Activation of Humoral Immunity during the Pathogenesis of Experimental Chronic Lung Allograft Dysfunction
Abstract
1. Introduction
2. Results
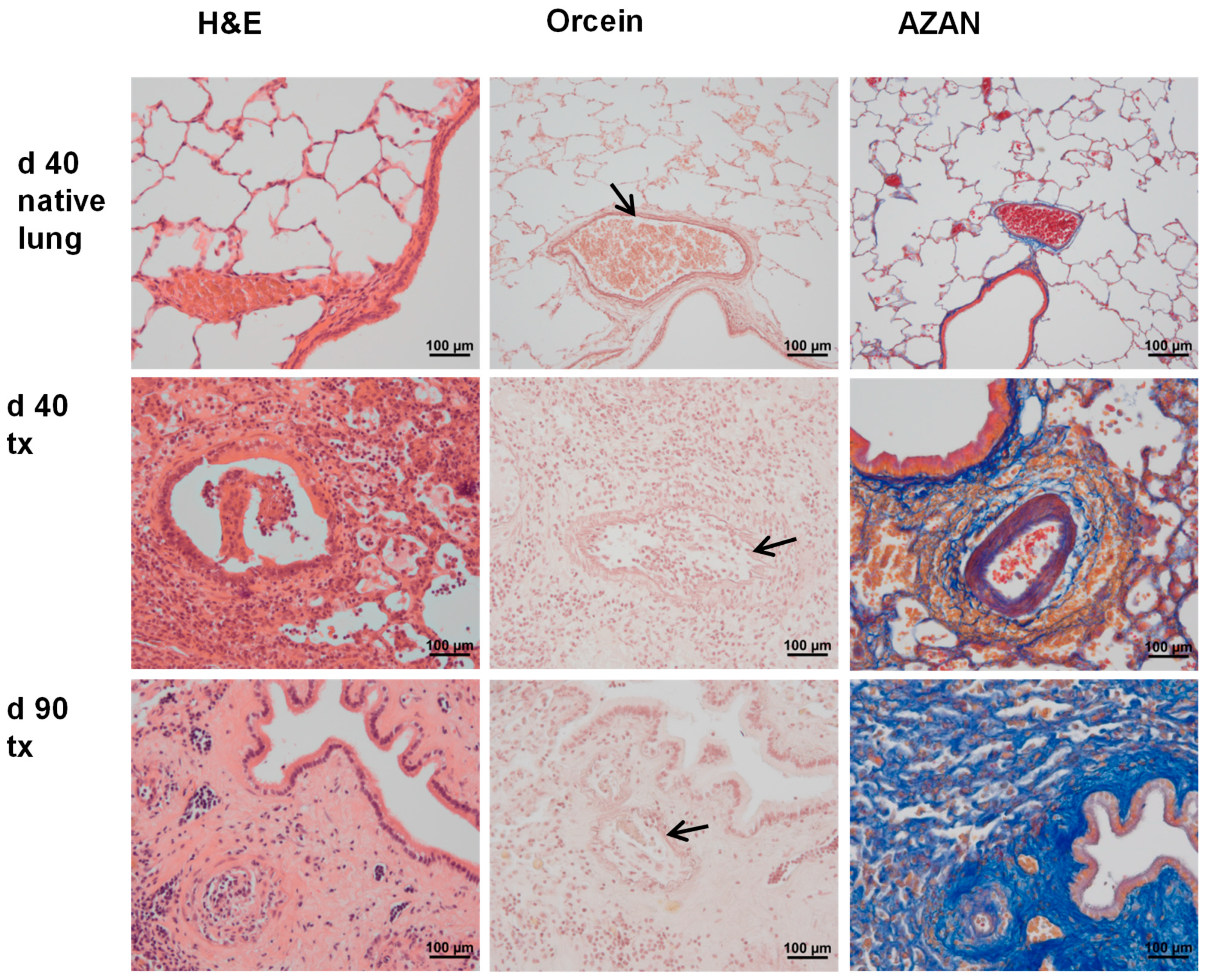
2.1. Graft Histopathology
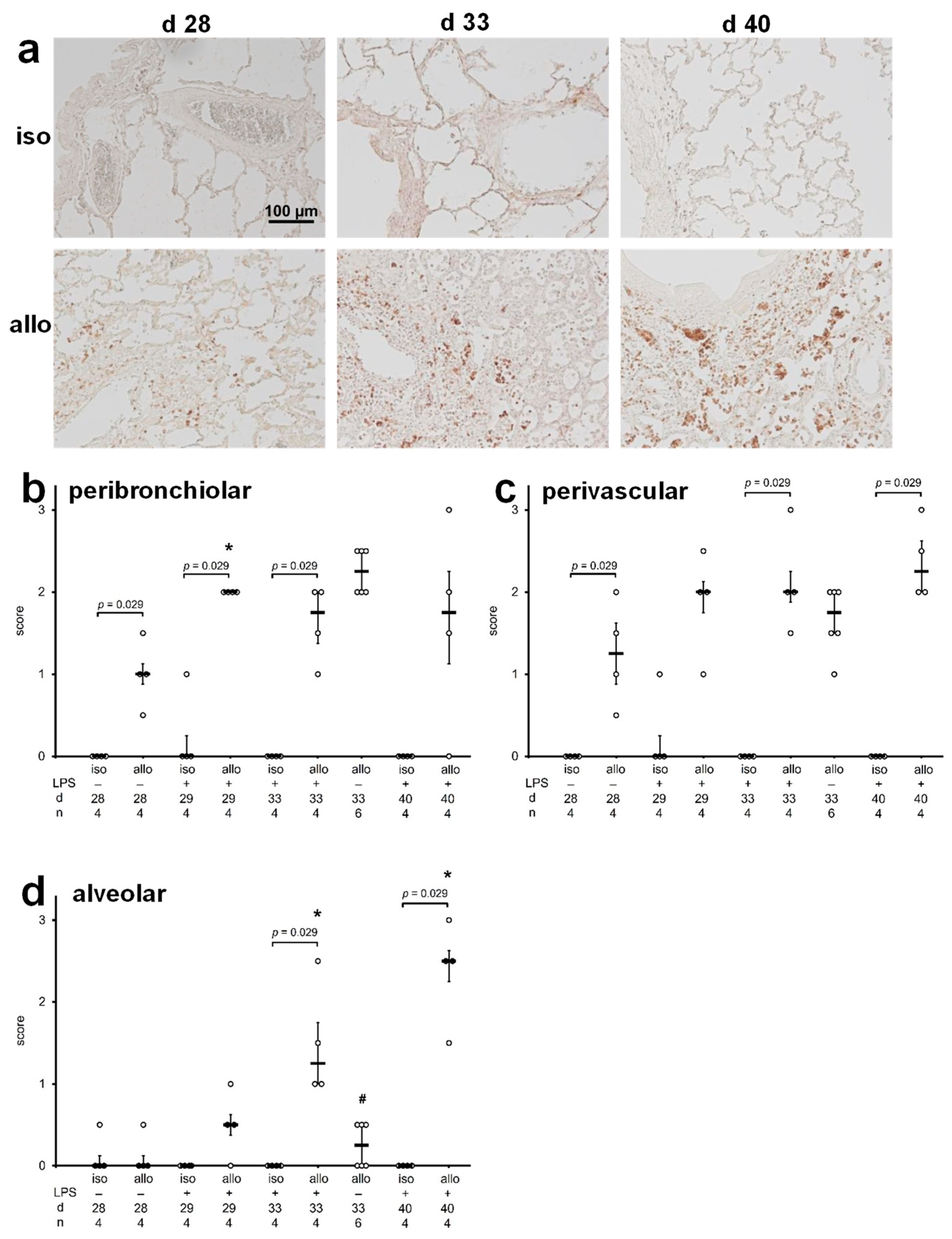
2.2. B Cell Infiltration into Lungs
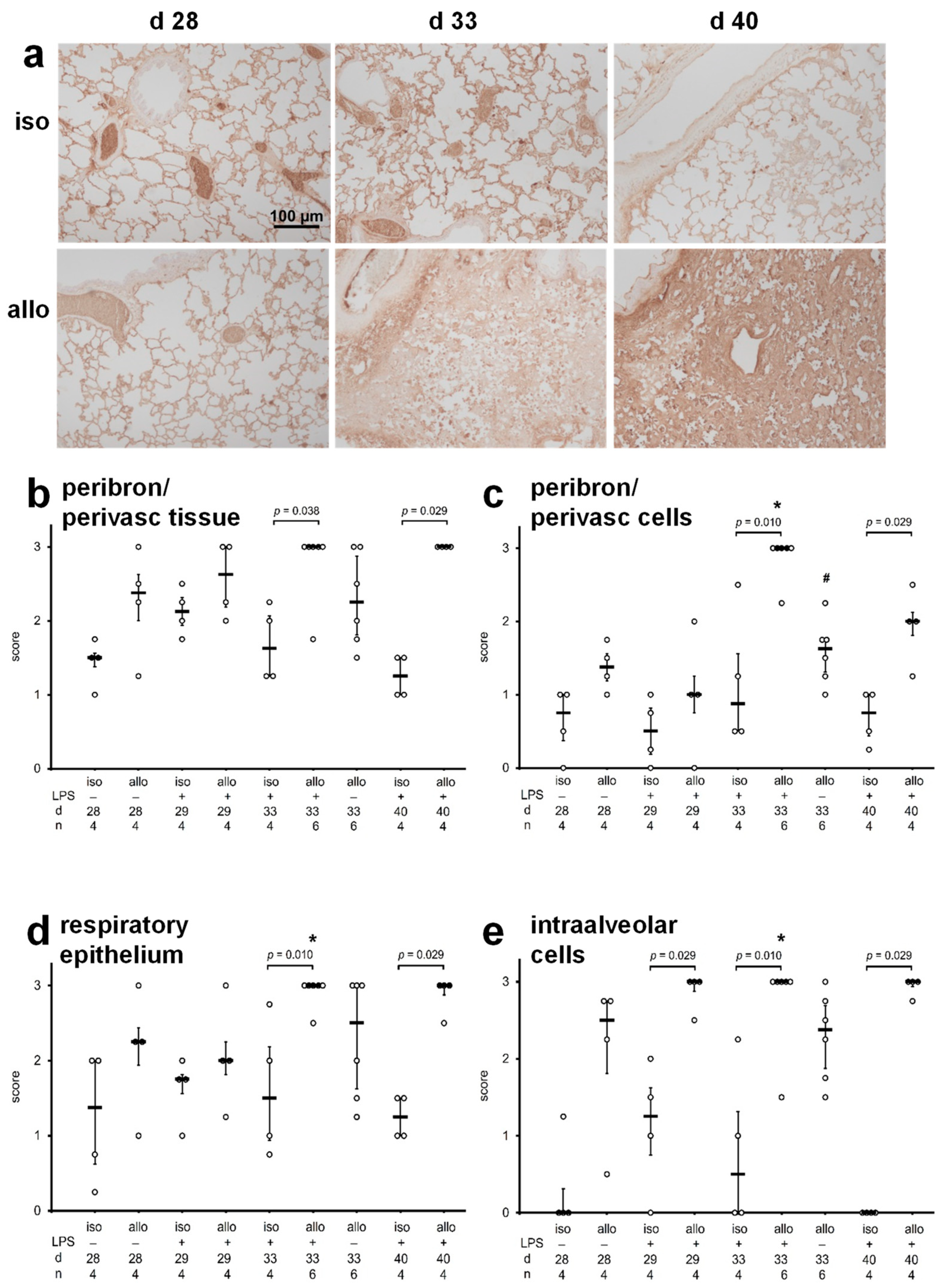
2.3. Accumulation of Immunoglobulins in Lung Tissue
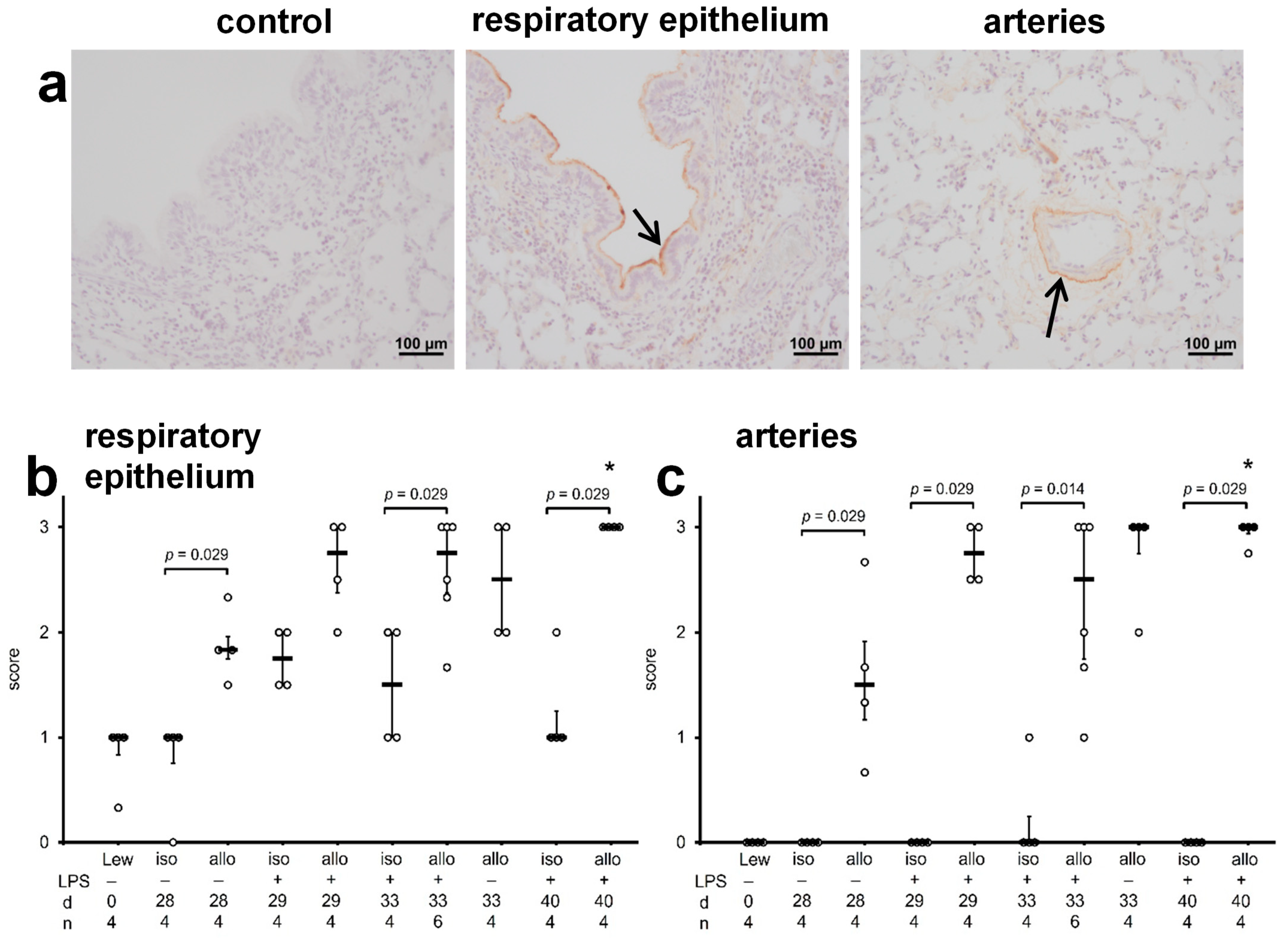
2.4. C4d Deposits in Lung Tissue
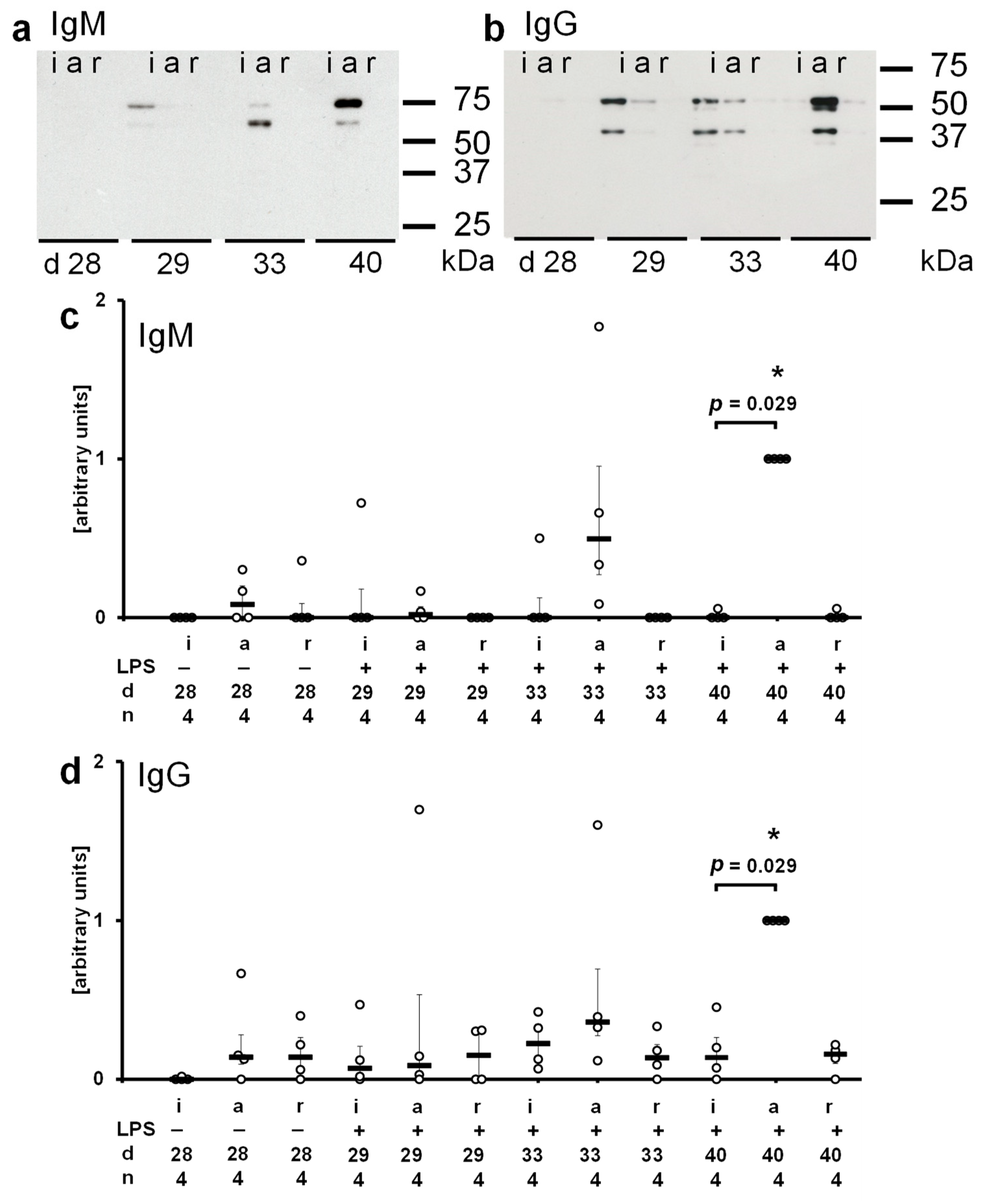
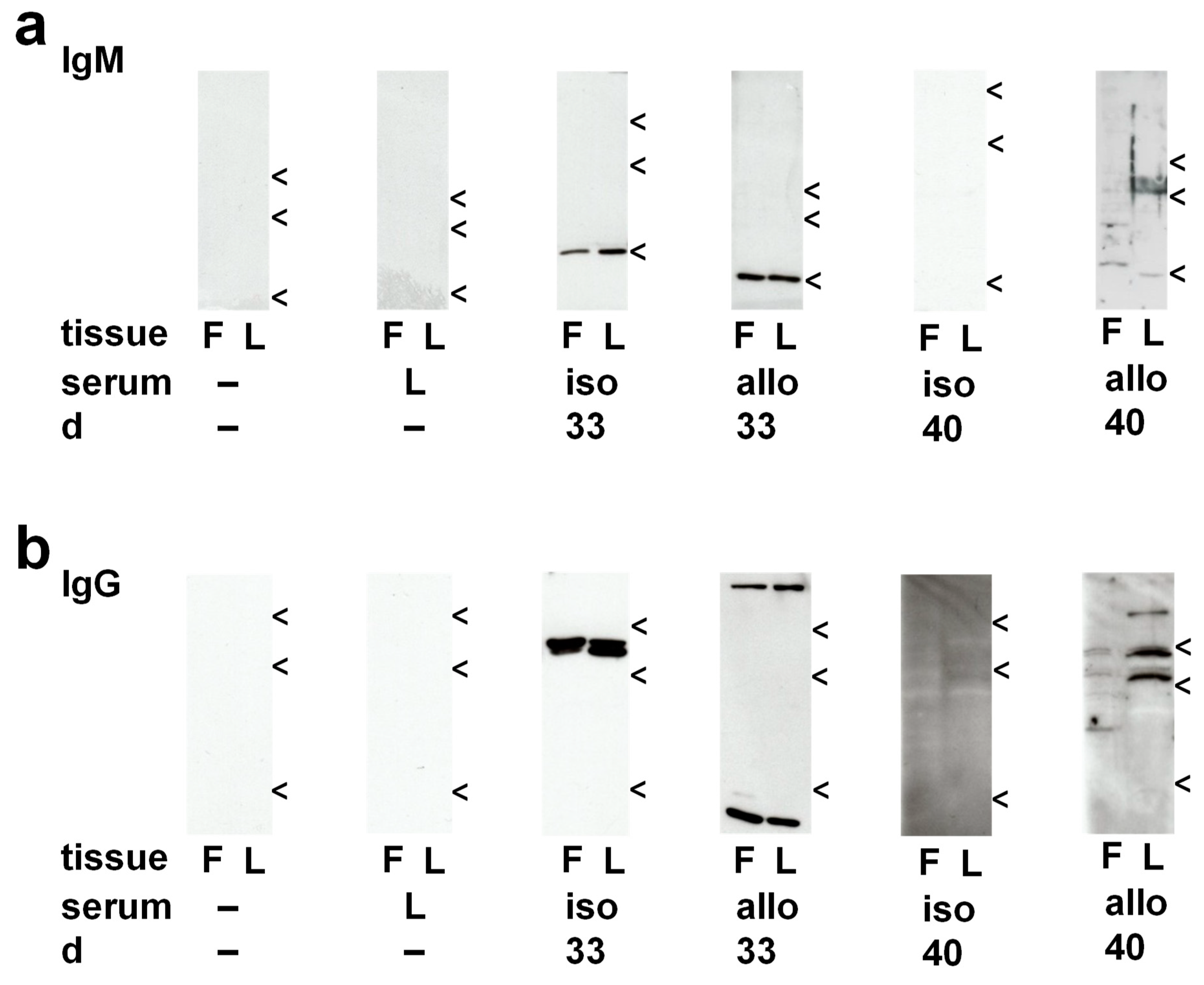
2.5. Antigens Recognized by Recipient Sera
2.5.1. Circulating IgM in Isograft Recipients
2.5.2. Circulating IgM in Allograft Recipients
| Postoperative Day, Graft | Intratracheal Treatment | n | Immunoreactivity |
|---|---|---|---|
| day 28, isograft | none | 4 | none |
| day 29, isograft | LPS | 6 | none |
| day 33, isograft | LPS | 5 1 | ~25 kDa (autoreactivity) * ~25 kDa, ~55 kDa double-band (autoreactivity) |
| day 40, isograft | LPS | 4 | none * |
| day 28, allograft | none | 4 | none |
| day 29, allograft | LPS | 6 | none |
| day 33, allograft | vehicle | 3 | none |
| 1 | ~25 kDa (autoreactivity) * | ||
| 1 | ~55 kDa double-band (autoreactivity) | ||
| day 33, allograft | LPS | 2 4 | none ~25 kDa (autoreactivity) |
| day 40, allograft | LPS | 4 1 | ~25 kDa (autoreactivity) multiple bands (autoreactivity and alloreactivity) * |
2.5.3. Circulating IgG in Isograft Recipients
2.5.4. Circulating IgG in Allograft Recipients
| Postoperative Day, Graft | Intratracheal Treatment | n | Immunoreactivity |
|---|---|---|---|
| day 28, isograft | none | 5 | none |
| day 29, isograft | LPS | 3 1 | ~55 kDa double-band (autoreactivity) ~55 kDa double-band, ~35 kDa (autoreactivity) |
| day 33, isograft | LPS | 5 | ~55 kDa double-band (autoreactivity) * |
| day 40, isograft | LPS | 4 | strong background staining * |
| day 28, allograft | none | 2 | none |
| 1 | ~50 kDa (autoreactivity) | ||
| 1 | ~55 kDa (autoreactivity) | ||
| day 29, allograft | LPS | 5 | strong background staining |
| day 33, allograft | vehicle | 2 | none |
| 2 | ~55 kDa double-band (autoreactivity) | ||
| 1 | strong background staining | ||
| day 33, allograft | LPS | 1 | ~55 kDa (autoreactivity) |
| 3 | ~55 kDa double-band (autoreactivity) | ||
| 2 | >100 kDa, <10 kDa (autoreactivity) * | ||
| 1 | ~55 kDa double-band, >100 kDa, <10 kDa (autoreactivity) | ||
| day 40, allograft | LPS | 3 1 | ~55 kDa double-band (autoreactivity) multiple bands (autoreactivity and alloreactivity) * |
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Lung Transplantation
4.2. Perioperative Medication
4.3. Standard Computed Tomography
4.4. Micro-Computed Tomography
4.5. Histopathology and Immunohistochemistry
4.6. Electrophoresis and Western Blotting
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment—A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Yusen, R.D.; Cherikh, W.S.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Khusch, K.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-fourth adult lung and heart-lung transplantation report—2017; Focus theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef]
- Verleden, S.E.; Von der Thüsen, J.; Roux, A.; Brouwers, E.S.; Braubach, P.; Kuehnel, M.; Laenger, F.; Jonigk, D. When tissue is the issue: A histological review of chronic lung allograft dysfunction. Am. J. Transplant. 2020, 20, 2644–2651. [Google Scholar] [CrossRef]
- Glanville, A.R.; Verleden, G.M.; Todd, J.L.; Benden, C.; Calabrese, F.; Gottlieb, J.; Hachem, R.R.; Levine, D.; Meloni, F.; Palmer, S.M.; et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome—A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 483–492. [Google Scholar] [CrossRef]
- Leuschner, G.; Lauseker, M.; Howanietz, A.-S.; Milger, K.; Veit, T.; Munker, D.; Schneider, C.; Weig, T.; Michel, S.; Barton, J.; et al. Longitudinal lung function measurements in single lung transplant recipients with chronic lung allograft dysfunction. J. Heart Lung Transplant. 2020, 39, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Martinu, T.; Howell, D.N.; Davis, R.D.; Steele, M.P.; Palmer, S.M. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest 2006, 129, 1016–1023. [Google Scholar] [CrossRef]
- Yousem, S.A.; Paradis, I.L.; Dauber, J.H.; Zeevi, A.; Duquesnoy, R.J.; Dal Col, R.; Armitage, J.; Hardesty, R.L.; Griffith, B.P. Pulmonary arteriosclerosis in long-term human heart-lung transplant recipients. Transplantation 1989, 47, 564–569. [Google Scholar] [PubMed]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Ius, F.; Verboom, M.; Sommer, W.; Poyanmehr, R.; Knoefel, A.-K.; Salman, J.; Kuehn, C.; Avsar, M.; Siemeni, T.; Erdfelder, C.; et al. Preemptive treatment of early donor-specific antibodies with IgA- and IgM-enriched intravenous human immunoglobulins in lung transplantation. Am. J. Transplant. 2018, 18, 2295–2304. [Google Scholar] [CrossRef]
- Milross, L.; Hachem, R.; Levine, D.; Glanville, A.R. Lung autoantibodies: Ready for prime time? J. Heart Lung Transplant. 2018, 37, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Sacreas, A.; Taupin, J.-L.; Emonds, M.-P.; Daniëls, L.; Van Raemdonck, D.E.; Vos, R.; Verleden, G.M.; Vanaudenaerde, B.M.; Roux, A.; Verleden, S.E. Intragraft donor-specific anti-HLA antibodies in phenotypes of chronic lung allograft dysfunction. Eur. Respir. J. 2019, 54, 1900847. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.R. Lung allograft rejection: Diagnosis and management. Curr. Opin. Organ Transplant. 2009, 14, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.R. Donor-specific antibodies in lung transplantation. Curr. Opin. Organ Transplant. 2020, 25, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, T.L.; Wilkes, D.S. Role of autoimmunity in organ allograft rejection: A focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1129–L1139. [Google Scholar] [CrossRef][Green Version]
- Goers, T.A.; Ramachandran, S.; Aloush, A.; Trulock, E.; Patterson, G.A.; Mohanakumar, T. De novo production of K-alpha1 tubulin-specific antibodies: Role in chronic lung allograft rejection. J. Immunol. 2008, 180, 4487–4494. [Google Scholar] [CrossRef]
- Hachem, R.R.; Tiriveedhi, V.; Patterson, G.A.; Aloush, A.; Trulock, E.P.; Mohanakumar, T. Antibodies to K-α 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am. J. Transplant. 2012, 12, 2164–2171. [Google Scholar] [CrossRef]
- Lama, V.N.; Belperio, J.A.; Christie, J.D.; El-Chemaly, S.; Fishbein, M.C.; Gelman, A.E.; Hancock, W.W.; Keshavjee, S.; Kreisel, D.; Laubach, V.E.; et al. Models of lung transplant research: A consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight 2017, 2, e93121. [Google Scholar] [CrossRef]
- Atanasova, S.; Hirschburger, M.; Jonigk, D.; Obert, M.; Petri, K.; Evers, A.; Hecker, A.; Schmitz, J.; Kaufmann, A.; Wilhelm, J.; et al. A relevant experimental model for human bronchiolitis obliterans syndrome. J. Heart Lung Transplant. 2013, 32, 1131–1139. [Google Scholar] [CrossRef]
- Evers, A.; Atanasova, S.; Fuchs-Moll, G.; Petri, K.; Wilker, S.; Zakrzewicz, A.; Hirschburger, M.; Padberg, W.; Grau, V. Adaptive and innate immune responses in a rat orthotopic lung transplant model of chronic lung allograft dysfunction. Transplant. Int. 2015, 28, 95–107. [Google Scholar] [CrossRef]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D.J.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.D.; Li, N.; Andersen, C.B.; Arrossi, A.V.; Askar, M.; Berry, G.J.; DeNicola, M.M.; Neil, D.A.; Pavlisko, E.N.; Reed, E.F.; et al. Banff study of pathologic changes in lung allograft biopsy specimens with donor-specific antibodies. J. Heart Lung Transplant. 2016, 35, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Levine, D.J.; Zeevi, A.; Hachem, R.; Halloran, K.; Halloran, P.F.; Gibault, L.; Taupin, J.L.; Neil, D.A.H.; Loupy, A.; et al. Banff Lung Report: Current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR). Am. J. Transplant. 2019, 19, 21–31. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Jankowska-Gan, E.; Hegde, S.; Pestrak, M.A.; Agashe, V.V.; Park, A.C.; Brown, M.E.; Kernien, J.F.; Wilkes, D.S.; Kaufman, D.B.; et al. Th17 responses to collagen type V, kα1-Tubulin, and Vimentin are present early in human development and persist throughout life. Am. J. Transplant. 2017, 17, 944–956. [Google Scholar] [CrossRef]
- Subramanian, V.; Ramachandran, S.; Banan, B.; Bharat, A.; Wang, X.; Benshoff, N.; Kreisel, D.; Gelman, A.E.; Mohanakumar, T. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am. J. Transplant. 2014, 14, 2359–2366. [Google Scholar] [CrossRef]
- Cascalho, M.I.; Chen, B.J.; Kain, M.; Platt, J.L. The paradoxical functions of B cells and antibodies in transplantation. J. Immunol. 2013, 190, 875–879. [Google Scholar] [CrossRef]
- Schmidt, A.; Sucke, J.; Fuchs-Moll, G.; Freitag, P.; Hirschburger, M.; Kaufmann, A.; Garn, H.; Padberg, W.; Grau, V. Macrophages in experimental rat lung isografts and allografts: Infiltration and proliferation in situ. J. Leukoc. Biol. 2007, 81, 186–194. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichert, M.; Atanasova, S.; Petri, K.; Kampschulte, M.; Kojonazarov, B.; Fuchs-Moll, G.; Krombach, G.A.; Padberg, W.; Grau, V. Activation of Humoral Immunity during the Pathogenesis of Experimental Chronic Lung Allograft Dysfunction. Int. J. Mol. Sci. 2022, 23, 8111. https://doi.org/10.3390/ijms23158111
Reichert M, Atanasova S, Petri K, Kampschulte M, Kojonazarov B, Fuchs-Moll G, Krombach GA, Padberg W, Grau V. Activation of Humoral Immunity during the Pathogenesis of Experimental Chronic Lung Allograft Dysfunction. International Journal of Molecular Sciences. 2022; 23(15):8111. https://doi.org/10.3390/ijms23158111
Chicago/Turabian StyleReichert, Martin, Srebrena Atanasova, Kathrin Petri, Marian Kampschulte, Baktybek Kojonazarov, Gabriele Fuchs-Moll, Gabriele A. Krombach, Winfried Padberg, and Veronika Grau. 2022. "Activation of Humoral Immunity during the Pathogenesis of Experimental Chronic Lung Allograft Dysfunction" International Journal of Molecular Sciences 23, no. 15: 8111. https://doi.org/10.3390/ijms23158111
APA StyleReichert, M., Atanasova, S., Petri, K., Kampschulte, M., Kojonazarov, B., Fuchs-Moll, G., Krombach, G. A., Padberg, W., & Grau, V. (2022). Activation of Humoral Immunity during the Pathogenesis of Experimental Chronic Lung Allograft Dysfunction. International Journal of Molecular Sciences, 23(15), 8111. https://doi.org/10.3390/ijms23158111






