Dipeptide IF and Exercise Training Attenuate Hypertension in SHR Rats by Inhibiting Fibrosis and Hypertrophy and Activating AMPKα1, SIRT1, and PGC1α
Abstract
:1. Introduction
2. Results
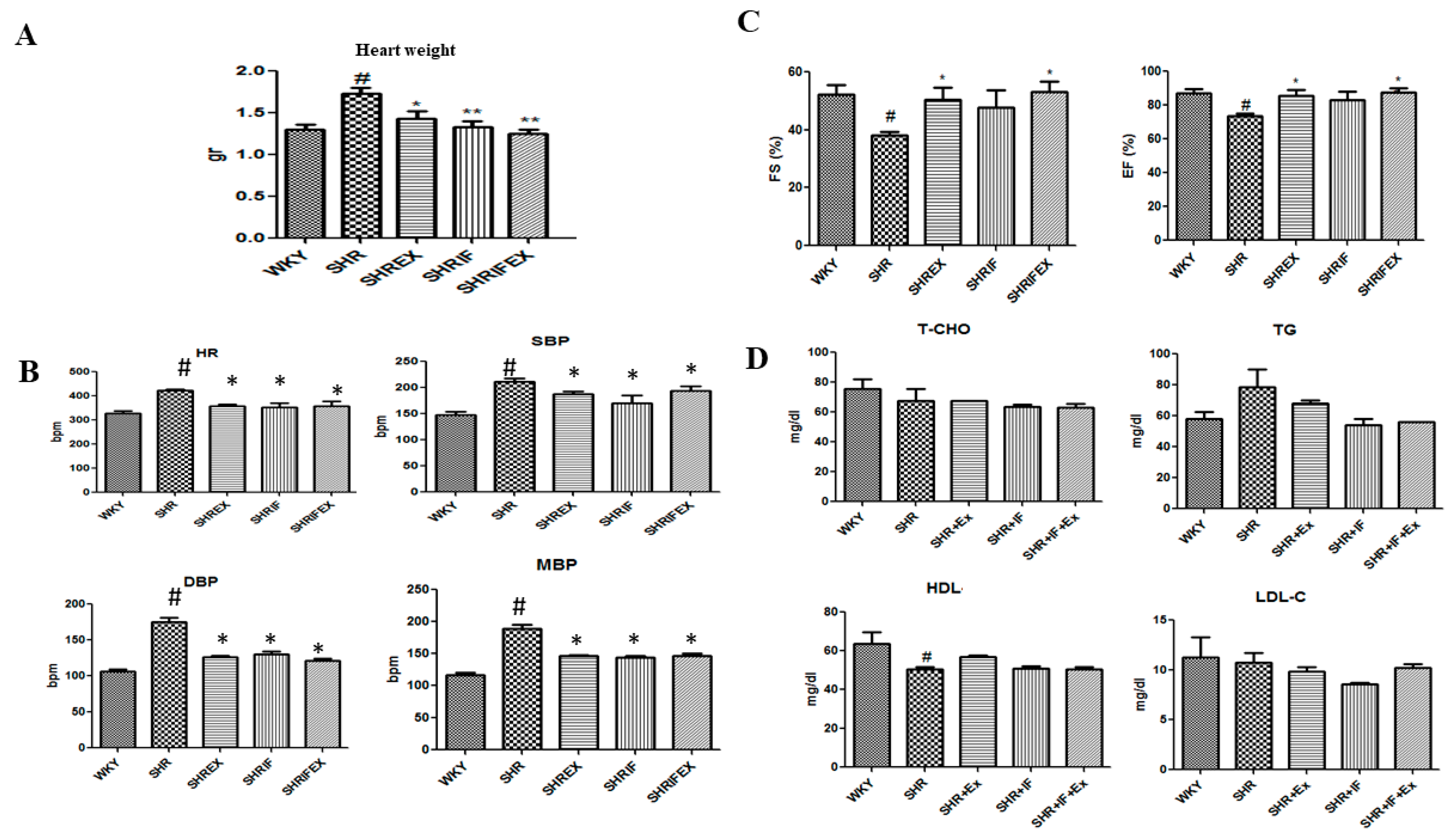
2.1. Effect of IF Peptide and Exercise Training on Heart Weight, Blood Lipid Profile, Blood Pressure Ejection Fraction, and Fractional Shortening Parameters in Hypertension Rats
2.2. Effect of IF Peptide and Exercise on Heart, Liver, and Kidney Functional Markers
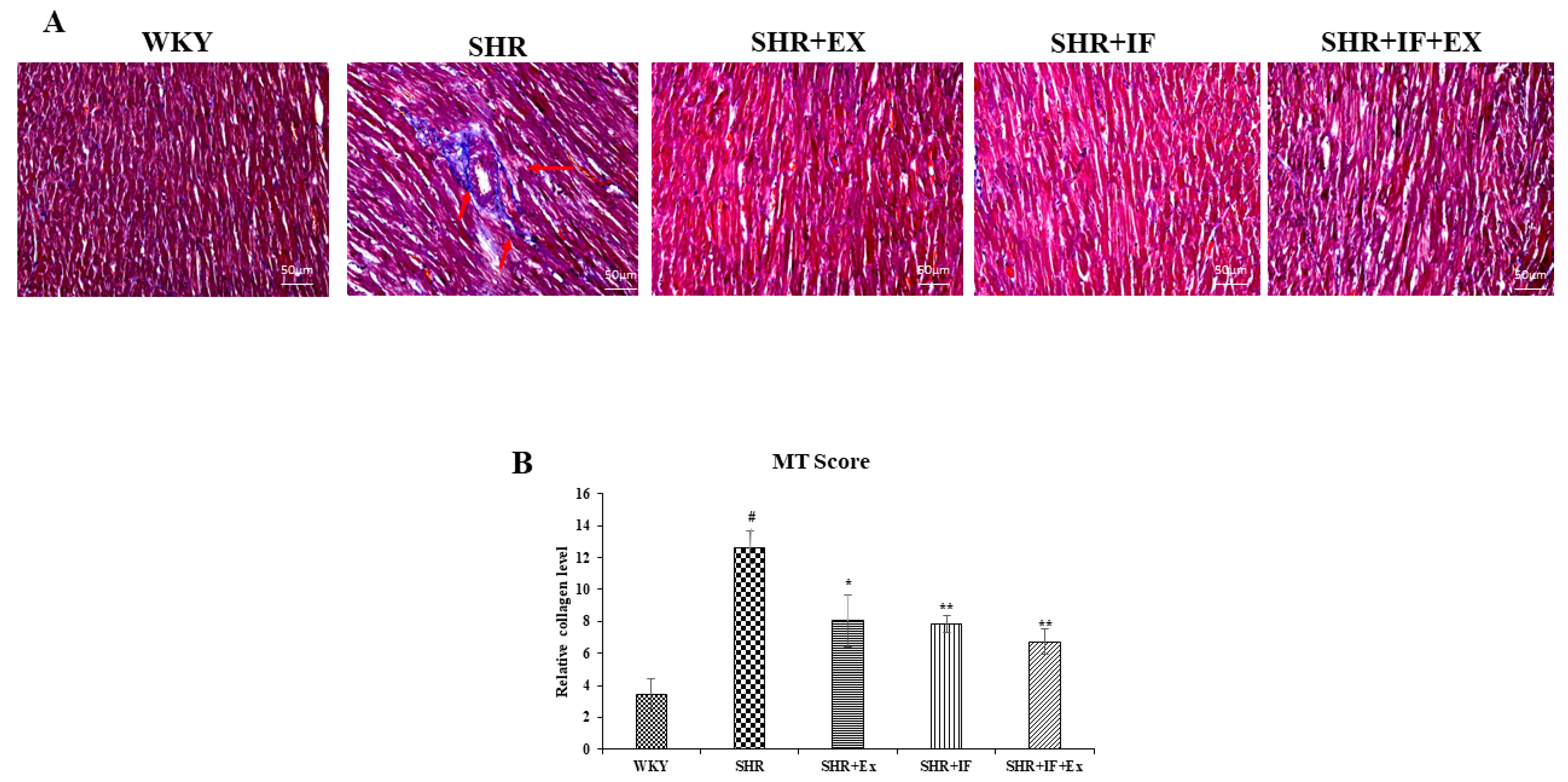
2.3. Effect of IF Peptide and Exercise Training on Histological Changes in Heart Morphology of Hypertension Rats
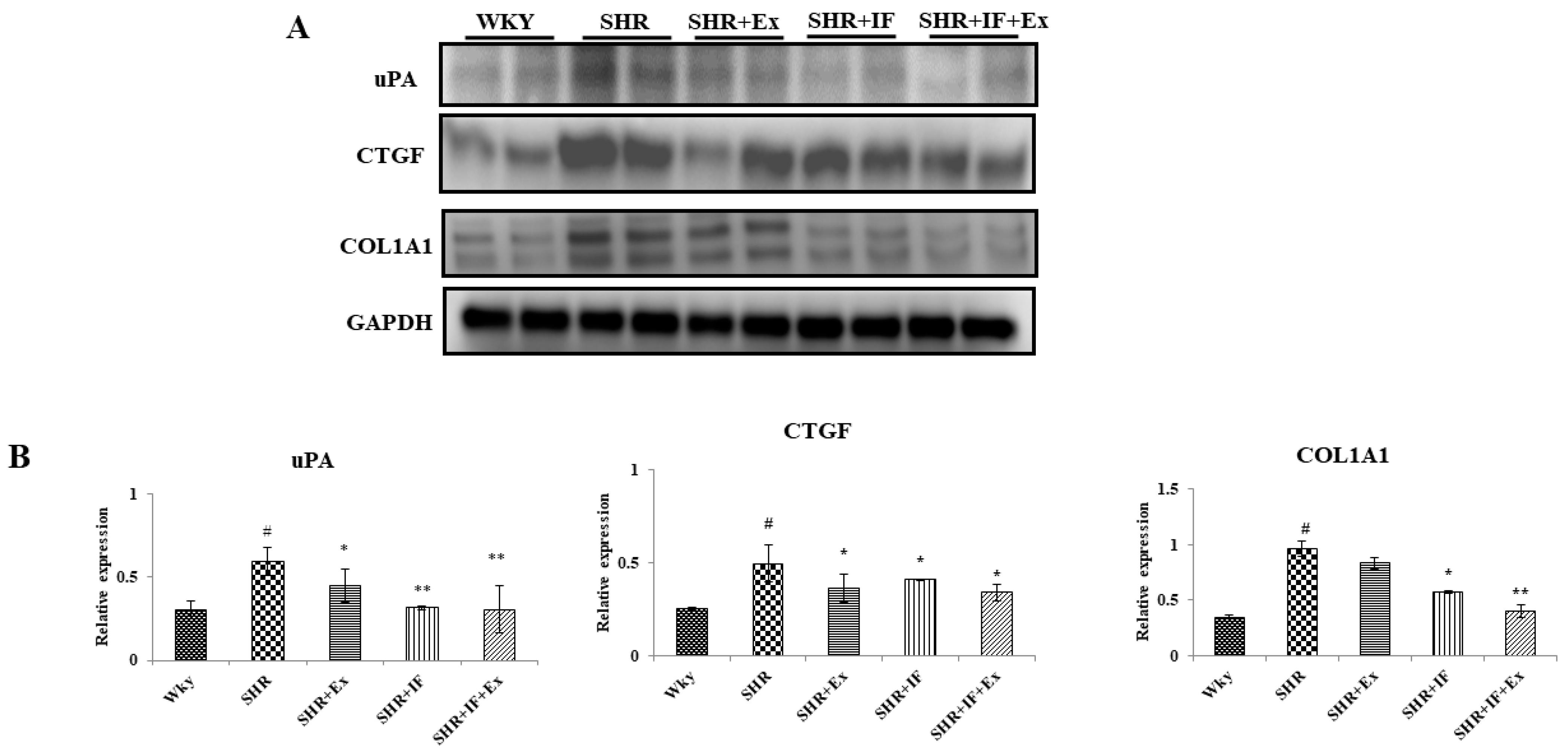
2.4. IF Treatment and Exercise Prevent Fibrosis in SHR Rats
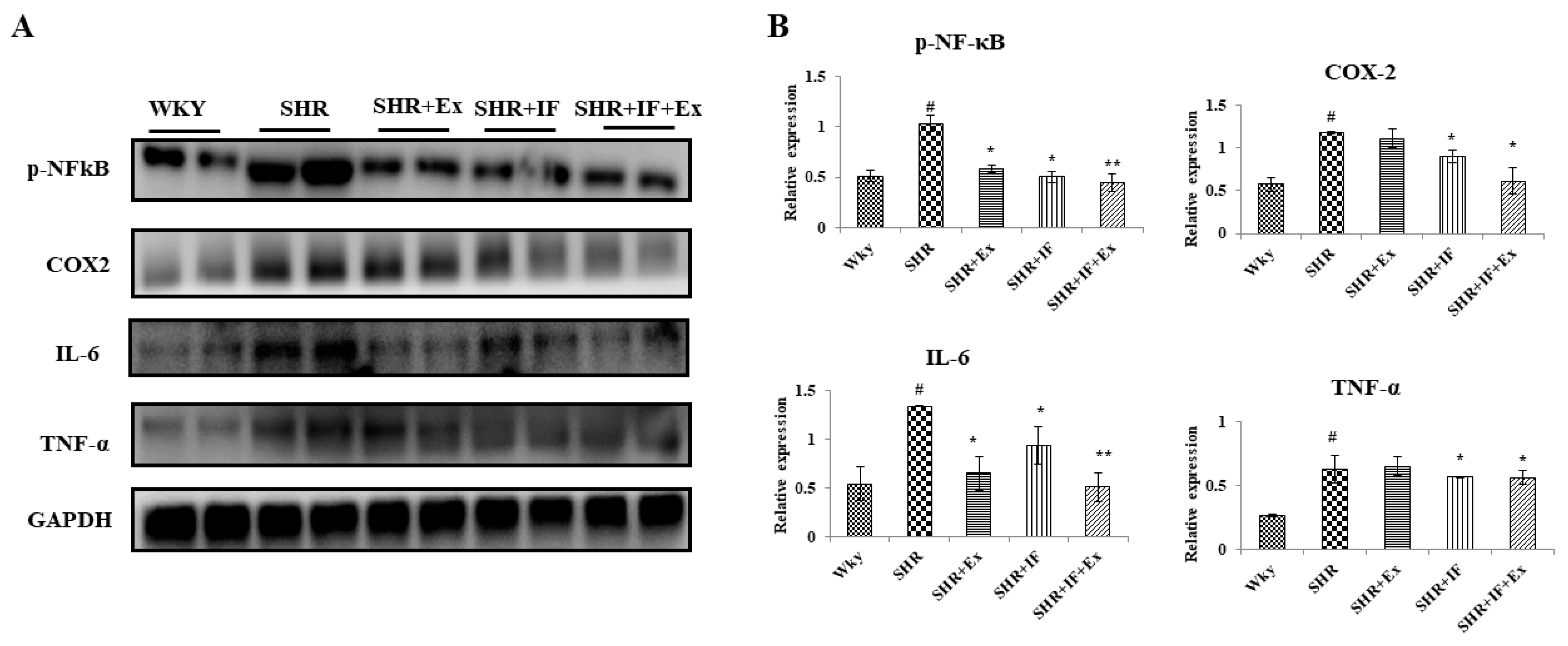
2.5. Role of IF Peptide and Exercise Training in Alleviating Hypertrophy-Mediated Inflammation in Hypertensive Rats
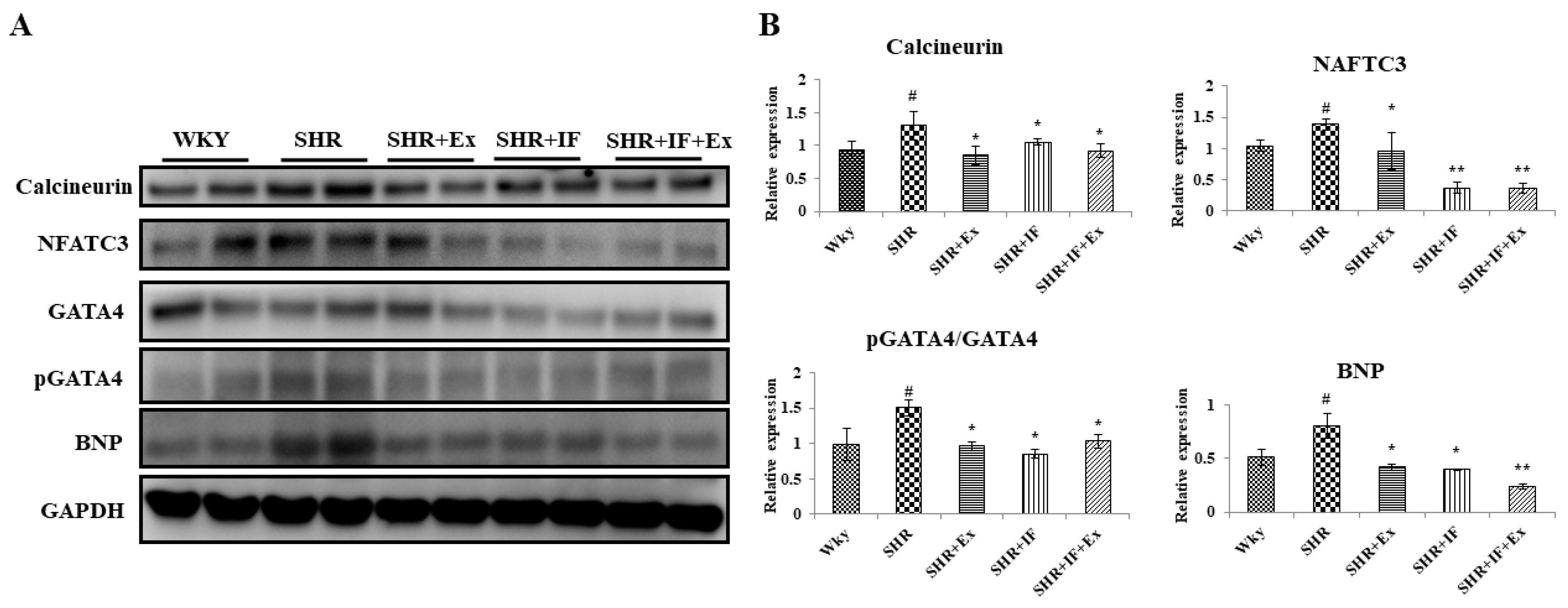
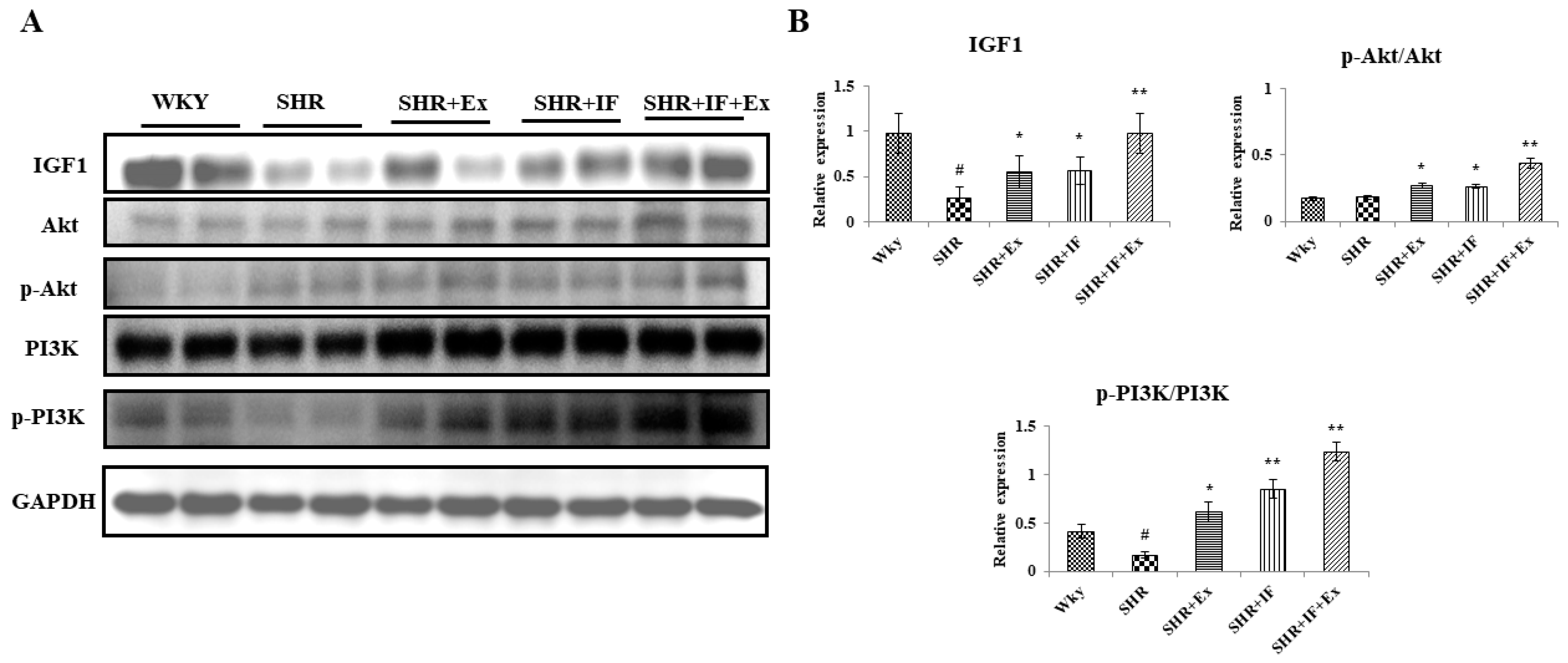
2.6. Effect of IF and Exercise Training on the Cell Survival Pathway in the Heart Tissue of SHR Animals
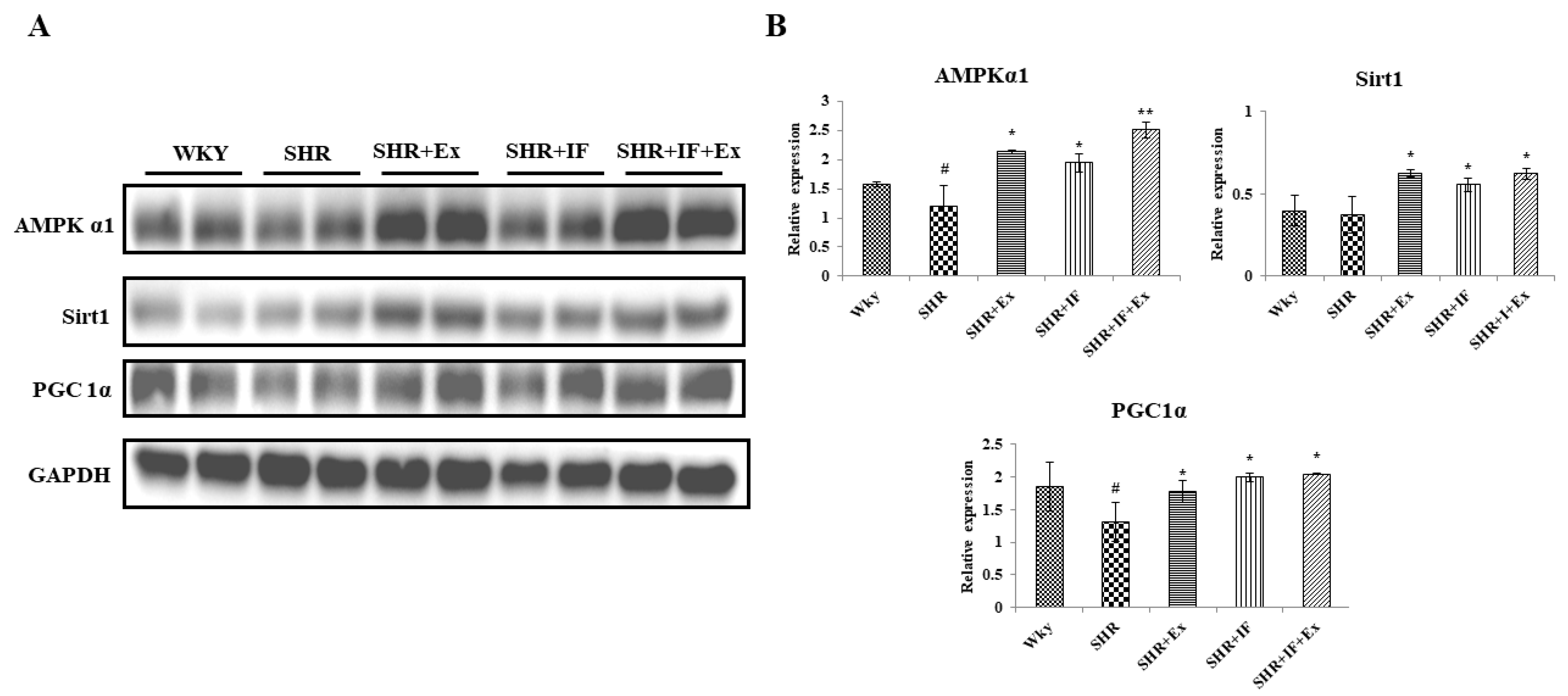
2.7. IF Treatment Combined with Exercise Training Alleviates Hypertension through Upregulating AMPKα1, SIRT1, and PGC1α Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Treatment Schedule
4.4. Blood Pressure Measurement
4.5. Measurement of Blood Serum Biochemical Parameters
4.6. Echocardiography
4.7. Histology Staining
4.8. Western Blot
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egan, B.M.; Kjeldsen, S.E.; Grassi, G.; Esler, M.; Mancia, G. The global burden of hypertension exceeds 1.4 billion people: Should a systolic blood pressure target below 130 become the universal standard? J. Hypertens. 2019, 37, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Jekell, A.; Nilsson, P.M.; Kahan, T. Treatment of hypertensive left ventricular hypertrophy. Curr. Pharm. Des. 2018, 24, 4391–4396. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Ma, M.; Chen, W.; Hua, Y.; Jia, H.; Song, Y.; Wang, Y. Aerobic exercise ameliorates cardiac hypertrophy by regulating mitochondrial quality control and endoplasmic reticulum stress through M2AChR. J. Cell. Physiol. 2021, 236, 6581–6596. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Siwik, D.A.; Xiao, L.; Pimentel, D.R.; Singh, K.; Colucci, W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell. Cardiol. 2002, 34, 379–388. [Google Scholar] [CrossRef]
- Karamanlidis, G.; Bautista-Hernandez, V.; Fynn-Thompson, F.; Del Nido, P.; Tian, R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ. Heart Fail. 2011, 4, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Anastasiou, D.; Krek, W. SIRT1: Linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology 2006, 21, 404–410. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.-H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [Green Version]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Chinsomboon, J.; Ruas, J.; Gupta, R.K.; Thom, R.; Shoag, J.; Rowe, G.C.; Sawada, N.; Raghuram, S.; Arany, Z. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA 2009, 106, 21401–21406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; LeBrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Bredin, S.S.; Guan, Y.; Dickinson, K.; Kim, D.D.; Chua, Z.; Kaufman, K.; Warburton, D.E. Cardiovascular health benefits of exercise training in persons living with type 1 diabetes: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 253. [Google Scholar] [CrossRef] [Green Version]
- Pagano, G.; Pallardó, F.V.; Lyakhovich, A.; Tiano, L.; Fittipaldi, M.R.; Toscanesi, M.; Trifuoggi, M. Aging-related disorders and mitochondrial dysfunction: A critical review for prospect mitoprotective strategies based on mitochondrial nutrient mixtures. Int. J. Mol. Sci. 2020, 21, 7060. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Thomson, D.M. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-Y.; Kuo, W.-W.; Baskaran, R.; Kuo, C.-H.; Chen, Y.-A.; Chen, W.S.-T.; Ho, T.-J.; Day, C.H.; Mahalakshmi, B.; Huang, C.-Y. Swimming exercise stimulates IGF1/PI3K/Akt and AMPK/SIRT1/PGC1α survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852. [Google Scholar] [CrossRef]
- Tanaka, H.; Bassett Jr, D.R.; Howley, E.T.; Thompson, D.L.; Ashraf, M.; Rawson, F.L. Swimming training lowers the resting blood pressure in individuals with hypertension. J. Hypertens. 1997, 15, 651–657. [Google Scholar] [CrossRef]
- Kokkinos, P.F.; Papademetriou, V. Exercise and hypertension. Coron. Artery Dis. 2000, 11, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Corpuz, H.M.; Nakamura, S. Potential of plant-derived peptides for the improvement of memory and cognitive function. Peptides 2021, 142, 170571. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-F.; Baskaran, R.; Kuo, C.-H.; Day, C.H.; Chen, R.-J.; Ho, T.-J.; Yeh, Y.-L.; Padma, V.V.; Lai, C.-H.; Huang, C.-Y. Bioactive dipeptide from potato protein hydrolysate combined with swimming exercise prevents high fat diet induced hepatocyte apoptosis by activating PI3K/Akt in SAMP8 mouse. Mol. Biol. Rep. 2021, 48, 2629–2637. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Lin, W.T.; Nithiyanantham, S.; Hsieh, D.J.Y.; Chen, R.J.; Day, C.H.; Liao, J.Y.; Kuo, C.H.; Mahalakshmi, B.; Kuo, W.W.; Huang, C.Y. Bioactive peptides attenuate cardiac apoptosis in spontaneously hypertensive rat hearts through activation of autophagy and mitochondrial biogenesis pathway. Environ. Toxicol. 2020, 35, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Marthandam Asokan, S.; Wang, T.; Su, W.-T.; Lin, W.-T. Antidiabetic effects of a short peptide of potato protein hydrolysate in STZ-induced diabetic mice. Nutrients 2019, 11, 779. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Mi, C.; Liu, J.; Gao, F.; Long, J. Compromised mitochondrial remodeling in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Cardiovasc. Pathol. 2014, 23, 101–106. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Zhu, Y.-Z.; Gohlke, P.; Stauss, H.M.; Unger, T. Effects of angiotensin-converting enzyme inhibition and angiotensin II AT1 receptor antagonism on cardiac parameters in left ventricular hypertrophy. Am. J. Cardiol. 1997, 80, 110A–117A. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Ren, Y.; Zeng, Y.; Huang, Q.; Wang, C. Antihypertensive effect of soybean bioactive peptides: A review. Curr. Opin. Pharmacol. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Asokan, S.M.; Wang, T.; Wang, M.-F.; Lin, W.-T. A novel dipeptide from potato protein hydrolysate augments the effects of exercise training against high-fat diet-induced damages in senescence-accelerated mouse-prone 8 by boosting pAMPK/SIRT1/PGC-1α/pFOXO3 pathway. Aging 2020, 12, 7334. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Gavlighi, H.A.; Sarteshnizi, R.A.; Barzegar, M.; Udenigwe, C.C. In vitro antioxidant activity and antidiabetic effect of fractionated potato protein hydrolysate via ultrafiltration and adsorption chromatography. LWT 2022, 154, 112765. [Google Scholar] [CrossRef]
- Chiang, W.-D.; Shih, C.-J.; Chu, Y.-H. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999, 65, 189–194. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Pünsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2674–2687. [Google Scholar] [CrossRef]
- Chen, X.; Qian, J.; Wang, L.; Li, J.; Zhao, Y.; Han, J.; Khan, Z.; Chen, X.; Wang, J.; Liang, G. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine 2018, 60, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Nithiyanantham, S.; Liao, J.Y.; Lin, W.T. Bioactive peptides attenuate cardiac hypertrophy and fibrosis in spontaneously hypertensive rat hearts. J. Food Drug Anal. 2020, 28, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Lin, Y.-C.; Kuo, W.-W.; Shen, C.-Y.; Cheng, Y.-C.; Lin, Y.-M.; Chang, R.-L.; Padma, V.V.; Huang, C.-Y.; Huang, C.-Y. Platycodin D reverses pathological cardiac hypertrophy and fibrosis in spontaneously hypertensive rats. Am. J. Chin. Med. 2018, 46, 537–549. [Google Scholar] [CrossRef]
- Shehzad, A.; Rehmat, S.; Ul-Islam, S.; Ahmad, R.; Aljafary, M.; Alrushaid, N.A.; Al-Suhaimi, E.A. Lirioresinol B dimethyl ether inhibits NF-κB and COX-2 and activates IκBα expression in CCl4-induced hepatic fibrosis. BMC Complementary Med. Ther. 2020, 20, 49. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Deng, X.; Li, Y.; Li, R.; Yang, Z.; Li, X.; Song, S.; Shi, Y.; Duan, H.; Wu, H. PP2 Ameliorates Renal Fibrosis by Regulating the NF-κB/COX-2 and PPARγ/UCP2 Pathway in Diabetic Mice. Oxidative Med. Cell. Longev. 2021, 2021, 7394344. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α signaling in heart disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Zohouri, A. Carvedilol prevents pancreatic β-cell damage and the development of type 1 diabetes in mice by the inhibition of proinflammatory cytokines, NF-κB, COX-2, iNOS and oxidative stress. Cytokine 2021, 138, 155394. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Hong, Y.; Zhou, M.-C.; Huang, H.-L.; Shyu, W.-C.; Chen, J.-S.; Ting, H.; Cheng, Y.-J.; Yang, A.-L.; Lee, S.-D. Exercise training attenuates cardiac inflammation and fibrosis in hypertensive ovariectomized rats. J. Appl. Physiol. 2020, 128, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Lupu, F.; Terclavers, S.; Vanwetswinkel, B.; Herbert, J.-M.; Baker, A.; Collen, D.; Carmeliet, P.; Moons, L. Loss or Inhibition of uPA or MMP-9 Attenuates LV Remodeling and Dysfunction after Acute Pressure Overload in Mice. Am. J. Pathol. 2005, 166, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Soares, L.; Drummond, F.; Lavorato, V.; Carneiro-Junior, M.; Natali, A. Exercise training and pulmonary arterial hypertension: A review of the cardiac benefits. Sci. Sports 2018, 33, 197–206. [Google Scholar] [CrossRef]
- Palabiyik, O.; Tastekin, E.; Doganlar, Z.B.; Tayfur, P.; Dogan, A.; Vardar, S.A. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed. Rep. 2019, 10, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.H.; Wu, K.L.; Chang, A.Y.; Tai, M.-H.; Chan, J.Y. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 2009, 53, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Puddu, P.; Puddu, G.M.; Cravero, E.; De Pascalis, S.; Muscari, A. The putative role of mitochondrial dysfunction in hypertension. Clin. Exp. Hypertens. 2007, 29, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Sarrion, I.; Milian, L.; Juan, G.; Ramon, M.; Naufal, D.; Gil, J.; Ridocci, F.; Fabregat-Andres, O.; Cortijo, J. PGC-1α induction in pulmonary arterial hypertension. Oxidative Med. Cell. Longev. 2012, 2012, 236572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 modulates redox homeostasis during ischemia/reperfusion in the aging heart. Antioxidants 2020, 9, 858. [Google Scholar] [CrossRef]
- Hajializadeh, Z.; Khaksari, M. The protective effects of 17-β estradiol and SIRT1 against cardiac hypertrophy: A review. Heart Fail. Rev. 2021, 27, 725–738. [Google Scholar] [CrossRef]
- Cheng, B.; Gao, W.; Wu, X.; Zheng, M.; Yu, Y.; Song, C.; Miao, W.; Yang, Z.; He, Y.; Liu, C. Ginsenoside Rg2 ameliorates high-fat diet-induced metabolic disease through SIRT1. J. Agric. Food Chem. 2020, 68, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lin, C.-C.; Ting, W.-J.; Pai, P.-Y.; Kuo, C.-H.; Ho, T.-J.; Kuo, W.-W.; Chang, C.-H.; Huang, C.-Y.; Lin, W.-T. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age 2014, 36, 9705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Lennon, S.L.; Quindry, J.; Mehta, J.L. Exercise and cardioprotection. Curr. Opin. Cardiol. 2002, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Redington, A.; George, K.P.; Hopman, M.T.; Jones, H. Association of exercise preconditioning with immediate cardioprotection: A review. JAMA Cardiol. 2018, 3, 169–176. [Google Scholar] [CrossRef]
- Improta Caria, A.C.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; Souza, B.S.d.F. Exercise training-induced changes in microRNAs: Beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, J.-H.; Baskaran, R.; Wang, M.-F.; Mohammedsaleh, Z.M.; Yang, H.-S.; Balasubramanian, B.; Lin, W.-T. Dipeptide IF and Exercise Training Attenuate Hypertension in SHR Rats by Inhibiting Fibrosis and Hypertrophy and Activating AMPKα1, SIRT1, and PGC1α. Int. J. Mol. Sci. 2022, 23, 8167. https://doi.org/10.3390/ijms23158167
Ho J-H, Baskaran R, Wang M-F, Mohammedsaleh ZM, Yang H-S, Balasubramanian B, Lin W-T. Dipeptide IF and Exercise Training Attenuate Hypertension in SHR Rats by Inhibiting Fibrosis and Hypertrophy and Activating AMPKα1, SIRT1, and PGC1α. International Journal of Molecular Sciences. 2022; 23(15):8167. https://doi.org/10.3390/ijms23158167
Chicago/Turabian StyleHo, Jou-Hsuan, Rathinasamy Baskaran, Ming-Fu Wang, Zuhair M. Mohammedsaleh, Hong-Siang Yang, Balamuralikrishnan Balasubramanian, and Wan-Teng Lin. 2022. "Dipeptide IF and Exercise Training Attenuate Hypertension in SHR Rats by Inhibiting Fibrosis and Hypertrophy and Activating AMPKα1, SIRT1, and PGC1α" International Journal of Molecular Sciences 23, no. 15: 8167. https://doi.org/10.3390/ijms23158167
APA StyleHo, J.-H., Baskaran, R., Wang, M.-F., Mohammedsaleh, Z. M., Yang, H.-S., Balasubramanian, B., & Lin, W.-T. (2022). Dipeptide IF and Exercise Training Attenuate Hypertension in SHR Rats by Inhibiting Fibrosis and Hypertrophy and Activating AMPKα1, SIRT1, and PGC1α. International Journal of Molecular Sciences, 23(15), 8167. https://doi.org/10.3390/ijms23158167







