 × 106 cells | 24 weeks | Remission in the ITT (53 of 107 [50%] vs. 36 of 105 [34%]; difference 15.2%, 97.5% CI 0.2–30.3; p = 0·024) C × 601 vs. placebo. |
| Panés (2018) [106] | Fistulising Crohn’s disease | AT-MSC | Single intrafistular injection | 120 × 106 cells | 52 weeks | C × 601 achieved combined remission (56.3%) vs. controls (38.6%) (a difference of 17.7 percentage points; 95% CI 4.2–31.2; p = 0.010). |
| Park (2018) [107] | DSS-induced chronic colitis | AT-MSC | IP | 10 × 106 cells | 20 days | In DSS-induced chronic colitis model, hASCs decreased the frequency of macrophage transition, specially M1 macrophages. The results suggest that PGE2, produced by co-culture of ASCs and THP-1, reduces M1 population. |
| Park (2018) [107] | DSS-induced colitis | AT-MSC | IP | 2 × 106 cells | 20 day | ASCs can suppress the inflammatory response by controlling the macrophage population, and ASCs may be therapeutically useful for the treatment of IBD. |
| Pouya (2018) [75] | DSS-induced colitis | MSC-CM | IP | 500 μL, ×3 | 10 days | After infusion, colon inflammation was reduced and histopathological analysis showed a decrease in mucosal degeneration. |
| Song (2017) [72] | DSS-induced acute/chronic colitis | MSC-Ex/UC-MSC | IP | 10 × 106 cells; 150 μg/mouse | 36 days | MSC-Ex ameliorates the clinical parameters in DSS-induced colitis; the treated group showed significantly less MPO activity. The level of IL-17 was significantly decreased, whereas those of IL-10 and TGF-Beta1 were increased. MSC-Ex is superior to UC-MSC in chronic IBD models, without differences in the colon length. |
| Song (2018) [85] | DSS-induced colitis | canine AT-MSC | IP | 2 × 106 + TSG-6 siRNA | 10 days | AT-MSC-secreted TSG-6 reduced inflammatory response and apoptosis in the colon; intraperitoneally infused cAT-MSC did not migrate to the inflamed colon; increased M2 macrophages in the inflamed colon. |
| Soontararak (2018) [80] | DSS-induced colitis | iMSC/AT-MSC | IV | 3 × 106 cells | 19 days | The clinical illness scores were significantly reduced (iMSC-treated p = 0.003, adMSC-treated p = 0.001); colonic tissues from mice treated with either iMSC or adMSC exhibited an overall reduction in transmural inflammation, with significantly less infiltration of inlammatory cells in the lamina propria, diminished mucosal ulceration and decreased mucosal collapse and granulation tissue formation. |
| Tanaka (2008) [68] | DSS-induced colitis | BM-MSC | IV | 5 × 106 cells | 7 days | In the rectum of treated rats the mRNA expression of TNF-alpha and IL-1Beta was markedly decreased to (43.7 ± 25.5% p 0.05 and 14.5 ± 12% p 0.01 respectively), as well as COX-2 16.5 ± 15.2% (p 0.01). |
| Tanaka (2008) [68] | DSS-induced colitis | MSC | IV | 5 × 106 cells | 7 days | Exogenous MSC accumulated in inflamed tissues and ameliorated DSS-induced colitis via a local anti-inflammatory action. |
| Wang (2016) [108] | DSS-induced colitis | BM-MSC | IP | 0.5 × 106 cells | 10 days | Intraperitoneal injection is the best delivery way for MSC: showed better mucosa recovery and higher cell engraftment at inflamed colon. |
| Wu (2018) [109] | DSS-induced colitis | UCB-MSC | IV | 400 μg UC-MSC | 11 days | Exosomes from hucMSC have profound effects on alleviating DSS-induced IBD and may exert their function by regulating the ubiquitin modification level. |
| Xu (2018) [86] | DSS-induced colitis | Endometrial regenerative cells (ERC) | IV | 3 × 106 | 10 days | ERC treatment significantly reduced the levels of TNF-Alpha, IL-1Beta and IL-6; ERCs downregulated the expanded Th1 and Th17 cells in colitis, and elevated the proportion of Tregs in lymphocytes; ERCs inhibited B-cell activation, differentiation and IgG production in colitis. ERC treatment enhanced the concentration of IL-10 in the colon and spleen, as well as CD1dhiCD5 + B cells in the spleen, peritoneal cavity and MLN. |
| Yu (2017) [73] | DSS-induced colitis | Tonsil-MSC | IP | 20 × 106/40 × 106 cells | 30 days | Co-culture with T-MSC clearly inhibited the PMA-stimulated proliferation of splenocytes by 60%; TMSC [×4] treated mice’s survival rate was improved to that of the normal. TMSC [×2] injection also significantly improved the survival rate to 89% of the control. TMSC [×4] treatment inhibits DSS-induced colon shortening; TMSC injection does no inhibit histopathological alterations in the distal colon in the chronic colitis mouse model, although it ameliorates IL-1Beta and IL-6 mRNA production in chronic colitis mice. |
3.1. Potential Therapeutic Mechanisms of MSC in IBD
3.1.1. Anti-Inflammatory Effects
3.1.2. Regenerative Effects
3.1.3. Antifibrotic Effects
3.1.4. Anti-Apoptotic Effects
3.1.5. Anti-Oxidative Stress Effects
3.1.6. Antimicrobial Effects
3.1.7. Anti-Tumor Effects
3.1.8. IBD and Colon Cancer
3.2. Clinical Trials
3.2.1. Clinical Trials on MSC Transplantation in IBD
3.2.2. MSC in Fistulizing and Perianal CD
3.2.3. MSC Transplantation in Luminal Crohn’s Disease
3.2.4. MSC Transplantation in Ulcerative Colitis
4. Limitations of MSC Therapy in IBD
Regulatory Aspects
5. New Perspectives for MSC Therapy in IBD
5.1. Importance of the Type of MSC
5.2. Cell-Free Therapy
5.2.1. Cell Extracts
5.2.2. Conditioned Medium
5.2.3. Extracellular Vesicles
6. New Technologies for MSC Cultures and Mass Production of Secretome Derived Products
6.1. MSC In Vitro Production
6.2. Ex Vivo MSC Modifications toward More Specific Therapeutic Applications
6.3. Standardization and Functional Tests Research for Specific Applications
6.4. Route of Administration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Pedersen, N.; Čuković-Čavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonová, O.; Vind, I.; Avnstrøm, S.; Thorsgaard, N.; et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, G.J.; Masedo, Á.; Yela, C.; Martínez-Montiel Mdel, P.; Casís, B. Current stage in inflammatory bowel disease: What is next? World J. Gastroenterol. 2015, 21, 11282–11303. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef]
- Abdel Salam, A.G.; Ata, H.M.; Salman, T.M.; Rashed, L.A.; Sabry, D.; Schaalan, M.F. Potential therapeutic utility of mesenchymal stem cells in inflammatory bowel disease in mice. Int. Immunopharmacol. 2014, 22, 515–521. [Google Scholar] [CrossRef]
- Zhu, J.F.; Xu, Y.; Zhao, J.; Li, X.; Meng, X.; Wang, T.Q.; Zou, B.Y.; Zhao, P.Y.; Liu, Q.; Lu, C.L.; et al. IL-33 Protects Mice against DSS-Induced Chronic Colitis by Increasing Both Regulatory B Cell and Regulatory T Cell Responses as Well as Decreasing Th17 Cell Response. J. Immunol. Res. 2018, 2018, 1827901. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, Y.; Zhao, N.; He, X.; Lu, A.; Wei, W.; Jiang, M. The Th17/Treg Immune Imbalance in Ulcerative Colitis Disease in a Chinese Han Population. Mediat. Inflamm 2016, 2016, 7089137. [Google Scholar] [CrossRef]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 2014, 2014, 928461. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef]
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Liver Physiol. 2016, 311, G59–G73. [Google Scholar] [CrossRef]
- Thomas, A.; Lodhia, N. Advanced therapy for inflammatory bowel disease: A guide for the primary care physician. J. Am. Board Fam. Med. 2014, 27, 411–420. [Google Scholar] [CrossRef][Green Version]
- Jneid, H.; Anderson, J.L.; Wright, R.S.; Adams, C.D.; Bridges, C.R.; Casey, D.E., Jr.; Ettinger, S.M.; Fesmire, F.M.; Ganiats, T.G.; Lincoff, A.M.; et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2012, 60, 645–681. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Rifas-Shiman, S.L.; Porter, C.Q.; Ollendorf, D.A.; Sandler, R.S.; Galanko, J.A.; Finkelstein, J.A. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 2008, 135, 1907–1913. [Google Scholar] [CrossRef]
- Mozaffari, S.; Nikfar, S.; Abdolghaffari, A.H.; Abdollahi, M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin. Biol. Ther. 2014, 14, 583–600. [Google Scholar] [CrossRef]
- Bernstein, C.N. Treatment of IBD: Where we are and where we are going. Am. J. Gastroenterol. 2015, 110, 114–126. [Google Scholar] [CrossRef]
- Abdu-Allah, H.; El-Shorbagi, A.-N.; Abdel-Moty, S.; El-awady, R.; Abdel-Alim, A.-A. 5-Aminosalyclic Acid (5-ASA): A Unique Anti-Inflammatory Salicylate. Med. Chem. 2016, 6, 306–315. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Costa, L.; Esparza, P.; Landin, M.; Diaz-Rodriguez, P.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. Int. J. Mol. Sci. 2019, 20, 3738. [Google Scholar] [CrossRef]
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef]
- Marmont, A.M. New horizons in the treatment of autoimmune diseases: Immunoablation and stem cell transplantation. Annu Rev. Med. 2000, 51, 115–134. [Google Scholar] [CrossRef]
- Snowden, J.A.; Badoglio, M.; Labopin, M.; Giebel, S.; McGrath, E.; Marjanovic, Z.; Burman, J.; Moore, J.; Rovira, M.; Wulffraat, N.M.; et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017, 1, 2742–2755. [Google Scholar] [CrossRef]
- Balassa, K.; Danby, R.; Rocha, V. Haematopoietic stem cell transplants: Principles and indications. Br. J. Hosp. Med. 2019, 80, 33–39. [Google Scholar] [CrossRef]
- Kashyap, A.; Forman, S.J. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Br. J. Haematol. 1998, 103, 651–652. [Google Scholar] [CrossRef]
- Musso, M.; Porretto, F.; Crescimanno, A.; Bondì, F.; Polizzi, V.; Scalone, R. Crohn’s disease complicated by relapsed extranodal Hodgkin’s lymphoma: Prolonged complete remission after unmanipulated PBPC autotransplant. Bone Marrow Transplant. 2000, 26, 921–923. [Google Scholar] [CrossRef][Green Version]
- Söderholm, J.D.; Malm, C.; Juliusson, G.; Sjödahl, R. Long-term endoscopic remission of crohn disease after autologous stem cell transplantation for acute myeloid leukaemia. Scand. J. Gastroenterol. 2002, 37, 613–616. [Google Scholar] [CrossRef]
- Hawkey, C.J.; Allez, M.; Clark, M.M.; Labopin, M.; Lindsay, J.O.; Ricart, E.; Rogler, G.; Rovira, M.; Satsangi, J.; Danese, S.; et al. Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease: A Randomized Clinical Trial. Jama 2015, 314, 2524–2534. [Google Scholar] [CrossRef]
- López-García, A.; Rovira, M.; Jauregui-Amezaga, A.; Marín, P.; Barastegui, R.; Salas, A.; Ribas, V.; Feu, F.; Elizalde, J.I.; Fernández-Avilés, F.; et al. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn’s Disease: Efficacy in a Single-Centre Cohort. J. Crohn’s Colitis 2017, 11, 1161–1168. [Google Scholar] [CrossRef]
- Burt, R.K.; Kaiser, R.L., Jr.; Ruiz, M.A. Stem-cell transplantation for Crohn’s disease: Same authors, different conclusions? Lancet Gastroenterol. Hepatol. 2017, 2, 386–387. [Google Scholar] [CrossRef]
- Clerici, M.; Cassinotti, A.; Onida, F.; Trabattoni, D.; Annaloro, C.; Della Volpe, A.; Rainone, V.; Lissoni, F.; Duca, P.; Sampietro, G.; et al. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig. Liver Dis. 2011, 43, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Marmont, A.M. Immunoablation followed or not by hematopoietic stem cells as an intense therapy for severe autoimmune diseases. New perspectives, new problems. Haematologica 2001, 86, 337–345. [Google Scholar] [PubMed]
- Cassinotti, A.; Passamonti, F.; Segato, S. CELL THERAPY IN INFLAMMATORY BOWEL DISEASE. Pharmacol. Res. 2021, 163, 105247. [Google Scholar] [CrossRef]
- Gooley, T.A.; Chien, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.J.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef]
- Hahn, T.; McCarthy, P.L., Jr.; Hassebroek, A.; Bredeson, C.; Gajewski, J.L.; Hale, G.A.; Isola, L.M.; Lazarus, H.M.; Lee, S.J.; Lemaistre, C.F.; et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J. Clin. Oncol. 2013, 31, 2437–2449. [Google Scholar] [CrossRef]
- Mehta, K.; Jaiswal, P.; Briggs, F.; Faubion, W.A.; Tabibian, J.H.; Cominelli, F.; Dave, M. In-patient outcomes of Hematopoietic Stem Cell Transplantation in Patients with Immune Mediated Inflammatory Diseases: A Nationwide Study. Sci. Rep. 2018, 8, 6825. [Google Scholar] [CrossRef]
- Penack, O.; Smith, O.M.; Cunningham-Bussel, A.; Liu, X.; Rao, U.; Yim, N.; Na, I.K.; Holland, A.M.; Ghosh, A.; Lu, S.X.; et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J. Exp. Med. 2009, 206, 2101–2110. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Montcuquet, N.; Roy, M.; Dussaillant, M.; Hugot, J.P.; Barreau, F. Complementary Roles of Nod2 in Hematopoietic and Nonhematopoietic Cells in Preventing Gut Barrier Dysfunction Dependent on MLCK Activity. Inflamm. Bowel Dis. 2017, 23, 1109–1119. [Google Scholar] [CrossRef]
- Lee, C.; Choi, C.; Kang, H.S.; Shin, S.W.; Kim, S.Y.; Park, H.C.; Hong, S.N. NOD2 Supports Crypt Survival and Epithelial Regeneration after Radiation-Induced Injury. Int. J. Mol. Sci. 2019, 20, 4297. [Google Scholar] [CrossRef]
- Jansen, S.A.; Nieuwenhuis, E.E.S.; Hanash, A.M.; Lindemans, C.A. Challenges and opportunities targeting mechanisms of epithelial injury and recovery in acute intestinal graft-versus-host disease. Mucosal Immunol. 2022, 15, 605–619. [Google Scholar] [CrossRef]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Lotfinegad, P.; Shamsasenjan, K.; Movassaghpour, A.; Majidi, J.; Baradaran, B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: A hope in cell therapy. Adv. Pharm. Bull. 2014, 4, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- De Bari, C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res. Ther. 2015, 17, 113. [Google Scholar] [CrossRef]
- Cheng, R.J.; Xiong, A.J.; Li, Y.H.; Pan, S.Y.; Zhang, Q.P.; Zhao, Y.; Liu, Y.; Marion, T.N. Mesenchymal Stem Cells: Allogeneic MSC May Be Immunosuppressive but Autologous MSC Are Dysfunctional in Lupus Patients. Front. Cell Dev. Biol. 2019, 7, 285. [Google Scholar] [CrossRef]
- Rennert, R.C.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef]
- Hou, R.; Liu, R.; Niu, X.; Chang, W.; Yan, X.; Wang, C.; Li, J.; An, P.; Li, X.; Yin, G.; et al. Biological characteristics and gene expression pattern of bone marrow mesenchymal stem cells in patients with psoriasis. Exp. Dermatol. 2014, 23, 521–523. [Google Scholar] [CrossRef]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef]
- Beswick, E.J.; Johnson, J.R.; Saada, J.I.; Humen, M.; House, J.; Dann, S.; Qiu, S.; Brasier, A.R.; Powell, D.W.; Reyes, V.E.; et al. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J. Immunol. 2014, 193, 2218–2229. [Google Scholar] [CrossRef]
- Thomson, C.A.; Nibbs, R.J.; McCoy, K.D.; Mowat, A.M. Immunological roles of intestinal mesenchymal cells. Immunology 2020, 160, 313–324. [Google Scholar] [CrossRef]
- Biancheri, P.; Di Sabatino, A.; Corazza, G.R.; MacDonald, T.T. Proteases and the gut barrier. Cell Tissue Res. 2013, 351, 269–280. [Google Scholar] [CrossRef]
- Hidalgo-Garcia, L.; Molina-Tijeras, J.A.; Huertas-Peña, F.; Ruiz-Malagón, A.J.; Diez-Echave, P.; Vezza, T.; Rodríguez-Sojo, M.J.; Morón, R.; Becerra-Massare, P.; Rodríguez-Nogales, A.; et al. Intestinal mesenchymal cells regulate immune responses and promote epithelial regeneration in vitro and in dextran sulfate sodium-induced experimental colitis in mice. Acta Physiol. 2021, 233, e13699. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Lacroix, V.; Cassard, A.; Mas, E.; Barreau, F. Multi-Omics Analysis of Gut Microbiota in Inflammatory Bowel Diseases: What Benefits for Diagnostic, Prognostic and Therapeutic Tools? Int. J. Mol. Sci. 2021, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Wang, L.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: An enhanced therapeutic effect. Clin. Transl. Med. 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Han, C.; Li, Y.; Qian, W.; Hou, X. Cancer-Preventive Role of Bone Marrow-Derived Mesenchymal Stem Cells on Colitis-Associated Colorectal Cancer: Roles of Gut Microbiota Involved. Front. Cell Dev. Biol. 2021, 9, 642948. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; He, L.; Wu, Q.; Li, J.; He, Y.; Zhao, L.; Chen, S.; An, J.; Liu, Y.; Chen, C.; et al. Microbiota regulates bone marrow mesenchymal stem cell lineage differentiation and immunomodulation. Stem Cell Res. Ther. 2017, 8, 213. [Google Scholar] [CrossRef]
- Lauro, M.L.; Burch, J.M.; Grimes, C.L. The effect of NOD2 on the microbiota in Crohn’s disease. Curr. Opin. Biotechnol. 2016, 40, 97–102. [Google Scholar] [CrossRef]
- Sidiq, T.; Yoshihama, S.; Downs, I.; Kobayashi, K.S. Nod2: A Critical Regulator of Ileal Microbiota and Crohn’s Disease. Front. Immunol. 2016, 7, 367. [Google Scholar] [CrossRef]
- Gu, L.; Ren, F.; Fang, X.; Yuan, L.; Liu, G.; Wang, S. Exosomal MicroRNA-181a Derived From Mesenchymal Stem Cells Improves Gut Microbiota Composition, Barrier Function, and Inflammatory Status in an Experimental Colitis Model. Front. Med. 2021, 8, 660614. [Google Scholar] [CrossRef]
- Tanaka, F.; Tominaga, K.; Ochi, M.; Tanigawa, T.; Watanabe, T.; Fujiwara, Y.; Ohta, K.; Oshitani, N.; Higuchi, K.; Arakawa, T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008, 83, 771–779. [Google Scholar] [CrossRef]
- Wang, M.; Liang, C.; Hu, H.; Zhou, L.; Xu, B.; Wang, X.; Han, Y.; Nie, Y.; Jia, S.; Liang, J.; et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci. Rep. 2016, 6, 30696. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, S.H.; Jang, H.W.; Kwon, J.H.; Lee, K.J.; Kim, C.H.; Park, S.J.; Hong, S.P.; Cheon, J.H.; Kim, T.I.; et al. Long-Term Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-Induced Murine Chronic Colitis. Gut Liver 2016, 10, 412–419. [Google Scholar] [CrossRef]
- Chao, K.; Zhang, S.; Qiu, Y.; Chen, X.; Zhang, X.; Cai, C.; Peng, Y.; Mao, R.; Pevsner-Fischer, M.; Ben-Horin, S.; et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells. Stem Cell Res. Ther. 2016, 7, 109. [Google Scholar] [CrossRef]
- Song, J.Y.; Kang, H.J.; Hong, J.S.; Kim, C.J.; Shim, J.Y.; Lee, C.W.; Choi, J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci. Rep. 2017, 7, 9412. [Google Scholar] [CrossRef]
- Yu, Y.; Song, E.M.; Lee, K.E.; Joo, Y.H.; Kim, S.E.; Moon, C.M.; Kim, H.Y.; Jung, S.A.; Jo, I. Therapeutic potential of tonsil-derived mesenchymal stem cells in dextran sulfate sodium-induced experimental murine colitis. PLoS ONE 2017, 12, e0183141. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, T.; Yang, D. Cotransfer of regulatory T cells improve the therapeutic effectiveness of mesenchymal stem cells in treating a colitis mouse model. Exp. Anim. 2017, 66, 167–176. [Google Scholar] [CrossRef][Green Version]
- Pouya, S.; Heidari, M.; Baghaei, K.; Asadzadeh Aghdaei, H.; Moradi, A.; Namaki, S.; Zali, M.R.; Hashemi, S.M. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int. Immunopharmacol. 2018, 54, 86–94. [Google Scholar] [CrossRef]
- Heidari, M.; Pouya, S.; Baghaei, K.; Aghdaei, H.A.; Namaki, S.; Zali, M.R.; Hashemi, S.M. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J. Cell. Physiol. 2018, 233, 8754–8766. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Anderson, P.; González, M.A.; Rico, L.; Büscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef]
- Fu, Z.W.; Zhang, Z.Y.; Ge, H.Y. Mesenteric injection of adipose-derived mesenchymal stem cells relieves experimentally-induced colitis in rats by regulating Th17/Treg cell balance. Am. J. Transl. Res. 2018, 10, 54–66. [Google Scholar]
- Lee, B.C.; Shin, N.; Lee, J.Y.; Kang, I.; Kim, J.J.; Lee, S.E.; Choi, S.W.; Webster, G.A.; Kang, K.S. MIS416 Enhances Therapeutic Functions of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Experimental Colitis by Modulating Systemic Immune Milieu. Front. Immunol. 2018, 9, 1078. [Google Scholar] [CrossRef]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef]
- Martín Arranz, E.; Martín Arranz, M.D.; Robredo, T.; Mancheño-Corvo, P.; Menta, R.; Alves, F.J.; Suárez de Parga, J.M.; Mora Sanz, P.; de la Rosa, O.; Büscher, D.; et al. Endoscopic submucosal injection of adipose-derived mesenchymal stem cells ameliorates TNBS-induced colitis in rats and prevents stenosis. Stem Cell Res. Ther. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Su, J.; Hu, Y.; Huang, Q.; Shi, H.; Wang, L.; Ren, J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am. J. Transl. Res. 2017, 9, 4149–4160. [Google Scholar] [PubMed]
- Legaki, E.; Roubelakis, M.G.; Theodoropoulos, G.E.; Lazaris, A.; Kollia, A.; Karamanolis, G.; Marinos, E.; Gazouli, M. Therapeutic Potential of Secreted Molecules Derived from Human Amniotic Fluid Mesenchymal Stem/Stroma Cells in a Mice Model of Colitis. Stem Cell Rev. Rep. 2016, 12, 604–612. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, C.F.; Castoldi, A.; Andrade-Oliveira, V.; Ignacio, A.; da Cunha, F.F.; Felizardo, R.J.F.; Bassi, Ê.J.; Câmara, N.O.S.; de Almeida, D.C. Mesenchymal stromal cells modulate gut inflammation in experimental colitis. Inflammopharmacology 2018, 26, 251–260. [Google Scholar] [CrossRef]
- Song, W.-J.; Li, Q.; Ryu, M.-O.; Ahn, J.-O.; Bhang, D.H.; Jung, Y.C.; Youn, H.-Y. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res. Ther. 2018, 9, 91. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, B.; Lan, X.; Lu, S.; Sun, P.; Li, X.; Shi, G.; Zhao, Y.; Han, H.; et al. Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res. Ther. 2018, 9, 146. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ohnishi, S.; Onishi, R.; Tsuchiya, I.; Hosono, H.; Katsurada, T.; Yamahara, K.; Takeda, H.; Sakamoto, N. Therapeutic effects of human amnion-derived mesenchymal stem cell transplantation and conditioned medium enema in rats with trinitrobenzene sulfonic acid-induced colitis. Am. J. Transl. Res. 2017, 9, 940–952. [Google Scholar]
- Pak, S.; Hwang, S.W.; Shim, I.; Bae, S.; Ryu, Y.; Kim, H.-B.; Do, E.-J.; Son, H.-N.; Choi, E.-j.; Park, S.-h.; et al. Endoscopic Transplantation of Mesenchymal Stem Cell Sheets in Experimental Colitis in Rats. Sci. Rep. 2018, 8, 11314. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef]
- de la Portilla, F.; Yuste, Y.; Pereira, S.; Olano, C.; Maestre, M.V.; Padillo, F.J. Local Mesenchymal Stem Cell Therapy in Experimentally Induced Colitis in the Rat. Int. J. Stem Cells 2018, 11, 39–47. [Google Scholar] [CrossRef]
- Barnhoorn, M.; de Jonge-Muller, E.; Molendijk, I.; van Gulijk, M.; Lebbink, O.; Janson, S.; Schoonderwoerd, M.; van der Helm, D.; van der Meulen-de Jong, A.; Hawinkels, L.; et al. Endoscopic Administration of Mesenchymal Stromal Cells Reduces Inflammation in Experimental Colitis. Inflamm. Bowel Dis. 2018, 24, 1755–1767. [Google Scholar] [CrossRef]
- Pan, X.H.; Li, Q.Q.; Zhu, X.Q.; Li, Z.A.; Cai, X.M.; Pang, R.Q.; Ruan, G.P. Mechanism and therapeutic effect of umbilical cord mesenchymal stem cells in inflammatory bowel disease. Sci. Rep. 2019, 9, 17646. [Google Scholar] [CrossRef]
- Ko, I.K.; Kim, B.G.; Awadallah, A.; Mikulan, J.; Lin, P.; Letterio, J.J.; Dennis, J.E. Targeting improves MSC treatment of inflammatory bowel disease. Mol. Ther. 2010, 18, 1365–1372. [Google Scholar] [CrossRef]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Clinical and laboratory outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef]
- Cristobal, J.I.; Duque, F.J.; Uson-Casaus, J.M.; Ruiz, P.; Nieto, E.L.; Perez-Merino, E.M. Effects of Allogeneic Mesenchymal Stem Cell Transplantation in Dogs with Inflammatory Bowel Disease Treated with and without Corticosteroids. Animals 2021, 11, 2061. [Google Scholar] [CrossRef]
- Wang, R.; Yao, Q.; Chen, W.; Gao, F.; Li, P.; Wu, J.; Yu, J.; Cao, H. Stem cell therapy for Crohn’s disease: Systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res. Ther. 2021, 12, 463. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Wasser, M.; Roelofs, H.; Maljaars, P.W.J.; Molendijk, I.; Bonsing, B.A.; Oosten, L.E.M.; Dijkstra, G.; van der Woude, C.J.; Roelen, D.L.; et al. Long-term Evaluation of Allogeneic Bone Marrow-derived Mesenchymal Stromal Cell Therapy for Crohn’s Disease Perianal Fistulas. J. Crohn’s Colitis 2020, 14, 64–70. [Google Scholar] [CrossRef]
- de la Portilla, F.; Alba, F.; García-Olmo, D.; Herrerías, J.M.; González, F.X.; Galindo, A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int. J. Color. Dis. 2013, 28, 313–323. [Google Scholar] [CrossRef]
- Gregoire, C.; Briquet, A.; Pirenne, C.; Lechanteur, C.; Louis, E.; Beguin, Y. Allogeneic mesenchymal stromal cells for refractory luminal Crohn’s disease: A phase I-II study. Dig. Liver. Dis. 2018, 50, 1251–1255. [Google Scholar] [CrossRef]
- Heidari, N.; Abbasi-Kenarsari, H.; Namaki, S.; Baghaei, K.; Zali, M.R.; Ghaffari Khaligh, S.; Hashemi, S.M. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J. Cell. Physiol. 2021, 236, 5906–5920. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, G.; Zhang, L.; Qiao, C.; Di, A.; Gao, H.; Xu, H. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp. Ther. Med. 2016, 12, 2983–2989. [Google Scholar] [CrossRef]
- Mao, F.; Wu, Y.; Tang, X.; Kang, J.; Zhang, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed Res. Int. 2017, 2017, 5356760. [Google Scholar] [CrossRef]
- Martin, C.; Olmos, É.; Collignon, M.-L.; De Isla, N.; Blanchard, F.; Chevalot, I.; Marc, A.; Guedon, E. Revisiting MSC expansion from critical quality attributes to critical culture process parameters. Process Biochem. 2017, 59, 231–243. [Google Scholar] [CrossRef]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.; Wasser, M.N.; Dijkstra, G.; van der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.J.; et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927.e916. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342.e4. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.W.; Jun, H.S.; Roh, J.Y.; Lee, H.Y.; Hong, I.S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Wang, W.Q.; Dong, K.; Zhou, L.; Jiao, G.H.; Zhu, C.Z.; Li, W.W.; Yu, G.; Wu, W.T.; Chen, S.; Sun, Z.N.; et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol. Sin. 2015, 36, 1377–1387. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, W.; Xu, X.; Kang, J.; Wang, J.; Wen, Y.; Tang, X.; Yan, Y.; Qian, H.; Zhang, X.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am. J. Transl. Res. 2018, 10, 2026–2036. [Google Scholar]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Marlow, G.J.; van Gent, D.; Ferguson, L.R. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J. Gastroenterol. 2013, 19, 3931–3941. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yan, L.; Wang, C.Z.; Wang, W.H.; Shi, H.; Su, B.B.; Zeng, Q.H.; Du, H.T.; Wan, J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013, 19, 4702–4717. [Google Scholar] [CrossRef]
- Alves, V.B.F.; de Sousa, B.C.; Fonseca, M.T.C.; Ogata, H.; Caliári-Oliveira, C.; Yaochite, J.N.U.; Rodrigues Júnior, V.; Chica, J.E.L.; da Silva, J.S.; Malmegrim, K.C.R.; et al. A single administration of human adipose tissue-derived mesenchymal stromal cells (MSC) induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin. Exp. Immunol. 2019, 196, 139–154. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, T.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Seo, M.S.; Hong, I.S.; Choi, S.W.; Seo, K.W.; et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology 2013, 145, 1392–1403.e8. [Google Scholar] [CrossRef]
- Bluguermann, C.; Wu, L.; Petrigliano, F.; McAllister, D.; Miriuka, S.; Evseenko, D.A. Novel aspects of parenchymal-mesenchymal interactions: From cell types to molecules and beyond. Cell Biochem. Funct. 2013, 31, 271–280. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Q.; Wang, F. Mesenchymal stem cells for the treatment of ulcerative colitis: A systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res. Ther. 2019, 10, 266. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Rio, L.G.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-β-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- Choi, A.; Park, S.E.; Jeong, J.B.; Choi, S.J.; Oh, S.Y.; Ryu, G.H.; Lee, J.; Jeon, H.B.; Chang, J.W. Anti-Fibrotic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1. Int. J. Mol. Sci. 2020, 21, 6269. [Google Scholar] [CrossRef]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. Ther. 2020, 11, 130. [Google Scholar] [CrossRef]
- Yao, X.; Wang, J.; Zhu, J.; Rong, X. The anti-fibrotic effect of human fetal skin-derived stem cell secretome on the liver fibrosis. Stem Cell Res. Ther. 2020, 11, 379. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef]
- Alfaifi, M.; Eom, Y.W.; Newsome, P.N.; Baik, S.K. Mesenchymal stromal cell therapy for liver diseases. J. Hepatol. 2018, 68, 1272–1285. [Google Scholar] [CrossRef]
- Jang, Y.J.; An, S.Y.; Kim, J.H. Identification of MFGE8 in mesenchymal stem cell secretome as an anti-fibrotic factor in liver fibrosis. BMB Rep. 2017, 50, 58–59. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Huang, Y.; Su, Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Exp. Mol. Pathol. 2020, 115, 104468. [Google Scholar] [CrossRef]
- Qian, C.; Meng, Q.; Lu, J.; Zhang, L.; Li, H.; Huang, B. Human amnion mesenchymal stem cells restore spermatogenesis in mice with busulfan-induced testis toxicity by inhibiting apoptosis and oxidative stress. Stem Cell Res. Ther. 2020, 11, 290. [Google Scholar] [CrossRef]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des. Dev. Ther. 2020, 14, 3143–3158. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.X.; Fan, H.; Tang, Q.; Shou, Z.X.; Zuo, D.M.; Zou, Z.; Xu, M.; Chen, Q.Y.; Peng, Y.; et al. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS ONE 2015, 10, e0140551. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in Redox Biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Reactions of myeloperoxidase-derived oxidants with biological substrates: Gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 2006, 13, 3271–3290. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Garrison, B.R.; McDaniel, D.P.; Zhai, M.; Liao, P.J.; Nurmemet, D.; Kiang, J.G. Adaptive redox response of mesenchymal stromal cells to stimulation with lipopolysaccharide inflammagen: Mechanisms of remodeling of tissue barriers in sepsis. Oxidative Med. Cell. Longev. 2013, 2013, 186795. [Google Scholar] [CrossRef]
- Guillén, M.I.; Platas, J.; Pérez Del Caz, M.D.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Oh, J.Y.; Ko, J.H.; Lee, H.J.; Yu, J.M.; Choi, H.; Kim, M.K.; Wee, W.R.; Prockop, D.J. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells 2014, 32, 1553–1563. [Google Scholar] [CrossRef]
- Wu, X.; Dao Thi, V.L.; Huang, Y.; Billerbeck, E.; Saha, D.; Hoffmann, H.H.; Wang, Y.; Silva, L.A.V.; Sarbanes, S.; Sun, T.; et al. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 2018, 172, 423–438.e425. [Google Scholar] [CrossRef]
- Bailey, C.C.; Zhong, G.; Huang, I.C.; Farzan, M. IFITM-Family Proteins: The Cell’s First Line of Antiviral Defense. Annu. Rev. Virol. 2014, 1, 261–283. [Google Scholar] [CrossRef]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Yagi, H.; Chen, A.F.; Hirsch, D.; Rothenberg, A.C.; Tan, J.; Alexander, P.G.; Tuan, R.S. Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus. Stem Cell Res. Ther. 2020, 11, 293. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Marrazzo, P.; Crupi, A.N.; Alviano, F.; Teodori, L.; Bonsi, L. Exploring the roles of MSCs in infections: Focus on bacterial diseases. J. Mol. Med. 2019, 97, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37--A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- López-García, B.; Lee, P.H.; Yamasaki, K.; Gallo, R.L. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Investig. Dermatol. 2005, 125, 108–115. [Google Scholar] [CrossRef]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Martin, A.; Contreras, L.; Figueroa, F.E.; Khoury, M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res. Ther. 2015, 6, 199. [Google Scholar] [CrossRef]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, O.; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Kelk, P.; Qu, C.; Akiyama, K.; Chen, C.; Atsuta, I.; Chen, W.; Zhou, Y.; Shi, S. A subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans effect. Cell Res. 2013, 23, 107–121. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Saberpour, M.; Bakhshi, B.; Najar-Peerayeh, S. Evaluation of the Antimicrobial and Antibiofilm Effect of Chitosan Nanoparticles as Carrier for Supernatant of Mesenchymal Stem Cells on Multidrug-Resistant Vibrio cholerae. Infect. Drug Resist. 2020, 13, 2251–2260. [Google Scholar] [CrossRef]
- Krasnodembskaya, A.; Samarani, G.; Song, Y.; Zhuo, H.; Su, X.; Lee, J.W.; Gupta, N.; Petrini, M.; Matthay, M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1003–L1013. [Google Scholar] [CrossRef]
- Skrahin, A.; Jenkins, H.E.; Hurevich, H.; Solodovnikova, V.; Isaikina, Y.; Klimuk, D.; Rohava, Z.; Skrahina, A. Effectiveness of a novel cellular therapy to treat multidrug-resistant tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 4, 21–27. [Google Scholar] [CrossRef]
- Harman, R.M.; Yang, S.; He, M.K.; Van de Walle, G.R. Antimicrobial peptides secreted by equine mesenchymal stromal cells inhibit the growth of bacteria commonly found in skin wounds. Stem Cell Res. Ther. 2017, 8, 157. [Google Scholar] [CrossRef]
- Schneider, J.; Mateo, E.; Marcos-Arias, C.; Eiró, N.; Vizoso, F.; Pérez-Fernández, R.; Eraso, E.; Quindós, G. Antifungal Activity of the Human Uterine Cervical Stem Cells Conditioned Medium (hUCESC-CM) Against Candida albicans and Other Medically Relevant Species of Candida. Front. Microbiol. 2018, 9, 2818, Correction in Front. Microbiol. 2019, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl. Med. 2020, 9, 235–249. [Google Scholar] [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Van de Walle, G.R. The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. Stem Cells Transl. Med. 2020, 9, 746–757. [Google Scholar] [CrossRef]
- Rhee, K.J.; Lee, J.I.; Eom, Y.W. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. Biomed Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Leng, L.; Wang, Y.; He, N.; Wang, D.; Zhao, Q.; Feng, G.; Su, W.; Xu, Y.; Han, Z.; Kong, D.; et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014, 35, 5162–5170. [Google Scholar] [CrossRef]
- Ren, C.; Kumar, S.; Chanda, D.; Chen, J.; Mountz, J.D.; Ponnazhagan, S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells 2008, 26, 2332–2338. [Google Scholar] [CrossRef]
- Eiró, N.; Sendon-Lago, J.; Seoane, S.; Bermúdez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Shimizu, C.; Saito, J.; Tanaka, Y.; Inoue, M.; Tanizawa, O. An immunohistochemical study of colon-ovarian tumor antigen and colon-specific antigen in gynecologic tumors. Gynecol. Oncol. 1989, 35, 90–92. [Google Scholar] [CrossRef]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered with Exosomes. Urology 2016, 91, 241.e1–241.e7. [Google Scholar] [CrossRef]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965. [Google Scholar] [CrossRef]
- Wu, S.; Ju, G.Q.; Du, T.; Zhu, Y.J.; Liu, G.H. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE 2013, 8, e61366. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. The role of anti-inflammatory drugs in colorectal cancer. Annu. Rev. Med. 2013, 64, 131–144. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; He, X.; Chen, X.; Lin, X.; Zou, Y.; Wu, X.; Lan, P. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem. Biophys. Res. Commun. 2014, 450, 1402–1408. [Google Scholar] [CrossRef]
- Nasuno, M.; Arimura, Y.; Nagaishi, K.; Isshiki, H.; Onodera, K.; Nakagaki, S.; Watanabe, S.; Idogawa, M.; Yamashita, K.; Naishiro, Y.; et al. Mesenchymal stem cells cancel azoxymethane-induced tumor initiation. Stem Cells 2014, 32, 913–925. [Google Scholar] [CrossRef]
- Tang, R.J.; Shen, S.N.; Zhao, X.Y.; Nie, Y.Z.; Xu, Y.J.; Ren, J.; Lv, M.M.; Hou, Y.Y.; Wang, T.T. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res. Ther. 2015, 6, 71. [Google Scholar] [CrossRef]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.; Lee, K.; Xu, Q.; Wu, X.; Roper, J.; Mason, J.B.; Crott, J.W. Parabacteroides distasonis attenuates toll-like receptor 4 signaling and Akt activation and blocks colon tumor formation in high-fat diet-fed azoxymethane-treated mice. Int. J. Cancer 2018, 143, 1797–1805. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Rao, X. Apoptosis induced by Staphylococcus aureus toxins. Microbiol. Res. 2017, 205, 19–24. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef]
- Mahadev, S.; Young, J.M.; Selby, W.; Solomon, M.J. Quality of life in perianal Crohn’s disease: What do patients consider important? Dis. Colon Rectum 2011, 54, 579–585. [Google Scholar] [CrossRef]
- Panés, J.; Rimola, J. Perianal fistulizing Crohn’s disease: Pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 652–664. [Google Scholar] [CrossRef]
- García-Olmo, D.; García-Arranz, M.; García, L.G.; Cuellar, E.S.; Blanco, I.F.; Prianes, L.A.; Montes, J.A.; Pinto, F.L.; Marcos, D.H.; García-Sancho, L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: A new cell-based therapy. Int. J. Color. Dis. 2003, 18, 451–454. [Google Scholar] [CrossRef]
- García-Olmo, D.; García-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodríguez-Montes, J.A. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Guadalajara, H.; Herreros, D.; De-La-Quintana, P.; Trebol, J.; Garcia-Arranz, M.; Garcia-Olmo, D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int. J. Color. Dis. 2012, 27, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Calliada, F.; et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Gallia, A.; Sgarella, A.; Kruzliak, P.; Gobbi, P.G.; Corazza, G.R. Long-Term Follow-Up of Crohn Disease Fistulas After Local Injections of Bone Marrow-Derived Mesenchymal Stem Cells. Mayo Clin. Proc. 2015, 90, 747–755. [Google Scholar] [CrossRef]
- Park, K.J.; Ryoo, S.B.; Kim, J.S.; Kim, T.I.; Baik, S.H.; Kim, H.J.; Lee, K.Y.; Kim, M.; Kim, W.H. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: A pilot clinical trial. Color. Dis. 2016, 18, 468–476. [Google Scholar] [CrossRef]
- Garcia-Arranz, M.; Garcia-Olmo, D.; Herreros, M.D.; Gracia-Solana, J.; Guadalajara, H.; de la Portilla, F.; Baixauli, J.; Garcia-Garcia, J.; Ramirez, J.M.; Sanchez-Guijo, F.; et al. Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: A randomized clinical trial with long-term follow-up. Stem Cells Transl. Med. 2020, 9, 295–301. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, B.G.; Chae, G.; Lee, S.J. The clinical efficacy of stem cell therapy for complex perianal fistulas: A meta-analysis. Tech. Coloproctol. 2019, 23, 411–427. [Google Scholar] [CrossRef]
- Cheng, F.; Huang, Z.; Li, Z. Efficacy and Safety of Mesenchymal Stem Cells in Treatment of Complex Perianal Fistulas: A Meta-Analysis. Stem Cells Int. 2020, 2020, 8816737. [Google Scholar] [CrossRef]
- Duijvestein, M.; Vos, A.C.; Roelofs, H.; Wildenberg, M.E.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.; Koning, F.; Zwaginga, J.J.; Fidder, H.H.; et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: Results of a phase I study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef]
- Dhere, T.; Copland, I.; Garcia, M.; Chiang, K.Y.; Chinnadurai, R.; Prasad, M.; Galipeau, J.; Kugathasan, S. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease—A phase 1 trial with three doses. Aliment. Pharmacol. Ther. 2016, 44, 471–481. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Wang, D.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Sun, L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012, 61, 468–469. [Google Scholar] [CrossRef]
- Forbes, G.M.; Sturm, M.J.; Leong, R.W.; Sparrow, M.P.; Segarajasingam, D.; Cummins, A.G.; Phillips, M.; Herrmann, R.P. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 2014, 12, 64–71. [Google Scholar] [CrossRef]
- Onken, J.; Gallup, D.; Hanson, J.; Pandak, M.; Custer, L. Successful outpatient treatment of refractory Crohn’s disease using adult mesenchymal stem cells. In Proceedings of the American College of Gastroenterology Conference, Las Vegas, NV, USA, 20–25 October 2006. [Google Scholar]
- Mayer, L.; Pandak, W.M.; Melmed, G.Y.; Hanauer, S.B.; Johnson, K.; Payne, D.; Faleck, H.; Hariri, R.J.; Fischkoff, S.A. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant crohn’s disease: A phase 1 study. Inflamm. Bowel Dis. 2013, 19, 754–760. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, S.; Liu, X.; Song, B.; Shi, L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn’s Disease: A Randomized Controlled Clinical Trial. Gut Liver 2018, 12, 73–78. [Google Scholar] [CrossRef]
- Otagiri, S.; Ohnishi, S.; Miura, A.; Hayashi, H.; Kumagai, I.; Ito, Y.M.; Katsurada, T.; Nakamura, S.; Okamoto, R.; Yamahara, K.; et al. Evaluation of amnion-derived mesenchymal stem cells for treatment-resistant moderate Crohn’s disease: Study protocol for a phase I/II, dual-centre, open-label, uncontrolled, dose-response trial. BMJ Open Gastroenterol. 2018, 5, e000206. [Google Scholar] [CrossRef]
- Lazebnik, L.B.; Kniazev, O.V.; Konopliannikov, A.G.; Parfenov, A.I.; Ruchkina, I.N.; Mikhaĭlova, Z.F.; Tsaregorodtseva, T.M.; Khomeriki, S.G.; Rogozina, V.A.; Gudkova, R.B.; et al. Allogeneic mesenchymal stromal cells in patients with ulcerative colitis: Two years of observation. Eksp. Klin. Gastroenterol. 2010, 3–15. [Google Scholar]
- Fadeeva, N.; Knyazev, O.; Kagramanova, A.; Babayan, A.; Lishchinskaya, A.; Ruchkina, I.; Konoplyannikov, A.; Parfenov, A. P413 Relative frequency of relapses in patients with ulcerative colitis and Crohn’s disease treated with mesenchymal stromal cells: Five-years of follow-up. J. Crohn’s Colitis 2018, 12, S313. [Google Scholar] [CrossRef]
- Lazebnik, L.; Knyazev, O.; Sagynbaeva, V.; Parfenov, A. Laboratory prediction of the effectiveness of transplantation of allogeneic mesenchymal stromal cells of bone marrow in patients with ulcerative colitis.: P-131. Inflamm. Bowel Dis. 2011, 17, S51. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Ferrara, G.; Lanni, M.; Emiliani, C. Exosome-based strategies for Diagnosis and Therapy. Recent Pat. CNS Drug Discov. 2015, 10, 10–27. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Poncelet, A.J.; Nizet, Y.; Vercruysse, J.; Hiel, A.L.; Saliez, A.; Gianello, P. Inhibition of humoral response to allogeneic porcine mesenchymal stem cell with 12 days of tacrolimus. Transplantation 2008, 86, 1586–1595. [Google Scholar] [CrossRef]
- Markovic, B.S.; Kanjevac, T.; Harrell, C.R.; Gazdic, M.; Fellabaum, C.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Involved in Mesenchymal Stem Cell-Based Therapy of Inflammatory Bowel Diseases. Stem Cell Rev. 2018, 14, 153–165. [Google Scholar] [CrossRef]
- Gregoire, C.; Lechanteur, C.; Briquet, A.; Baudoux, E.; Baron, F.; Louis, E.; Beguin, Y. Review article: Mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 45, 205–221. [Google Scholar] [CrossRef]
- Touboul, T.; Hannan, N.R.; Corbineau, S.; Martinez, A.; Martinet, C.; Branchereau, S.; Mainot, S.; Strick-Marchand, H.; Pedersen, R.; Di Santo, J.; et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 2010, 51, 1754–1765. [Google Scholar] [CrossRef]
- Martinez-Montiel Mdel, P.; Gomez-Gomez, G.J.; Flores, A.I. Therapy with stem cells in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1211–1227. [Google Scholar] [CrossRef]
- Kim, N.; Cho, S.G. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int. J. Stem Cells 2015, 8, 54–68. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.; Lee, H.J.; Jeong, S.J.; Cho, K.J.; Hwang, S.G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- López-Beas, J.; Guadix, J.A.; Clares, B.; Soriano-Ruiz, J.L.; Zugaza, J.L.; Gálvez-Martín, P. An overview of international regulatory frameworks for mesenchymal stromal cell-based medicinal products: From laboratory to patient. Med. Res. Rev. 2020, 40, 1315–1334. [Google Scholar] [CrossRef]
- FDA Rejects Mesoblast Flagship Treatment. 2020. Available online: https://www.pharmamanufacturing.com/industrynews/2020/fda-rejects-mesoblast-flagship-treatment/ (accessed on 2 July 2022).
- Lipsitz, Y.Y.; Timmins, N.E.; Zandstra, P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016, 34, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Temple, S.; Studer, L. Lessons Learned from Pioneering Neural Stem Cell Studies. Stem Cell Rep. 2017, 8, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, C.; Nagaya, N.; Ohnishi, S.; Yamahara, K.; Takabatake, S.; Konno, T.; Hayashi, K.; Kawashiri, M.A.; Tsubokawa, T.; Yamagishi, M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ. J. 2011, 75, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Al-Nbaheen, M.; Vishnubalaji, R.; Ali, D.; Bouslimi, A.; Al-Jassir, F.; Megges, M.; Prigione, A.; Adjaye, J.; Kassem, M.; Aldahmash, A. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. Rep. 2013, 9, 32–43. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef]
- Coppin, L.; Sokal, E.; Stephenne, X. Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells 2019, 8, 1160. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Musial-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Barekzai, J.; Petry, F.; Zitzmann, J.; Czermak, P.; Salzig, D. Bioprocess Development for Human Mesenchymal Stem Cell Therapy Products. New Adv. Ferment. Processes 2020, 1–25. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Stoddart, M.J.; D’Amora, U.; Ambrosio, L.; Alini, M.; Musumeci, G. Mesenchymal Stem Cell-Based Cartilage Regeneration Approach and Cell Senescence: Can We Manipulate Cell Aging and Function? Tissue Eng. Part B Rev. 2017, 23, 529–539. [Google Scholar] [CrossRef]
- Knuth, C.A.; Kiernan, C.H.; Palomares Cabeza, V.; Lehmann, J.; Witte-Bouma, J.; Ten Berge, D.; Brama, P.A.; Wolvius, E.B.; Strabbing, E.M.; Koudstaal, M.J.; et al. Isolating Pediatric Mesenchymal Stem Cells with Enhanced Expansion and Differentiation Capabilities. Tissue Eng. Part C Methods 2018, 24, 313–321. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Bourgine, P.; Le Magnen, C.; Pigeot, S.; Geurts, J.; Scherberich, A.; Martin, I. Combination of immortalization and inducible death strategies to generate a human mesenchymal stromal cell line with controlled survival. Stem Cell Res. 2014, 12, 584–598. [Google Scholar] [CrossRef]
- Skårn, M.; Noordhuis, P.; Wang, M.Y.; Veuger, M.; Kresse, S.H.; Egeland, E.V.; Micci, F.; Namløs, H.M.; Håkelien, A.M.; Olafsrud, S.M.; et al. Generation and characterization of an immortalized human mesenchymal stromal cell line. Stem Cells Dev. 2014, 23, 2377–2389. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef]
- Piñeiro-Ramil, M.; Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Rodríguez-Fernández, S.; Hermida-Gómez, T.; Blanco-García, F.J.; Fuentes-Boquete, I.; Díaz-Prado, S. Immortalizing Mesenchymal Stromal Cells from Aged Donors While Keeping Their Essential Features. Stem Cells Int. 2020, 2020, 5726947. [Google Scholar] [CrossRef]
- Schneider, J.; Eiró, N.; Pérez-Fernández, R.; Martínez-Ordóñez, A.; Vizoso, F. Human Uterine Cervical Stromal Stem Cells (hUCESCs): Why and How they Exert their Antitumor Activity. Cancer Genom. Proteom. 2016, 13, 331–337. [Google Scholar]
- Apel, C.; Forlenza, O.V.; de Paula, V.J.; Talib, L.L.; Denecke, B.; Eduardo, C.P.; Gattaz, W.F. The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. J. Neural Transm. 2009, 116, 71–78. [Google Scholar] [CrossRef]
- Boulaiz, H.; Marchal, J.A.; Prados, J.; Melguizo, C.; Aránega, A. Non-viral and viral vectors for gene therapy. Cell. Mol. Biol. 2005, 51, 3–22. [Google Scholar]
- Filho, D.M.; de Carvalho Ribeiro, P.; Oliveira, L.F.; Dos Santos, A.; Parreira, R.C.; Pinto, M.C.X.; Resende, R.R. Enhancing the Therapeutic Potential of Mesenchymal Stem Cells with the CRISPR-Cas System. Stem Cell Rev. Rep. 2019, 15, 463–473. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, M.; Yu, M.; Bai, W.; Zuo, L.; Bu, X.; Liu, Y.; Xia, L.; Hu, J.; Liu, L.; et al. Transplantation of CRISPRa system engineered IL10-overexpressing bone marrow-derived mesenchymal stem cells for the treatment of myocardial infarction in diabetic mice. J. Biol. Eng. 2019, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Lasda, E.; Parker, R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. PLoS ONE 2016, 11, e0148407. [Google Scholar] [CrossRef]
- Sun, B.; Shi, L.; Shi, Q.; Jiang, Y.; Su, Z.; Yang, X.; Zhang, Y. Circular RNAs are abundantly expressed and upregulated during repair of the damaged endometrium by Wharton’s jelly-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 314. [Google Scholar] [CrossRef]
- Bach, D.H.; Lee, S.K.; Sood, A.K. Circular RNAs in Cancer. Mol. Ther. Nucleic Acids 2019, 16, 118–129. [Google Scholar] [CrossRef]
- Ogino, H.; Nakamura, K.; Ihara, E.; Akiho, H.; Takayanagi, R. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig. Dis. Sci. 2011, 56, 376–386. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A., Jr.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e666. [Google Scholar] [CrossRef]
- Liu, G.; Ma, H.; Qiu, L.; Li, L.; Cao, Y.; Ma, J.; Zhao, Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol. Cell Biol. 2011, 89, 130–142. [Google Scholar] [CrossRef]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, e131273. [Google Scholar] [CrossRef]
- Yang, S.; Liang, X.; Song, J.; Li, C.; Liu, A.; Luo, Y.; Ma, H.; Tan, Y.; Zhang, X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res. Ther. 2021, 12, 315. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, H.; Wang, H.; Zhu, Y.; Wu, Y.; Li, Q.; Li, T.; Liu, L. Mesenchymal stem cell-derived microvesicles improve intestinal barrier function by restoring mitochondrial dynamic balance in sepsis rats. Stem Cell Res. Ther. 2021, 12, 299. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Hassan, M.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef]
- Chen, A.K.; Reuveny, S.; Oh, S.K. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol. Adv. 2013, 31, 1032–1046. [Google Scholar] [CrossRef]
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80. [Google Scholar] [CrossRef]
- Song, E.M.; Joo, Y.H.; Choe, A.R.; Park, Y.; Tae, C.H.; Hong, J.T.; Moon, C.M.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Three-dimensional culture method enhances the therapeutic efficacies of tonsil-derived mesenchymal stem cells in murine chronic colitis model. Sci. Rep. 2021, 11, 19589. [Google Scholar] [CrossRef]
- Barckhausen, C.; Rice, B.; Baila, S.; Sensebé, L.; Schrezenmeier, H.; Nold, P.; Hackstein, H.; Rojewski, M.T. GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor. Methods Mol. Biol. 2016, 1416, 389–412. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Reyes-Ramos, A.M.; Domenech, M.; Almodovar, J. Effects of Physical, Chemical, and Biological Stimulus on h-MSC Expansion and Their Functional Characteristics. Ann. Biomed. Eng. 2020, 48, 519–535. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131, Correction in Stem Cell Res. Ther. 2019, 10, 132. [Google Scholar] [CrossRef]
- Hoch, A.I.; Leach, J.K. Concise review: Optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl. Med. 2014, 3, 643–652. [Google Scholar] [CrossRef]
- Henn, A.; Darou, S.; Yerden, R. Full-time physioxic culture conditions promote MSC proliferation more than hypoxic preconditioning. Cytotherapy 2019, 21, S73–S74. [Google Scholar] [CrossRef]
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, J.; Pan, Q.; Yang, J.; Hao, G.; Wang, Y.; Li, L.; Cao, H. Hypoxia-inducible factor-2 alpha promotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci. Rep. 2016, 6, 35489. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.M.; Klose, K.; Bieback, K.; Korinth, D.; Schneider, M.; Seifert, M.; Choi, Y.H.; Kurtz, A.; Falk, V.; Stamm, C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS ONE 2015, 10, e0138477. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Xia, Y.; Kim, W.S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.S.; Sung, J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Kim, A.K.; Jeong, G.J.; Jeon, H.R.; Bhang, S.H.; Kim, D.I. Enhanced Anti-Cancer Effects of Conditioned Medium from Hypoxic Human Umbilical Cord-Derived Mesenchymal Stem Cells. Int. J. Stem Cells 2019, 12, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Vertelov, G.; Kharazi, L.; Muralidhar, M.G.; Sanati, G.; Tankovich, T.; Kharazi, A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res. Ther. 2013, 4, 5. [Google Scholar] [CrossRef]
- Ying, J.; You, Q.; Wang, Z.; Hu, Z. Hypoxic preconditioning promotes the immunosuppressive effects of mesenchymal stem cells in mice with colitis. Res. Vet. Sci. 2021, 144, 157–163. [Google Scholar] [CrossRef]
- Monfoulet, L.E.; Becquart, P.; Marchat, D.; Vandamme, K.; Bourguignon, M.; Pacard, E.; Viateau, V.; Petite, H.; Logeart-Avramoglou, D. The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng. Part A 2014, 20, 1827–1840. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Campaña-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef]
- Wu, H.; Fan, H.; Shou, Z.; Xu, M.; Chen, Q.; Ai, C.; Dong, Y.; Liu, Y.; Nan, Z.; Wang, Y.; et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019, 68, 204–212. [Google Scholar] [CrossRef]
- Joo, H.; Oh, M.K.; Kang, J.Y.; Park, H.S.; Chae, D.H.; Kim, J.; Lee, J.H.; Yoo, H.M.; Choi, U.; Kim, D.K.; et al. Extracellular Vesicles from Thapsigargin-Treated Mesenchymal Stem Cells Ameliorated Experimental Colitis via Enhanced Immunomodulatory Properties. Biomedicines 2021, 9, 209. [Google Scholar] [CrossRef]
- Kang, J.Y.; Oh, M.K.; Joo, H.; Park, H.S.; Chae, D.H.; Kim, J.; Lee, H.R.; Oh, I.H.; Yu, K.R. Xeno-Free Condition Enhances Therapeutic Functions of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells against Experimental Colitis by Upregulated Indoleamine 2,3-Dioxygenase Activity. J. Clin. Med. 2020, 9, 2913. [Google Scholar] [CrossRef]
- Wu, X.; Wu, D.; Mu, Y.; Zhao, Y.; Ma, Z. Serum-Free Medium Enhances the Therapeutic Effects of Umbilical Cord Mesenchymal Stromal Cells on a Murine Model for Acute Colitis. Front. Bioeng. Biotechnol. 2020, 8, 586. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Wang, G.; Liu, D.; Bao, L.; Jiao, C.; Luan, J.; Hou, Y.; Xu, Y.; Wang, H.; et al. Nrf2 deficiency aggravates the kidney injury induced by subacute cadmium exposure in mice. Arch. Toxicol. 2021, 95, 883–893. [Google Scholar] [CrossRef]
- Billing, A.M.; Ben Hamidane, H.; Dib, S.S.; Cotton, R.J.; Bhagwat, A.M.; Kumar, P.; Hayat, S.; Yousri, N.A.; Goswami, N.; Suhre, K.; et al. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci. Rep. 2016, 6, 21507. [Google Scholar] [CrossRef]
- Menard, C.; Pacelli, L.; Bassi, G.; Dulong, J.; Bifari, F.; Bezier, I.; Zanoncello, J.; Ricciardi, M.; Latour, M.; Bourin, P.; et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells Dev. 2013, 22, 1789–1801. [Google Scholar] [CrossRef]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Panchalingam, K.M.; Assunção-Silva, R.; Serra, S.C.; Mendes-Pinheiro, B.; Patrício, P.; Jung, S.; Anjo, S.I.; Manadas, B.; Pinto, L.; et al. Modulation of the Mesenchymal Stem Cell Secretome Using Computer-Controlled Bioreactors: Impact on Neuronal Cell Proliferation, Survival and Differentiation. Sci. Rep. 2016, 6, 27791. [Google Scholar] [CrossRef]
- Cunha, B.; Aguiar, T.; Carvalho, S.B.; Silva, M.M.; Gomes, R.A.; Carrondo, M.J.T.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Bieback, K.; Kuçi, S.; Schäfer, R. Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion 2019, 59, 2164–2173. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e120. [Google Scholar] [CrossRef]
- Castelo-Branco, M.T.; Soares, I.D.; Lopes, D.V.; Buongusto, F.; Martinusso, C.A.; do Rosario, A., Jr.; Souza, S.A.; Gutfilen, B.; Fonseca, L.M.; Elia, C.; et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS ONE 2012, 7, e33360. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; AJL, D.H.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panes, J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 27, 328–335. [Google Scholar] [CrossRef]
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: In vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug Dev. Ind. Pharm. 2017, 43, 399–408. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W.L.; Wang, J.C.; Zhang, X.; Zhang, H.; Wang, X.Q.; Zhou, T.Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Saa, J.; Vizoso, F.J.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Polymeric Hydrogels as Technology Platform for Drug Delivery Applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.; Kan, C.W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Del Rio, L.; Diaz-Rodriguez, P.; Landin, M. New tools to design smart thermosensitive hydrogels for protein rectal delivery in IBD. Mater. Sci. Eng. C 2020, 106, 110252. [Google Scholar] [CrossRef]
- Din, F.U.; Mustapha, O.; Kim, D.W.; Rashid, R.; Park, J.H.; Choi, J.Y.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Choi, H.G. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015, 94, 64–72. [Google Scholar] [CrossRef]
- Santos Akkari, A.C.; Ramos Campos, E.V.; Keppler, A.F.; Fraceto, L.F.; de Paula, E.; Tófoli, G.R.; de Araujo, D.R. Budesonide-hydroxypropyl-β-cyclodextrin inclusion complex in binary poloxamer 407/403 system for ulcerative colitis treatment: A physico-chemical study from micelles to hydrogels. Colloids Surf. B Biointerfaces 2016, 138, 138–147. [Google Scholar] [CrossRef]
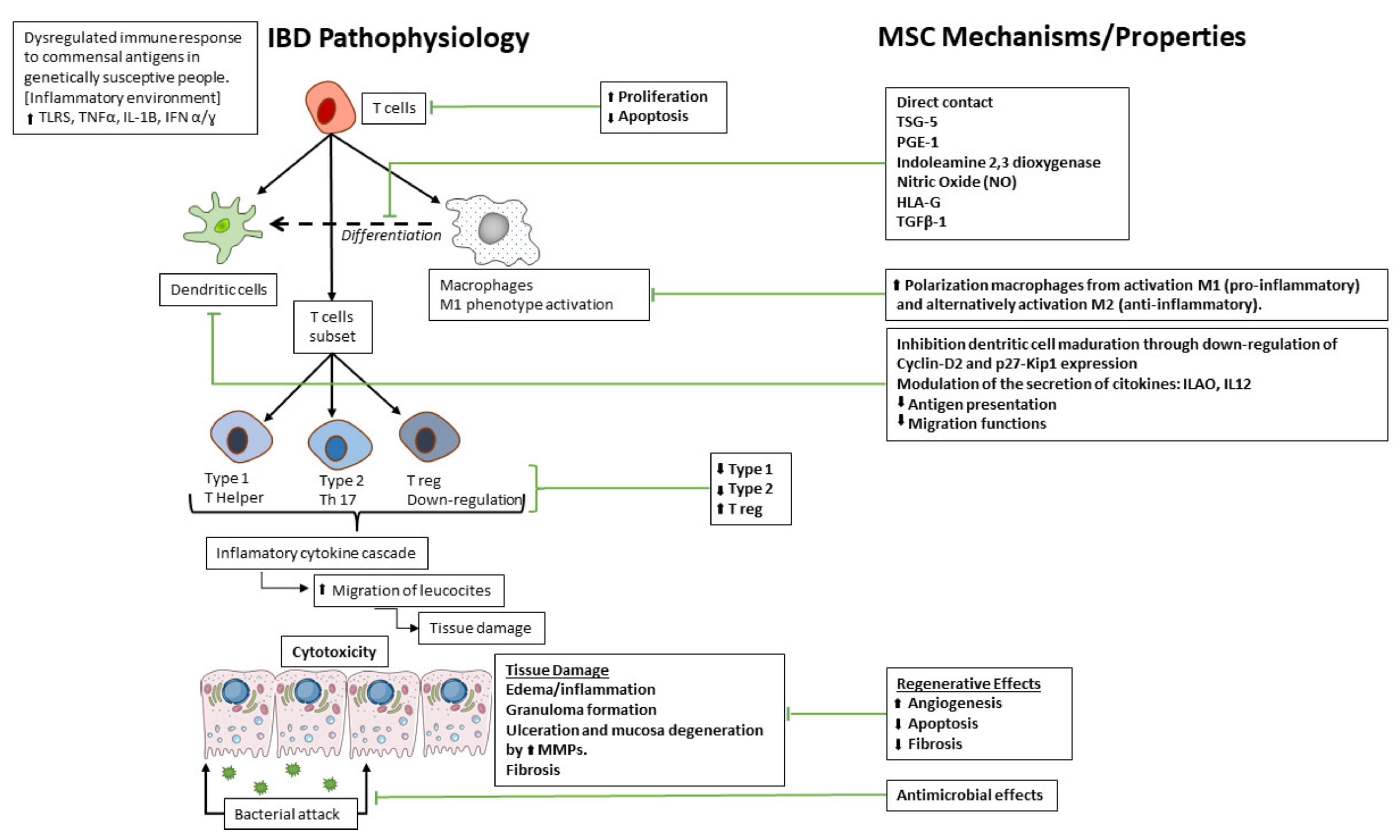
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiro, N.; Fraile, M.; González-Jubete, A.; González, L.O.; Vizoso, F.J. Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. Int. J. Mol. Sci. 2022, 23, 8905. https://doi.org/10.3390/ijms23168905
Eiro N, Fraile M, González-Jubete A, González LO, Vizoso FJ. Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. International Journal of Molecular Sciences. 2022; 23(16):8905. https://doi.org/10.3390/ijms23168905
Chicago/Turabian StyleEiro, Noemi, Maria Fraile, Alberto González-Jubete, Luis O. González, and Francisco J. Vizoso. 2022. "Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges" International Journal of Molecular Sciences 23, no. 16: 8905. https://doi.org/10.3390/ijms23168905
APA StyleEiro, N., Fraile, M., González-Jubete, A., González, L. O., & Vizoso, F. J. (2022). Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. International Journal of Molecular Sciences, 23(16), 8905. https://doi.org/10.3390/ijms23168905






