Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice
Abstract
1. Introduction
2. Results
2.1. Effects of Glabridin on Platelet Aggregation Stimulated by Various Agonists in Humans
2.2. Effects of Glabridin on ATP Release, Relative Intracellular Ca2+ Change, and P-Selectin Surface Expression and TxB2 Formation
2.3. Characteristics of Glabridin on Phospholipase Cγ2/Protein Kinase C Activation
2.4. Glabridin on Phosphoinositide 3-Kinase/Akt/Glycogen Synthase Kinase-3β and Mitogen-Activated Protein Kinases Activation
2.5. Effects of Glabridin on NF-κB Signaling
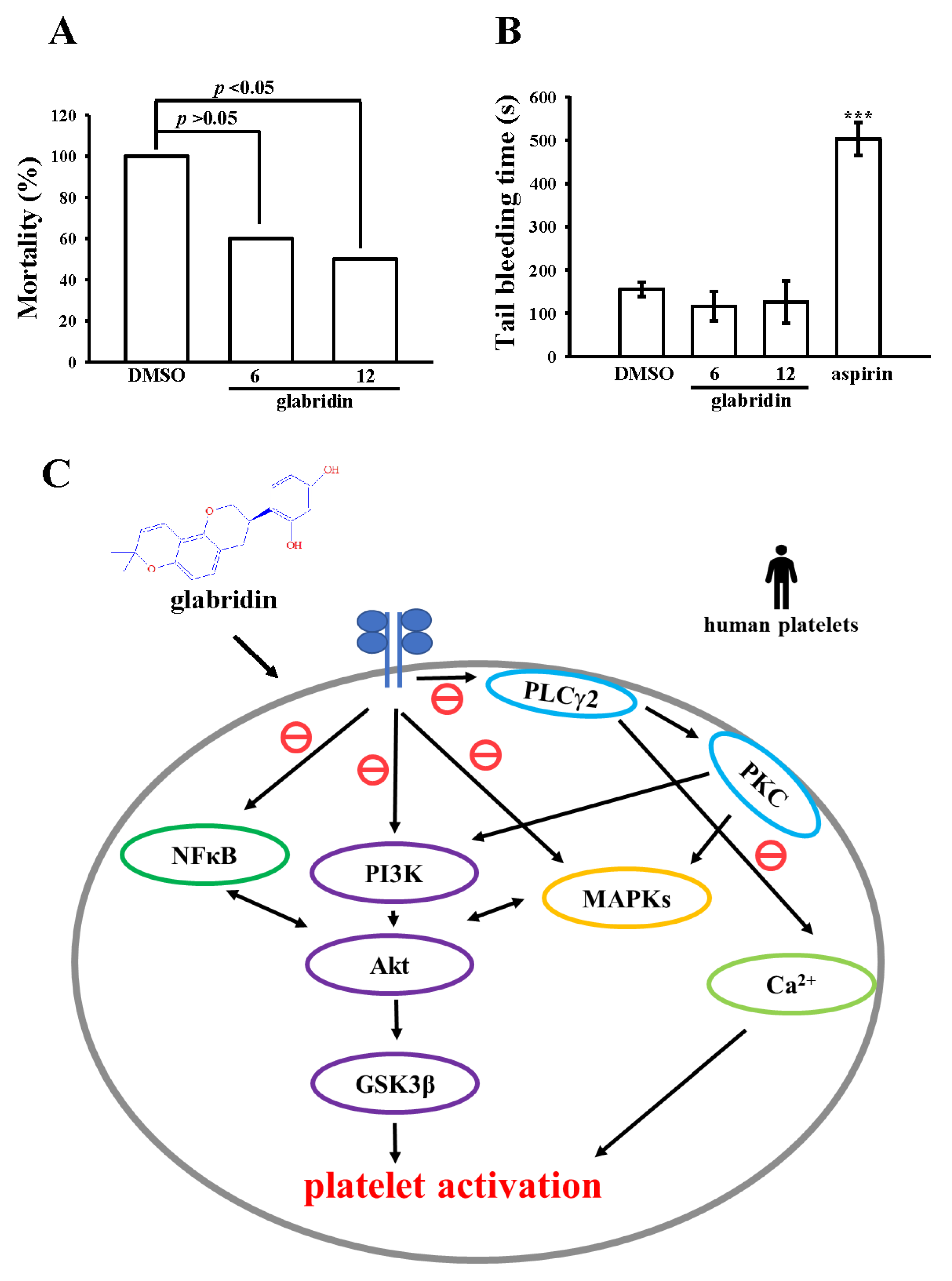
2.6. Activity of Glabridin on Experimental Acute Pulmonary Thrombosis and Bleeding Time
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Human Platelet Preparation and Aggregation
4.3. Change of [Ca2+]i and P-Selectin Surface Expression in Human Platelets
4.4. Measurement of TxB2 Formation
4.5. Immunoblotting
4.6. Confocal Laser Scanning Fluorescence Microscopy
4.7. ADP-Induced Acute Pulmonary Thromboembolism in Mice
4.8. Measurement of Tail Vein Bleeding Time
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagareddy, P.; Smyth, S.S. Inflammation and thrombosis in cardiovascular disease. Curr. Opin. Hematol. 2013, 20, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Rath, D.; Schlotterbeck, J.; Rheinlaender, J.; Walker-Allgaier, B.; Alnaggar, N.; Zdanyte, M.; Müller, I.; Borst, O.; Geisler, T.; et al. Regulation of oxidized platelet lipidome: Implications for coronary artery disease. Eur. Heart J. 2017, 38, 1993–2005. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Yen, M.H.; Hung, W.C.; Lee, Y.M.; Su, C.H.; Huang, T.F. Triflavin Inhibits Platelet-Induced Vasoconstriction in De-endothelialized Aorta. Arter. Thromb. Vasc. Biol. 1997, 17, 3461–3468. [Google Scholar] [CrossRef]
- Fukai, T.; Satoh, K.; Nomura, T.; Sakagami, H. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia 2003, 74, 624–629. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Zhang, X.; Li, X. Extraction of glabridin using imidazolium-based ionic liquids. Sep. Purif. Technol. 2012, 88, 146–150. [Google Scholar] [CrossRef]
- Li, C.X.; Li, T.H.; Zhu, M.; Lai, J.; Wu, Z.P. Pharmacological properties of glabridin (a flavonoid extracted from licorice): A comprehensive review. J. Funct. Foods 2021, 85, 104638. [Google Scholar] [CrossRef]
- Carmeli, E.; Harpaz, Y.; Kogan, N.N.; Fogelman, Y. The effect of endogenous antioxidant glabridin on oxidized LDL. J. Basic Clin. Physiol. Pharmacol. 2008, 19, 49–64. [Google Scholar] [CrossRef]
- Suo, T.; Liu, J.; Chen, X.; Yu, H.; Wang, T.; Li, C.; Wang, Y.; Wang, C.; Li, Z. Combining Chemical Profiling and Network Analysis to Investigate the Pharmacology of Complex Prescriptions in Traditional Chinese Medicine. Sci. Rep. 2017, 7, 40529. [Google Scholar] [CrossRef] [PubMed]
- Lien, L.-M.; Lin, K.-H.; Huang, L.-T.; Tseng, M.-F.; Chiu, H.-C.; Chen, R.-J.; Lu, W.-J. Licochalcone A Prevents Platelet Activation and Thrombus Formation through the Inhibition of PLCγ2-PKC, Akt, and MAPK Pathways. Int. J. Mol. Sci. 2017, 18, 1500. [Google Scholar] [CrossRef] [PubMed]
- Cosemans, J.M.E.M.; Iserbyt, B.F.; Deckmyn, H.; Heemskerk, J.W. Multiple ways to switch platelet integrins on and off. J. Thromb. Haemost. 2008, 6, 1253–1261. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.-A.; Severin, S.; Gratacap, M.-P.; Payrastre, B. Class I PI 3-kinases signaling in platelet activation and thrombosis: PDK1/Akt/GSK3 axis and impact of PTEN and SHIP1. Adv. Biol. Regul. 2014, 54, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83, Correction in Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Stoclet, J.-C.; Chataigneau, T.; Ndiaye, M.; Oak, M.-H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef]
- Kang, M.R.; Park, K.H.; Oh, S.J.; Yun, J.; Lee, C.W.; Lee, M.Y.; Han, S.-B.; Kang, J.S. Cardiovascular protective effect of glabridin: Implications in LDL oxidation and inflammation. Int. Immunopharmacol. 2015, 29, 914–918. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Liang, J.; Yu, X.-Q.; Xu, A.-L.; Chan, E.; Wei, D.; Huang, M.; Wen, J.-Y.; Yu, X.-Y.; et al. Role of P-glycoprotein in the Intestinal Absorption of Glabridin, an Active Flavonoid from the Root of Glycyrrhiza glabra. Drug Metab. Dispos. 2007, 35, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, D.A.; Yin, W. Platelet-Activation Mechanisms and Vascular Remodeling. Compr. Physiol. 2011, 8, 1117–1156. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Ding, K.; Liu, Z.; Li, Y.; He, T.; Zhang, W.; Fan, Y.; Ma, W.; Cui, L.; et al. Phospholipase Cγ2 Signaling Cascade Contribute to the Antiplatelet Effect of Notoginsenoside Fc. Front. Pharmacol. 2018, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.; Severin, S.; Gratacap, M.-P.; Aguado, E.; Malissen, M.; Jandrot-Perrus, M.; Malissen, B.; Ragab-Thomas, J.; Payrastre, B. Roles of the C-terminal tyrosine residues of LAT in GPVI-induced platelet activation: Insights into the mechanism of PLCγ2 activation. Blood 2007, 110, 2466–2474. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C.; Shi, P.; Gao, W.; Gu, J.; Geng, Y.; Yang, W.; Wu, N.; Wang, Y.; Xu, Y.; et al. Platelet MEKK3 regulates arterial thrombosis and myocardial infarct expansion in mice. Blood Adv. 2018, 2, 1439–1448. [Google Scholar] [CrossRef]
- Hughes, P.E.; Renshaw, M.W.; Pfaff, M.; Forsyth, J.; Keivens, V.M.; Schwartz, M.A.; Ginsberg, M.H. Suppression of Integrin Activation: A Novel Function of a Ras/Raf-Initiated MAP Kinase Pathway. Cell 1997, 88, 521–530. [Google Scholar] [CrossRef]
- Adam, F.; Kauskot, A.; Rosa, J.-P.; Bryckaert, M. Mitogen-activated protein kinases in hemostasis and thrombosis. J. Thromb. Haemost. 2008, 6, 2007–2016. [Google Scholar] [CrossRef]
- Jackson, S.P.; Schoenwaelder, S.; Goncalves, I.; Nesbitt, W.; Yap, C.L.; Wright, C.; Kenche, V.; Anderson, K.; Dopheide, S.M.; Yuan, Y.; et al. PI 3-kinase p110β: A new target for antithrombotic therapy. Nat. Med. 2005, 11, 507–514. [Google Scholar] [CrossRef]
- Gratacap, M.-P.; Guillermet-Guibert, J.; Martin, V.; Chicanne, G.; Tronchère, H.; Gaits-Iacovoni, F.; Payrastre, B. Regulation and roles of PI3Kβ, a major actor in platelet signaling and functions. Adv. Enzym. Regul. 2011, 51, 106–116. [Google Scholar] [CrossRef]
- Yin, H.; Stojanovic, A.; Hay, N.; Du, X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX–induced platelet activation. Blood 2008, 111, 658–665. [Google Scholar] [CrossRef]
- Jayakumar, T.; Chen, W.-F.; Lu, W.-J.; Chou, D.-S.; Hsiao, G.; Hsu, C.Y.; Sheu, J.-R.; Hsieh, C.-Y. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase: Ex vivo and in vivo studies. J. Nutr. Biochem. 2013, 24, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, D.S. Akt signaling in platelets and thrombosis. Expert Rev. Hematol. 2010, 3, 81–91. [Google Scholar] [CrossRef]
- Li, D.; August, S.; Woulfe, D.S. GSK3β is a negative regulator of platelet function and thrombosis. Blood 2008, 111, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Rivadeneyra, L.; Carestia, A.; Etulain, J.; Pozner, R.G.; Fondevila, C.; Negrotto, S.; Schattner, M. Regulation of platelet responses triggered by Toll-like receptor 2 and 4 ligands is another non-genomic role of nuclear factor-kappaB. Thromb. Res. 2014, 133, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Rojas, A.; Palomo, I. NF-κB signaling pathway as target for antiplatelet activity. Blood Rev. 2016, 30, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Malaver, E.; Romaniuk, M.A.; D’Atri, L.P.; Pozner, R.G.; Negrotto, S.; Benzadón, R.; Schattner, M. NF-κB inhibitors impair platelet activation responses. J. Thromb. Haemost. 2009, 7, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.-W.; Huang, W.-C.; Yang, C.-H.; Hsia, C.-H.; Jayakumar, T.; Bhavan, P.; Sheu, J.-R.; Chiou, K.-R. Comparison of the Potency of Pterostilbene with NF-κB Inhibitors in Platelet Activation: Mutual Activation by Akt-NF-κB Signaling in Human Platelets. Appl. Sci. 2021, 11, 6149. [Google Scholar] [CrossRef]
- Momi, S.; Falcinelli, E.; Giannini, S.; Ruggeri, L.; Cecchetti, L.; Corazzi, T.; Libert, C.; Gresele, P. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J. Exp. Med. 2009, 206, 2365–2379. [Google Scholar] [CrossRef]
- Chen, W.-F.; Lee, J.-J.; Chang, C.-C.; Lin, K.-H.; Wang, S.-H.; Sheu, J.-R. Platelet protease-activated receptor (PAR)4, but not PAR1, associated with neutral sphingomyelinase responsible for thrombin-stimulated ceramide-NF-B signaling in human platelets. Haematologica 2013, 98, 793–801. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Lee, C.-R.; Lin, C.-H.; Hsiao, G.; Ko, W.-C.; Chen, Y.-C.; Yen, M.-H. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human platelets. Thromb. Haemost. 2000, 83, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Poole, A.W. Physical and Functional Interaction between Protein Kinase C δ and Fyn Tyrosine Kinase in Human Platelets. J. Biol. Chem. 2003, 278, 24533–24541. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.-L.; Chen, J.-H.; Huang, W.-C.; Sheu, J.-R.; Hsia, C.-W.; Jayakumar, T.; Hsia, C.-H.; Chiou, K.-R.; Hou, S.-M. Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice. Int. J. Mol. Sci. 2022, 23, 11372. https://doi.org/10.3390/ijms231911372
Chung C-L, Chen J-H, Huang W-C, Sheu J-R, Hsia C-W, Jayakumar T, Hsia C-H, Chiou K-R, Hou S-M. Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice. International Journal of Molecular Sciences. 2022; 23(19):11372. https://doi.org/10.3390/ijms231911372
Chicago/Turabian StyleChung, Chi-Li, Jui-Hsuan Chen, Wei-Chieh Huang, Joen-Rong Sheu, Chih-Wei Hsia, Thanasekaran Jayakumar, Chih-Hsuan Hsia, Kuan-Rau Chiou, and Shaw-Min Hou. 2022. "Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice" International Journal of Molecular Sciences 23, no. 19: 11372. https://doi.org/10.3390/ijms231911372
APA StyleChung, C.-L., Chen, J.-H., Huang, W.-C., Sheu, J.-R., Hsia, C.-W., Jayakumar, T., Hsia, C.-H., Chiou, K.-R., & Hou, S.-M. (2022). Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice. International Journal of Molecular Sciences, 23(19), 11372. https://doi.org/10.3390/ijms231911372








