Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs
Abstract
:1. Introduction
2. Results
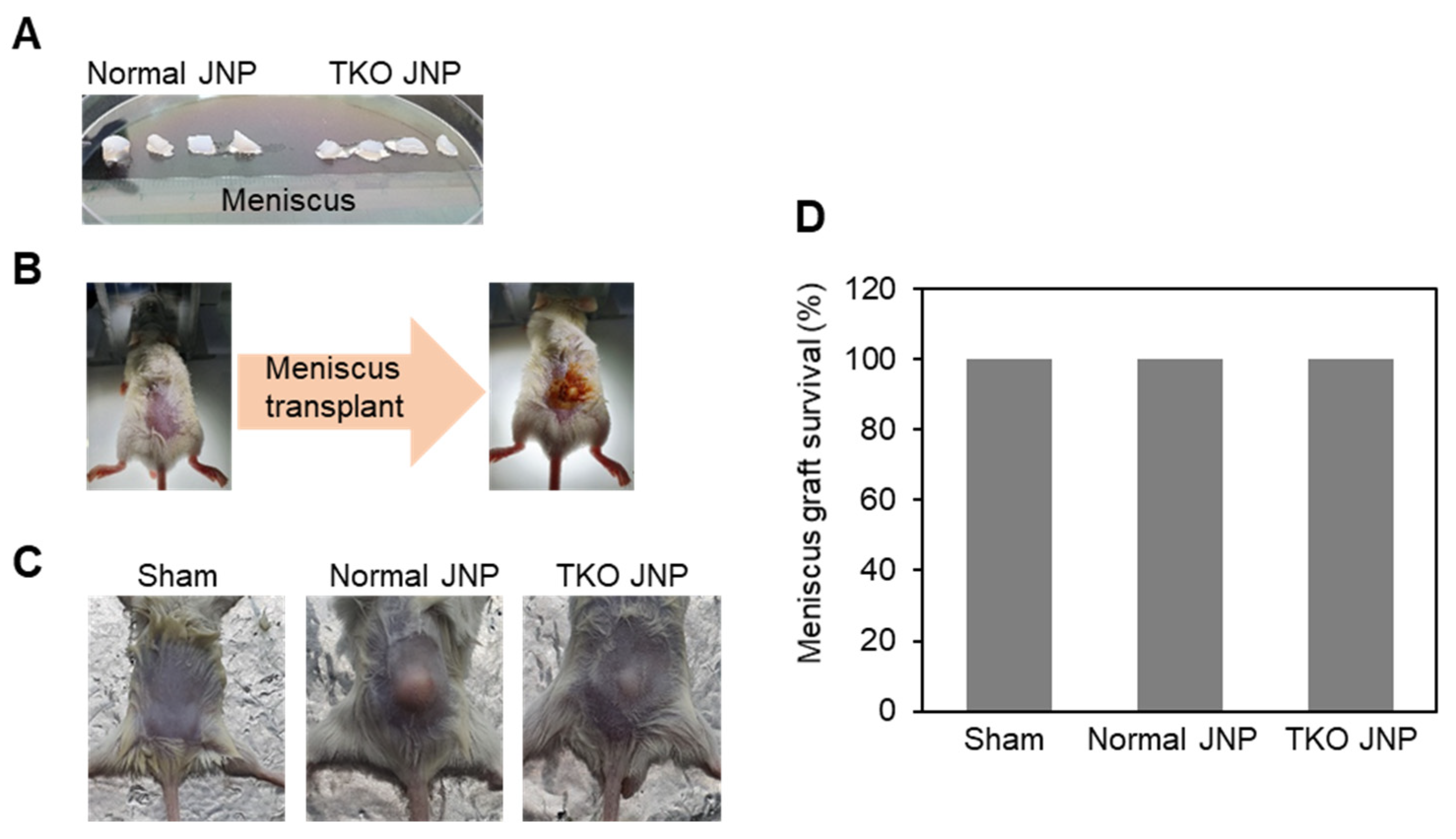
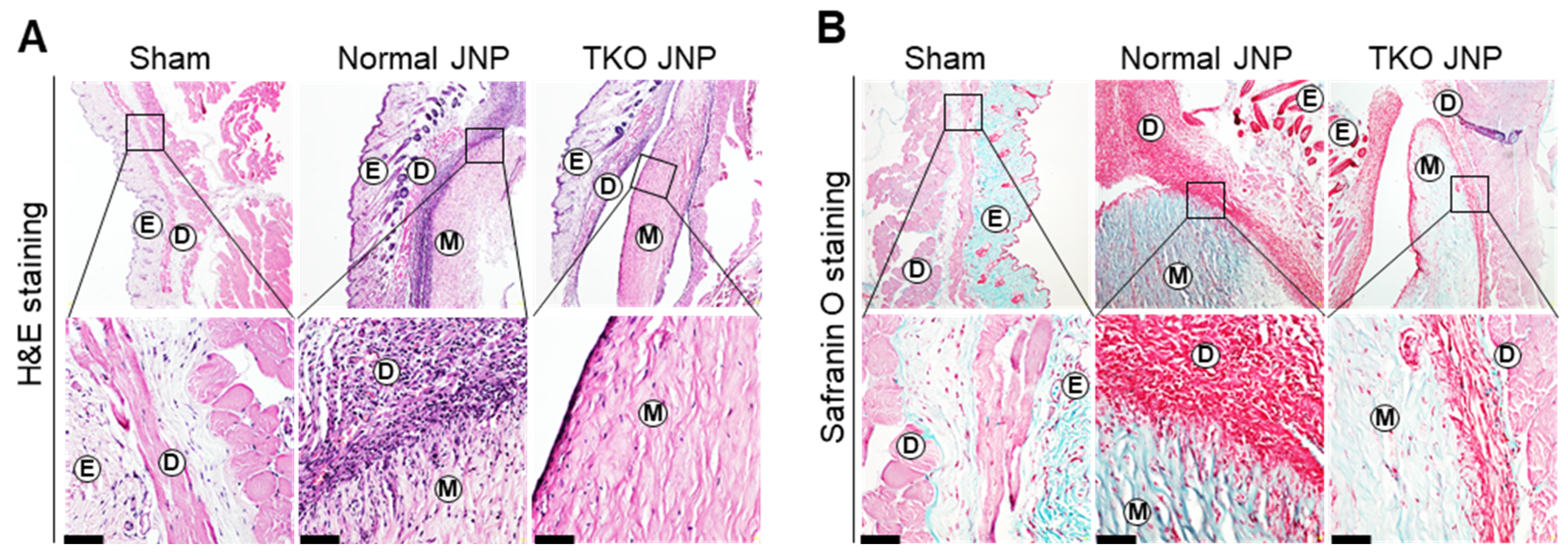
2.1. Histological Analysis and Meniscus Transplant Procedures
2.2. Analysis of Mast Cell Distribution across the Transplant Border
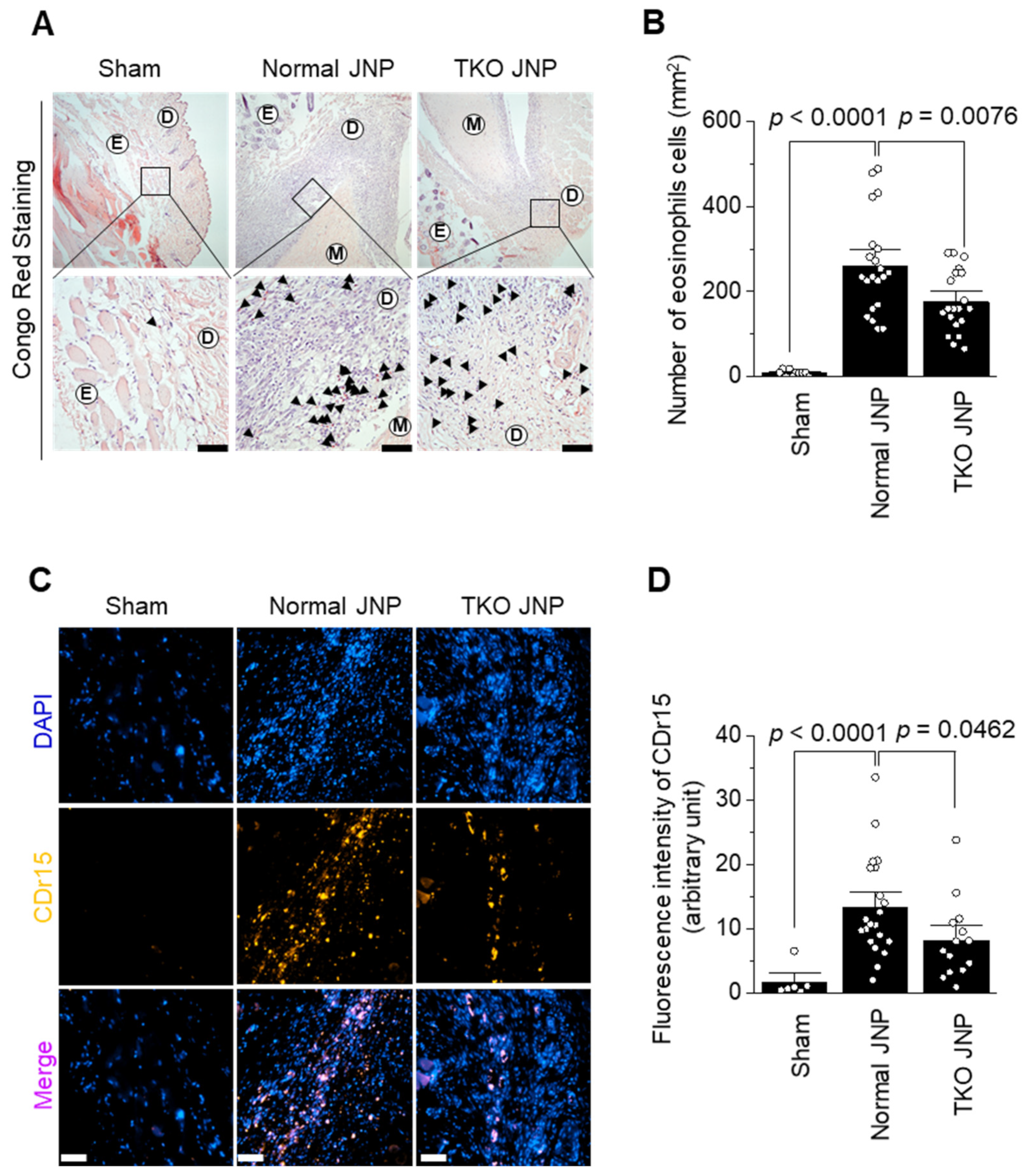
2.3. Analysis of Eosinophil and Neutrophil Distribution across the Transplant Border
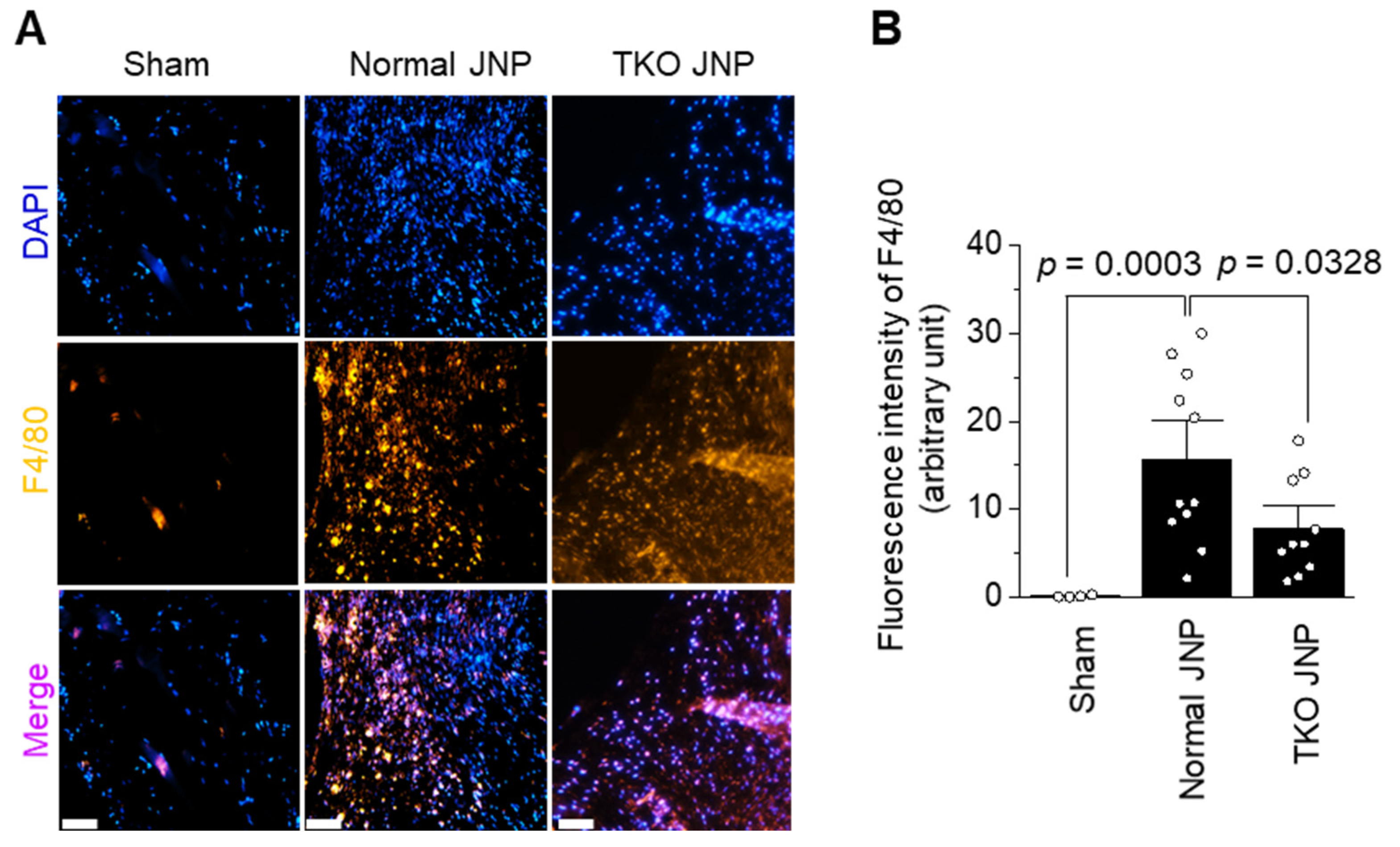
2.4. Analysis of Macrophage Distribution across the Transplant Border
3. Discussion
4. Materials and Methods
4.1. Animals and Materials
4.2. Meniscus Xenotransplantation
4.3. Histologic Analysis
4.4. Immunofluorescence Microscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Di Giancamillo, A.; Scotti, C.; Peretti, G.M.; Martin, I. Animal models for meniscus repair and regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Twomey-Kozak, J.; Jayasuriya, C.T. Meniscus Repair and Regeneration: A Systematic Review from a Basic and Translational Science Perspective. Clin. Sports Med. 2020, 39, 125–163. [Google Scholar] [CrossRef] [PubMed]
- Rongen, J.J.; Hannink, G.; van Tienen, T.G.; van Luijk, J.; Hooijmans, C.R. The protective effect of meniscus allograft transplantation on articular cartilage: A systematic review of animal studies. Osteoarthr. Cartil. 2015, 23, 1242–1253. [Google Scholar] [CrossRef]
- Rodeo, S.A. meniscal allografts-where do we stand? Am. Orthop. Soc. Sports Med. 2001, 29, 246–261. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Gollackner, B.; Sachs, D.H. Will the pig solve the transplantation blacking? Annu. Rev. Med. 2002, 53, 133–147. [Google Scholar] [CrossRef]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef]
- Ezzelarab, M.; Ayares, D.; Cooper, D.K. Carbohydrates in xenotransplantation. Immunol. Cell Biol. 2005, 83, 396–404. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Z.Z.; Zhao, F.; Wang, S.J.; Qi, Y.S.; Zhao, L.H.; Zhang, J.Y.; Yu, J.K. The Radiated Deep-frozen Xenogenic Meniscal Tissue Regenerated the Total Meniscus with Chondroprotection. Sci. Rep. 2018, 8, 9041. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Chen, Y.; Wan, X.; Zhao, C.; Qiu, P.; Lin, X.; Zhang, J.; Huang, Y. Preparation and Characterization of an Optimized Meniscal Extracellular Matrix Scaffold for Meniscus Transplantation. Front. Bioeng. Biotechnol. 2020, 8, 779. [Google Scholar] [CrossRef]
- Shim, J.; Ko, N.; Kim, H.J.; Lee, Y.; Lee, J.W.; Jin, D.I.; Kim, H.; Choi, K. Human immune reactivity of GGTA1/CMAH/A3GALT2 triple knockout Yucatan miniature pigs. Transgenic Res. 2021, 30, 619–634. [Google Scholar] [CrossRef]
- Hein, R.; Sake, H.J.; Pokoyski, C.; Hundrieser, J.; Brinkmann, A.; Baars, W.; Nowak-Imialek, M.; Lucas-Hahn, A.; Figueiredo, C.; Schuberth, H.J.; et al. Triple (GGTA1, CMAH, B2M) modified pigs expressing an SLA class Ilow phenotype-Effects on immune status and susceptibility to human immune responses. Am. J. Transplant. 2020, 20, 988–998. [Google Scholar] [CrossRef]
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of alpha-Gal epitope, anti-Gal antibody, alpha1,3 galactosyltransferase and its clinical exploitation (Review). Int. J. Mol. Med. 2016, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am. J. Phys. Anthr. 2001, 116 (Suppl. 33), 54–69. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwase, H.; Patel, D.; Jagdale, A.; Ayares, D.; Anderson, D.; Eckhoff, D.E.; Cooper, D.K.C.; Hara, H. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci. Rep. 2020, 10, 9771. [Google Scholar] [CrossRef]
- Byrne, G.; Ahmad-Villiers, S.; Du, Z.; McGregor, C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 2018, 25, e12394. [Google Scholar] [CrossRef]
- Hill, M.A.; Kwon, J.H.; Gerry, B.; Hardy, W.A.; Walkowiak, O.A.; Kavarana, M.N.; Nadig, S.N.; Rajab, T.K. Immune Privilege of Heart Valves. Front. Immunol. 2021, 12, 731361. [Google Scholar] [CrossRef]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Güell, M.; Niu, D.; George, H.; Lesha, E.; Grishin, D.; Aach, J.; Shrock, E.; Xu, W.; Poci, J.; et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015, 350, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Cooper, D.K.C.; Tector, A.J. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int. J. Surg. 2015, 23, 199–204. [Google Scholar] [CrossRef]
- Minai-Fleminger, Y.; Levi-Schaffer, F. Mast cells and eosinophils: The two key effector cells in allergic inflammation. Inflamm. Res. 2009, 58, 631–638. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Maeda, A.; Kogata, S.; Toyama, C.; Lo, P.C.; Okamatsu, C.; Yamamoto, R.; Masahata, K.; Kamiyama, M.; Eguchi, H.; Watanabe, M.; et al. The Innate Cellular Immune Response in Xenotransplantation. Front. Immunol. 2022, 13, 858604. [Google Scholar] [CrossRef]
- Sugaya, M. Macrophages and fibroblasts underpin skin immune responses. Explor. Immunol. 2021, 1, 226–242. [Google Scholar] [CrossRef]
- Stapleton, T.W.; Ingram, J.; Fisher, J.; Ingham, E. Investigation of the regenerative capacity of an acellular porcine medial meniscus for tissue engineering applications. Tissue Eng. Part A 2011, 17, 231–242. [Google Scholar] [CrossRef]
- Ochi, M.; Ikuta, Y.; Ishida, O.; Akiyama, M. Cellular and humoral immune responses after fresh meniscal allogragfts in mice. Arch. Orthop. Trauma Surg. 1993, 112, 163–166. [Google Scholar] [CrossRef]
- Wang, R.G.; Ruan, M.; Zhang, R.J.; Chen, L.; Li, X.X.; Fang, B.; Li, C.; Ren, X.Y.; Liu, J.Y.; Xiong, Q.; et al. Antigenicity of tissues and organs from GGTA1/CMAH/beta4GalNT2 triple gene knockout pigs. J. Biomed. Res. 2018, 33, 235–243. [Google Scholar] [CrossRef]
- Cui, Y.; Yamamoto, T.; Raza, S.S.; Morsi, M.; Nguyen, H.Q.; Ayares, D.; Cooper, D.K.C.; Hara, H. Evidence for GTKO/β4GalNT2KO Pigs as the Preferred Organ-source for Old World Nonhuman Primates as a Preclinical Model of Xenotransplantation. Transplant. Direct 2020, 6, e590. [Google Scholar] [CrossRef]
- Li, Q.; Iwase, H.; Yamamoto, T.; Nguyen, H.Q.; Ayares, D.; Wang, Y.; Cooper, D.K.C.; Hara, H. Anti-pig IgE and IgA Antibodies in Naive Primates and Nonhuman Primates with Pig Xenografts. Transplantation 2021, 105, 318–327. [Google Scholar] [CrossRef]
- Cooper, D.K.C. The 2021 IXA Keith Reemtsma Lecture: Moving xenotransplantation to the clinic. Xenotransplantation 2022, 29, e12723. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Min, Y.; Park, C.; Kim, D.; Heo, Y.; Kim, M.; Son, E.; Ghosh, M.; Son, Y.-O.; Hur, C.-G. Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs. Int. J. Mol. Sci. 2022, 23, 10416. https://doi.org/10.3390/ijms231810416
Yoon S, Min Y, Park C, Kim D, Heo Y, Kim M, Son E, Ghosh M, Son Y-O, Hur C-G. Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs. International Journal of Molecular Sciences. 2022; 23(18):10416. https://doi.org/10.3390/ijms231810416
Chicago/Turabian StyleYoon, Seungwon, Yunhui Min, Chungyu Park, Dahye Kim, Yunji Heo, Mangeun Kim, Eugene Son, Mrinmoy Ghosh, Young-Ok Son, and Chang-Gi Hur. 2022. "Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs" International Journal of Molecular Sciences 23, no. 18: 10416. https://doi.org/10.3390/ijms231810416
APA StyleYoon, S., Min, Y., Park, C., Kim, D., Heo, Y., Kim, M., Son, E., Ghosh, M., Son, Y.-O., & Hur, C.-G. (2022). Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs. International Journal of Molecular Sciences, 23(18), 10416. https://doi.org/10.3390/ijms231810416





