Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19
Abstract
:1. Introduction
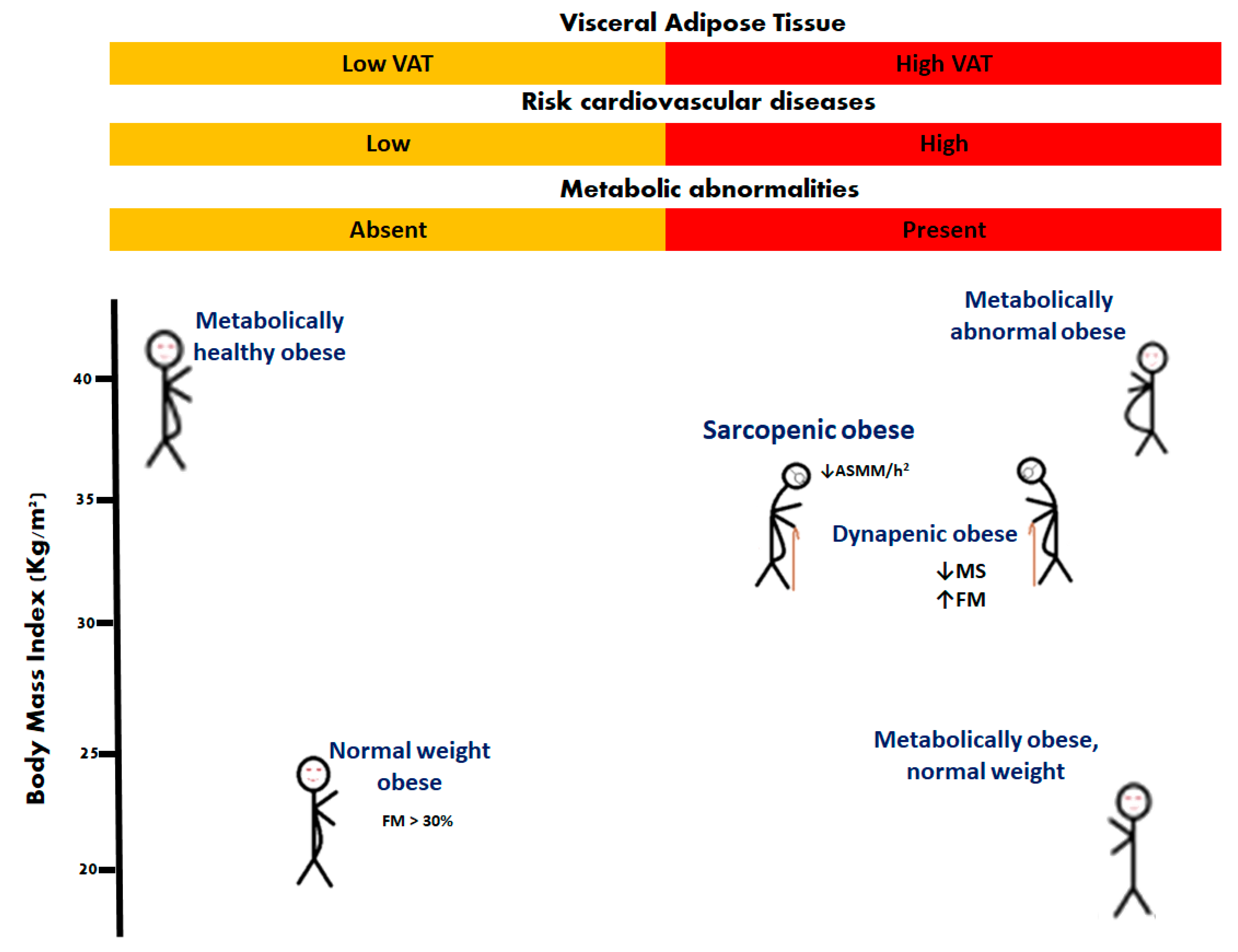
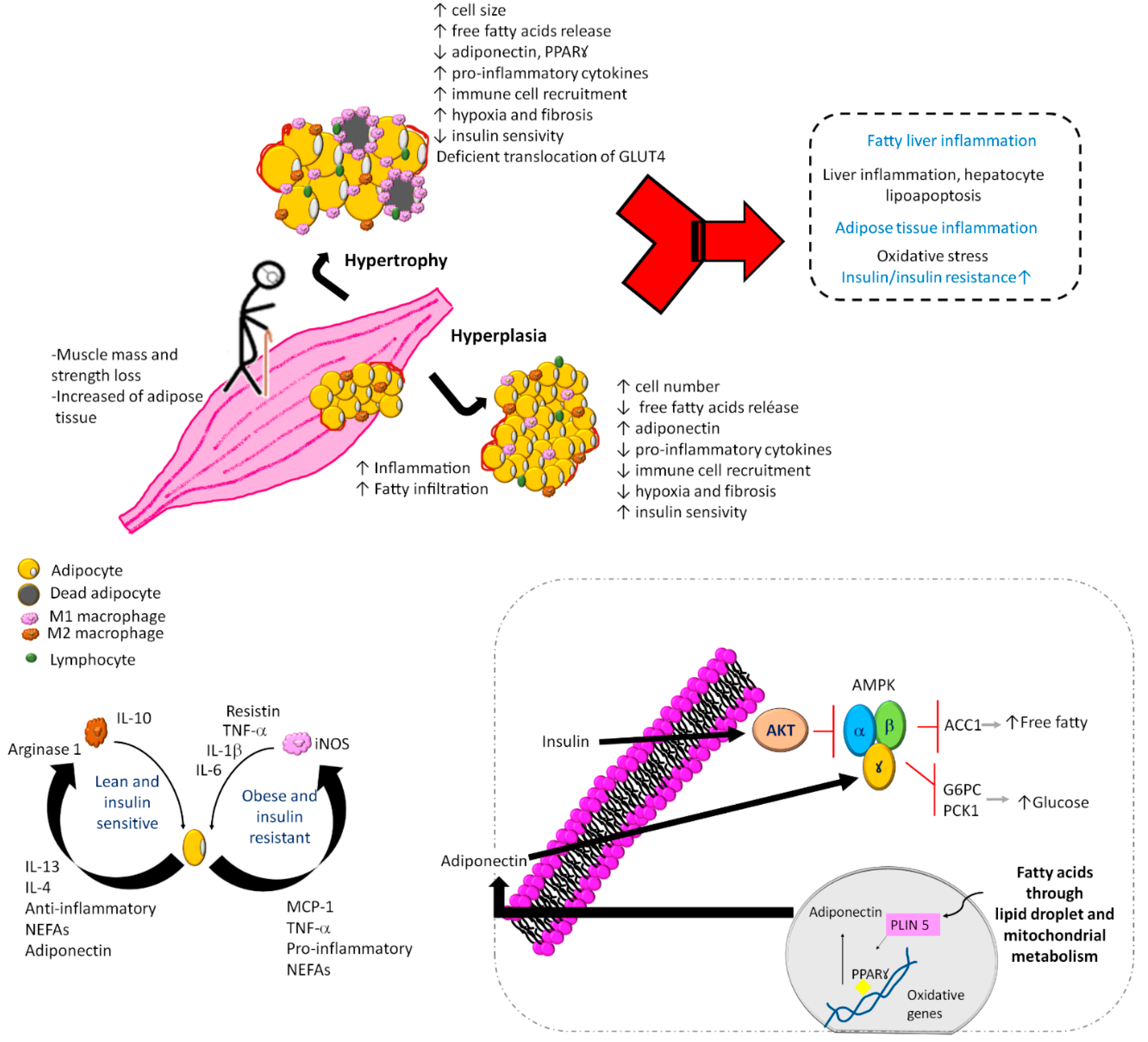
2. Sarcopenic Obesity and Dynapenic Obesity
3. Dynapenic and Sarcopenic Obesity and Their Association with Cardiovascular Disease Risk
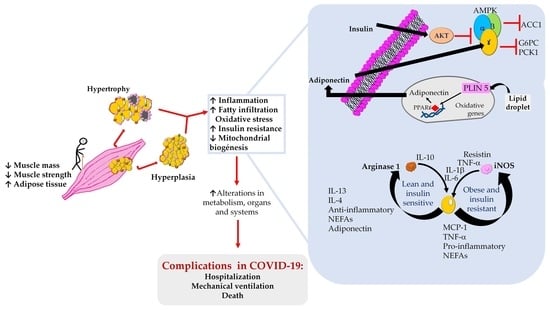
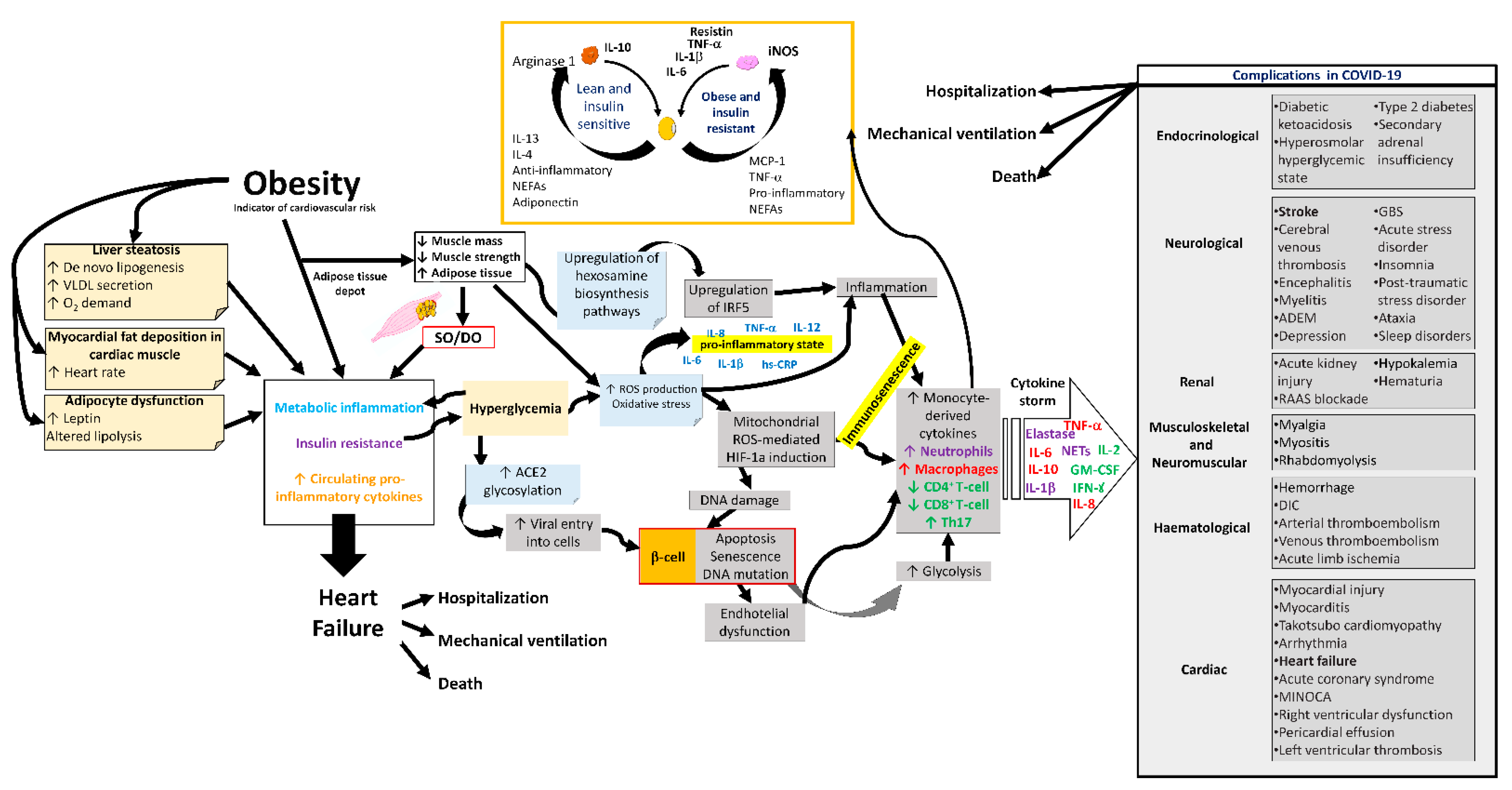
4. Sarcopenic Obesity, Dynapenic Obesity, and COVID-19
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Yang, S.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Sarcopenic obesity and its association with respiratory disease incidence and mortality. Clin. Nutr. 2020, 39, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Crimmins, E.M. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity 2012, 20, 2101–2106. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Lee, Y.; Chung, Y.S.; Lee, D.J.; Joo, N.S.; Hong, D.; Song, G.E.; Kim, H.J.; Choi, Y.J.; Kim, K.M. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1107–1113. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020, MA20013. [Google Scholar] [CrossRef]
- Shao, A.; Campbell, W.W.; Chen, C.-Y.O.; Mittendorfer, B.; Rivas, D.A.; Griffiths, J.C. The emerging global phenomenon of sarcopenic obesity: Role of functional foods; a conference report. J. Funct. Foods 2017, 33, 244–250. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 29 November 2021).
- Berumen, J.; Schmulson, M.; Alegre-Díaz, J.; Guerrero, G.; Larriva-Sahd, J.; Olaiz, G.; Wong-Chew, R.M.; Cantú-Brito, C.; Ochoa-Guzmán, A.; Garcilazo-Ávila, A.; et al. Risk of infection and hospitalization by COVID-19 in Mexico: A case-control study. medRxiv 2020. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Ata, A.M.; Özçakar, L. Sarcopenic obesity is the real problem in COVID-19! Eur. J. Intern. Med. 2021, 93, 103–104. [Google Scholar] [CrossRef]
- Laviano, A.; Koverech, A.; Zanetti, M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q. Definitions, Classification, and Epidemiology of Obesity. [Updated 12 April 2018]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279167/ (accessed on 29 November 2021).
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 November 2021).
- Lee, D.C.; Shook, R.P.; Drenowatz, C.; Blair, S.N. Physical activity and sarcopenic obesity: Definition, assessment, prevalence and mechanism. Future Sci. OA 2016, 2, FSO127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J.; et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 2021, 40, 571–580. [Google Scholar] [CrossRef]
- Chain, A.; Faerstein, E.; Wahrlich, V.; Bezerra, F.F. Obesity, dynapenia, and their combination: Implications for bone mineral density in Brazilian adults-the Pró-Saúde study. Nutrition 2021, 81, 110898. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; Kruizenga, H.M.; Konings, L.A.; Krebbers, D.; Jorissen, J.R.; Joosten, M.I.; van Aken, L.H.; Tan, F.M.; van Bodegraven, A.A.; Soeters, M.R.; et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN 2021, 43, 369–376. [Google Scholar] [CrossRef]
- Gungor, O.; Ulu, S.; Hasbal, N.B.; Anker, S.D.; Kalantar-Zadeh, K. Effects of hormonal changes on sarcopenia in chronic kidney disease: Where are we now and what can we do? J. Cachexia Sarcopenia Muscle 2021, 12, 1380–1392. [Google Scholar] [CrossRef]
- Gil, S.; Jacob, F.W.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira Júnior, G.N.; Swinton, P.; Gualano, B.; et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: A prospective observational study. J. Cachexia Sarcopenia Muscle 2021, 12, 1871–1878. [Google Scholar] [CrossRef]
- Rossi, A.P.; Urbani, S.; Fantin, F.; Nori, N.; Brandimarte, P.; Martini, A.; Zoico, E.; Mazzali, G.; Babbanini, A.; Muollo, V.; et al. Worsening Disability and Hospitalization Risk in Sarcopenic Obese and Dynapenic Abdominal Obese: A 5.5 Years Follow-Up Study in Elderly Men and Women. Front. Endocrinol. 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Baumgartner, R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011, 27, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, D.R.; Janssen, I. Dynapenic-obesity and physical function in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 71–77. [Google Scholar] [CrossRef]
- Sattar, N.; McInnes, I.B.; McMurray, J. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [Green Version]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef] [Green Version]
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Daghri, N.M. Sarcopenic Obesity. In Sarcopenia. Practical Issues in Geriatrics; Veronese, N., Beaudart, C., Sabico, S., Eds.; Springer: Cham, Switzerland, 2021; Volume 145–151. [Google Scholar] [CrossRef]
- Gumieiro, D.N.; Murino, R.B.; Buzati, P.B.; Cavallari, K.A.; Tanni, S.E.; Azevedo, P.S.; Polegato, B.F.; Mamede Zornoff, L.A.; Dinhane, D.I.; Innocenti Dinhane, K.G.; et al. Vitamin D serum levels are associated with handgrip strength but not with muscle mass or length of hospital stay after hip fracture. Nutrition 2015, 31, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, L.; Li, M.; Xiao, H. Relationship of vitamin D receptor gene polymorphism with sarcopenia and muscle traits based on propensity score matching. J. Clin. Lab. Anal. 2020, 34, e23485. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.Y.; Schuppan, D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J. Hepatol. 2013, 58, 619–621. [Google Scholar] [CrossRef] [Green Version]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143. [Google Scholar] [CrossRef]
- Asghar, A.; Sheikh, N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017, 315, 18–26. [Google Scholar] [CrossRef]
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Schrauwen, P.; Kooi, M.E. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring, Md.) 2006, 14, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Kern, P.A.; Nikolajczyk, B.S. The Immune System in Obesity: Developing Paradigms Amidst Inconvenient Truths. Curr. Diab. Rep. 2017, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenia—The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Weinsier, R.L.; Schutz, Y.; Bracco, D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am. J. Clin. Nutr. 1992, 55, 790–794. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.W.; Li, C.I.; Li, T.C.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Lin, C.C. Association of Sarcopenic Obesity with Higher Serum High-Sensitivity C-Reactive Protein Levels in Chinese Older Males—A Community-Based Study (Taichung Community Health Study-Elderly, TCHS-E). PLoS ONE 2015, 10, e0132908. [Google Scholar] [CrossRef]
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007, 102, 919–925. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Anton, S.; Beavers, D.P.; Manini, T.M.; Fielding, R.; Newman, A.; Church, T.; Kritchevsky, S.B.; Conroy, D.; McDermott, M.M.; et al. Dynapenia and Metabolic Health in Obese and Nonobese Adults Aged 70 Years and Older: The LIFE Study. J. Am. Med. Dir. Assoc. 2017, 18, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [Green Version]
- de Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.; Thomas, W.G. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. JASN 2017, 28, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Morton, A.B.; Hyatt, H.; Hinkley, M.J. The Renin-Angiotensin System and Skeletal Muscle. Exerc. Sport Sci. Rev. 2018, 46, 205–214. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Rivera, J.C.; Garcia, D. Skeletal muscle wasting: New role of nonclassical renin-angiotensin system. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tabony, A.M.; Galvez, S.; Mitch, W.E.; Higashi, Y.; Sukhanov, S.; Delafontaine, P. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: Potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2322–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, O.J.; Allwood, B.W.; Zemlin, A.E. COVID-19 and the renin-angiotensin system (RAS): A spark that sets the forest alight? Med. Hypotheses 2020, 144, 110231. [Google Scholar] [CrossRef] [PubMed]
- Sartiani, L.; Spinelli, V.; Laurino, A.; Blescia, S.; Raimondi, L.; Cerbai, E.; Mugelli, A. Pharmacological perspectives in sarcopenia: A potential role for renin-angiotensin system blockers? Clin. Cases Miner. Bone Metab. 2015, 12, 135–138. [Google Scholar] [CrossRef]
- Pan, M.; Vasbinder, A.; Anderson, E.; Catalan, T.; Shadid, H.R.; Berlin, H.; Padalia, K.; O’Hayer, P.; Meloche, C.; Azam, T.U.; et al. Angiotensin-Converting Enzyme Inhibitors, Angiotensin II Receptor Blockers, and Outcomes in Patients Hospitalized for COVID-19. J. Am. Heart Assoc. 2021, 10, e023535. [Google Scholar] [CrossRef]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef] [Green Version]
- Millward, D.J. Nutrition and sarcopenia: Evidence for an interaction. Proc. Nutr. Soc. 2012, 71, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.M.; de Oliveira, R.J.; Raposo, R.; Neri, S.; Gadelha, A.B. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch. Osteoporos. 2019, 14, 38. [Google Scholar] [CrossRef]
- von Berens, Å.; Obling, S.R.; Nydahl, M.; Koochek, A.; Lissner, L.; Skoog, I.; Frändin, K.; Skoglund, E.; Rothenberg, E.; Cederholm, T. Sarcopenic obesity and associations with mortality in older women and men—A prospective observational study. BMC Geriatr. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 15 December 2021).
- Curtis, A.B.; Karki, R.; Hattoum, A.; Sharma, U.C. Arrhythmias in Patients ≥80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018, 71, 2041–2057. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [Green Version]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Tzima, E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp. Gerontol. 2011, 46, 185–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczesny, B.; Bhakat, K.K.; Mitra, S.; Boldogh, I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech. Ageing Dev. 2004, 125, 755–765. [Google Scholar] [CrossRef]
- Evans, K.; Abdelhafiz, D.; Abdelhafiz, A.H. Sarcopenic obesity as a determinant of cardiovascular disease risk in older people: A systematic review. Postgrad. Med. 2021, 133, 831–842. [Google Scholar] [CrossRef]
- Lee, K. Sarcopenic obesity and 10-year cardiovascular disease risk scores in cancer survivors and non-cancer participants using a nationwide survey. Eur. J. Cancer Care 2021, 30, e13365. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kang, H.T.; Lee, D.C.; Lee, H.R.; Lee, Y.J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef]
- Tabibi, H.; As’habi, A.; Najafi, I.; Hedayati, M. Prevalence of dynapenic obesity and sarcopenic obesity and their associations with cardiovascular disease risk factors in peritoneal dialysis patients. Kidney Res. Clin. Pract. 2018, 37, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Connolly, T.M. Biomarkers of vulnerable atheromatous plaques: Translational medicine perspectives. Adv. Clin. Chem. 2010, 50, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Park, H.E.; Lee, H.; Kim, M.J.; Choi, S.Y.; Yim, J.Y.; Yoon, J.W. Sarcopenic Obesity Is Significantly Associated with Coronary Artery Calcification. Front. Med. 2021, 8, 651961. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Maezono, K.; Osman, A.; Pendergrass, M.; Patti, M.E.; Pratipanawatr, T.; DeFronzo, R.A.; Kahn, C.R.; Mandarino, L.J. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 2000, 105, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Di Pino, A.; De Fronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef] [Green Version]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Akbasheva, O.; Matveeva, V.; Karetnikova, V.; Kokov, A.; Barbarash, O. Relationship key factor of inflammation and the development of complications in the late period of myocardial infarction in patients with visceral obesity. BMC Cardiovasc. Disord. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, C.; Charnley, J.M.; Evans, W.J.; Crim, M.C. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am. J. Clin. Nutr. 1995, 62, 30–39. [Google Scholar] [CrossRef]
- Alexandre, T.D.; Aubertin-Leheudre, M.; Carvalho, L.P.; Máximo, R.D.; Corona, L.P.; Pereira de Brito, T.R.; Nunes, D.P.; Ferreira-Santos, J.L.; de Oliveira Duarte, Y.A.; Lebrão, M.L. Dynapenic obesity as an associated factor to lipid and glucose metabolism disorders and metabolic syndrome in older adults—Findings from SABE Study. Clin. Nutr. 2018, 37, 1360–1366. [Google Scholar] [CrossRef]
- Hanatani, S.; Izumiya, Y.; Yamamoto, M.; Araki, S.; Fujisue, K.; Arima, Y.; Takashio, S.; Yamamoto, E.; Kaikita, K.; Matsushita, K.; et al. A simple method of sarcopenia detection can predict adverse cardiovascular events in patients with abdominal obesity. Int. J. Obes. 2021, 45, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Banack, H.; Stokes, A. The ‘obesity paradox’ may not be a paradox at all. Int. J. Obes. 2017, 41, 1162–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzeh, B.; Pasdar, Y.; Mirzaei, N.; Faramani, R.S.; Najafi, F.; Shakiba, E.; Darbandi, M. Visceral adiposity index and atherogenic index of plasma as useful predictors of risk of cardiovascular diseases: Evidence from a cohort study in Iran. Lipids Health Dis. 2021, 20, 82. [Google Scholar] [CrossRef]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Chung, W.; Moon, S.; Ryu, O.H.; Kim, M.K.; Kang, J.G. Effect of sarcopenia and body shape on cardiovascular disease according to obesity phenotypes. Diabetes Metab. J. 2021, 45, 209–218. [Google Scholar] [CrossRef]
- Erdöl, M.A.; Kayaaslan, B.; Erdoğan, M.; Hasanoğlu, İ.; Yayla, Ç.; Civelek, E.F.; Beşler, M.S.; Kaya, K.A.; Gayretli, Y.K.; Erdöl, A.K.; et al. Sarcopenia and Its Prognostic Role on Hospitalization and In-Hospital Mortality in Coronavirus Disease 2019 Patients with At Least One Cardiovascular Risk Factor. Turk. Kardiyol. Dern. Ars. 2022, 50, 103–111. [Google Scholar] [CrossRef]
- Horn, J.W.; Feng, T.; Mørkedal, B.; Strand, L.B.; Horn, J.; Mukamal, K.; Janszky, I. Obesity and Risk for First Ischemic Stroke Depends on Metabolic Syndrome: The HUNT Study. Stroke 2021, 52, 3555–3561. [Google Scholar] [CrossRef]
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke 2021, 16, 137–149. [Google Scholar] [CrossRef]
- Mas, M.F.; González, J.; Frontera, W.R. Stroke and sarcopenia. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 452–460. [Google Scholar] [CrossRef]
- Nozoe, M.; Kubo, H.; Kanai, M.; Yamamoto, M. Relationships between Pre-Stroke SARC-F Scores, Disability, and Risk of Malnutrition and Functional Outcomes after Stroke-A Prospective Cohort Study. Nutrients 2021, 13, 3586. [Google Scholar] [CrossRef]
- Akyea, R.K.; Doehner, W.; Iyen, B.; Weng, S.F.; Qureshi, N.; Ntaios, G. Obesity and long-term outcomes after incident stroke: A prospective population-based cohort study. J. Cachexia Sarcopenia Muscle 2021, 12, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Lingvay, I.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Kahn, S.E.; Kushner, R.F.; Marso, S.; Plutzky, J.; Brown-Frandsen, K.; et al. Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) rationale and design. Am. Heart J. 2020, 229, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Chen, S.; Shao, H. Anti-diabetic drugs and sarcopenia: Emerging links, mechanistic insights, and clinical implications. J. Cachexia Sarcopenia Muscle 2021, 12, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef]
- Damanti, S.; Cristel, G.; Ramirez, G.A.; Bozzolo, E.P.; Da Prat, V.; Gobbi, A.; Centurioni, C.; Di Gaeta, E.; Del Prete, A.; Calabrò, M.G.; et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2021, S0261–5614, 00375–00377. [Google Scholar] [CrossRef]
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Wollina, U.; Karadağ, A.S.; Rowland-Payne, C.; Chiriac, A.; Lotti, T. Cutaneous signs in COVID-19 patients: A review. Dermatol. Ther. 2020, 33, e13549. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Matthai, J.; Shanmugam, N.; Sobhan, P.; Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition; Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Coronavirus Disease (COVID-19) and the Gastrointestinal System in Children. Indian Pediatr. 2020, 57, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Rhee, J.W.; Cheng, P.; Waliany, S.; Chang, A.; Witteles, R.M.; Maecker, H.; Davis, M.M.; Nguyen, P.K.; Wu, S.M. Cardiovascular Complications in Patients with COVID-19: Consequences of Viral Toxicities and Host Immune Response. Curr. Cardiol. Rep. 2020, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Run-Qian, L.; Hao-Ran, W.; Hao-Ran, C.; Ya-Bin, L.; Yang, G.; Fei, C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373. [Google Scholar] [CrossRef]
- See, A.; Toh, S.T. Respiratory sampling for severe acute respiratory syndrome coronavirus 2: An Overview. Head Neck 2020, 42, 1652–1656. [Google Scholar] [CrossRef]
- Elsevier Connect. El Nuevo Coronavirus SARS-CoV-2 y su Enfermedad, COVID-19, ¿a qué nos Enfrentamos? Available online: https://www.elsevier.com/es-es/connect/coronavirus/SARS-CoV-2-y-su-enfermedad-COVID19-a-que-nos-enfrentamos (accessed on 15 December 2021).
- Ekiz, T.; Pazarl, A.C. Relationship between COVID-19 and obesity. Diabetes Metab. Syndr. 2020, 14, 761–763. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Kreidieh, D.; Itani, L.; El Masri, D.; Tannir, H.; Citarella, R.; El Ghoch, M. Association between Sarcopenic Obesity, Type 2 Diabetes, and Hypertension in Overweight and Obese Treatment-Seeking Adult Women. J. Cardiovasc. Dev. Dis. 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020, 80, e14–e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frühbeck, G.; Baker, J.L.; Busetto, L.; Dicker, D.; Goossens, G.; Halford, J.; Handjieva-Darlenska, T.; Hassapidou, M.; Holm, J.C.; Lehtinen-Jacks, S.; et al. European Association for the Study of Obesity Position Statement on the Global COVID-19 Pandemic. Obes. Facts 2020, 13, 292–296. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, A.; Singh, A.K.; Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndr. 2020, 14, 211–212. [Google Scholar] [CrossRef]
- Molfino, A.; Imbimbo, G.; Rizzo, V.; Muscaritoli, M.; Alampi, D. The link between nutritional status and outcomes in COVID-19 patients in ICU: Is obesity or sarcopenia the real problem? Eur. J. Intern. Med. 2021, 91, 93–95. [Google Scholar] [CrossRef]
- Besutti, G.; Pellegrini, M.; Ottone, M.; Cantini, M.; Milic, J.; Bonelli, E.; Dolci, G.; Cassone, G.; Ligabue, G.; Spaggiari, L.; et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS ONE 2021, 16, e0251768. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Hosseini, F. Exercise against SARS-CoV-2 (COVID-19): Does workout intensity matter? (A mini review of some indirect evidence related to obesity). Obes. Med. 2020, 19, 100245. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhao, W.; Xu, Z.; Gu, J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020, 162, 108118. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Yates, T.; Baker, L.A.; Zaccardi, F.; Smith, A.C. Sarcopenic obesity and the risk of hospitalization or death from coronavirus disease 2019: Findings from UK Biobank. JCSM Rapid Commun. 2021, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cabrera, Á.J.; Amores-Hernández, L.; Fernández- Casteleiro, E. Immunosenescence and Frailty: A Current Glance. Med. Int. Mex. 2013, 29, 605–611. [Google Scholar]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Kara, Ö.; Kara, M.; Akın, M.E.; Özçakar, L. Grip strength as a predictor of disease severity in hospitalized COVID-19 patients. Heart Lung 2021, 50, 743–747. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Campos Mayoral, L.; Matias-Cervantes, C.A.; Pérez-Campos, E.; Romero Díaz, C.; Laguna Barrios, L.Á.; Pina Canseco, M.d.S.; Martínez Cruz, M.; Pérez-Campos Mayoral, E.; Solórzano Mata, C.J.; Rodal Canales, F.J.; et al. Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19. Int. J. Mol. Sci. 2022, 23, 8277. https://doi.org/10.3390/ijms23158277
Pérez-Campos Mayoral L, Matias-Cervantes CA, Pérez-Campos E, Romero Díaz C, Laguna Barrios LÁ, Pina Canseco MdS, Martínez Cruz M, Pérez-Campos Mayoral E, Solórzano Mata CJ, Rodal Canales FJ, et al. Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19. International Journal of Molecular Sciences. 2022; 23(15):8277. https://doi.org/10.3390/ijms23158277
Chicago/Turabian StylePérez-Campos Mayoral, Laura, Carlos Alberto Matias-Cervantes, Eduardo Pérez-Campos, Carlos Romero Díaz, Luis Ángel Laguna Barrios, María del Socorro Pina Canseco, Margarito Martínez Cruz, Eduardo Pérez-Campos Mayoral, Carlos Josué Solórzano Mata, Francisco Javier Rodal Canales, and et al. 2022. "Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19" International Journal of Molecular Sciences 23, no. 15: 8277. https://doi.org/10.3390/ijms23158277
APA StylePérez-Campos Mayoral, L., Matias-Cervantes, C. A., Pérez-Campos, E., Romero Díaz, C., Laguna Barrios, L. Á., Pina Canseco, M. d. S., Martínez Cruz, M., Pérez-Campos Mayoral, E., Solórzano Mata, C. J., Rodal Canales, F. J., Martínez Ruíz, H., & Hernández-Huerta, M. T. (2022). Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19. International Journal of Molecular Sciences, 23(15), 8277. https://doi.org/10.3390/ijms23158277