Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates
Abstract
:1. Introduction
2. Results
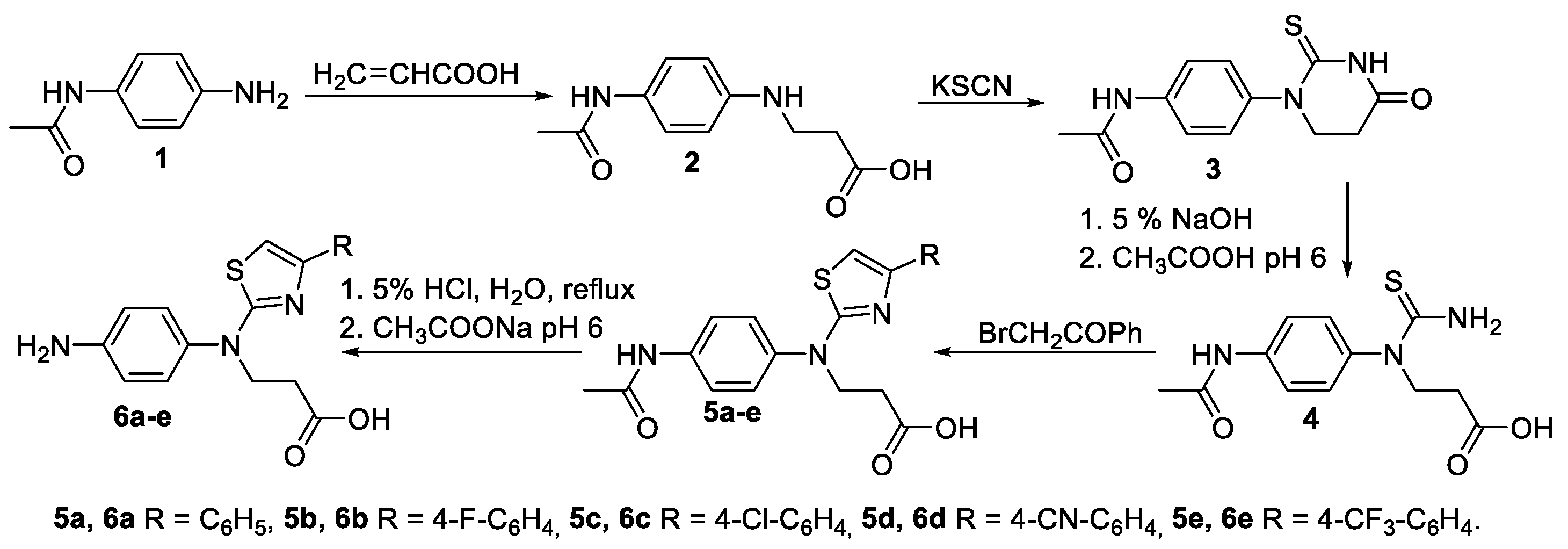
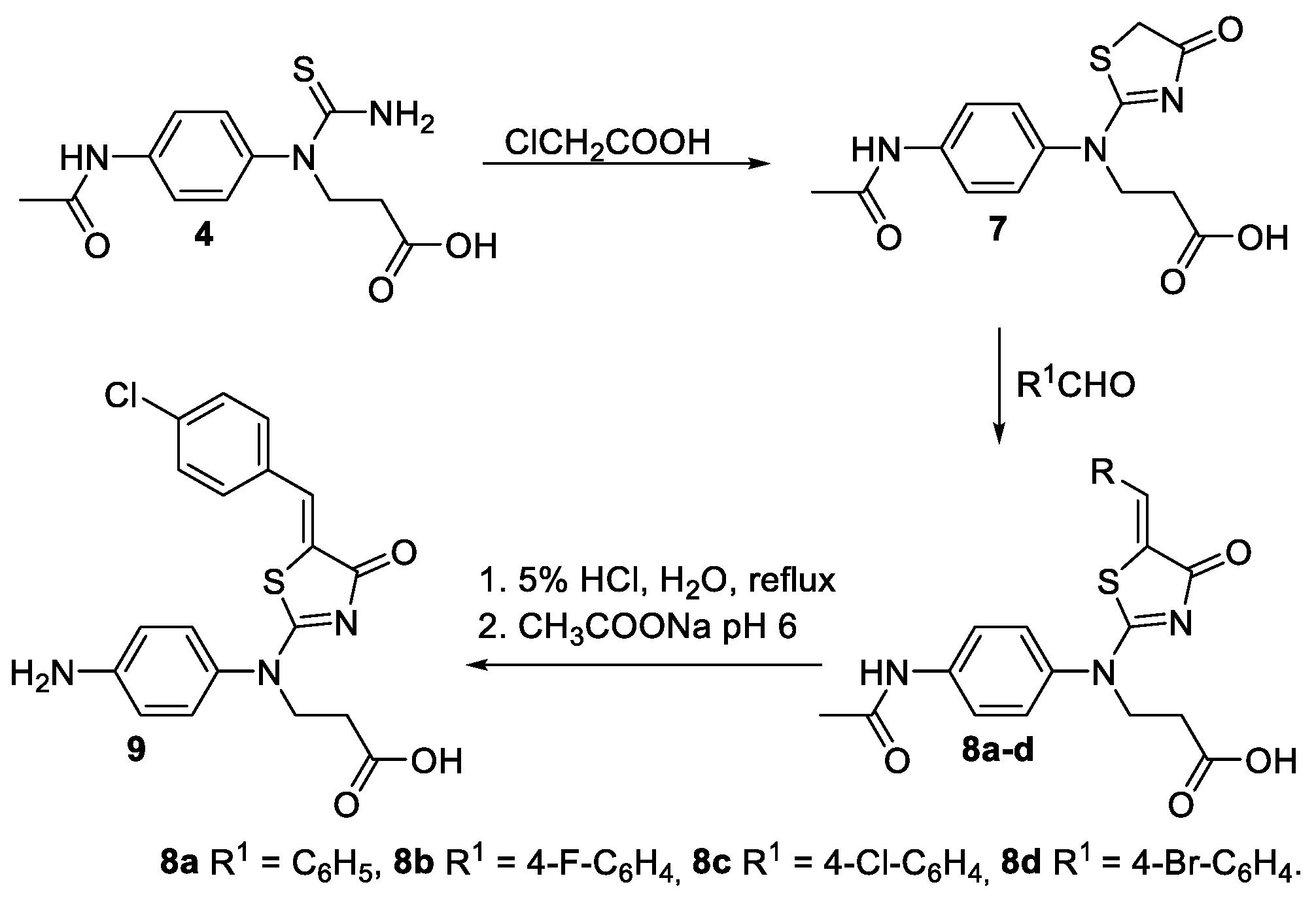
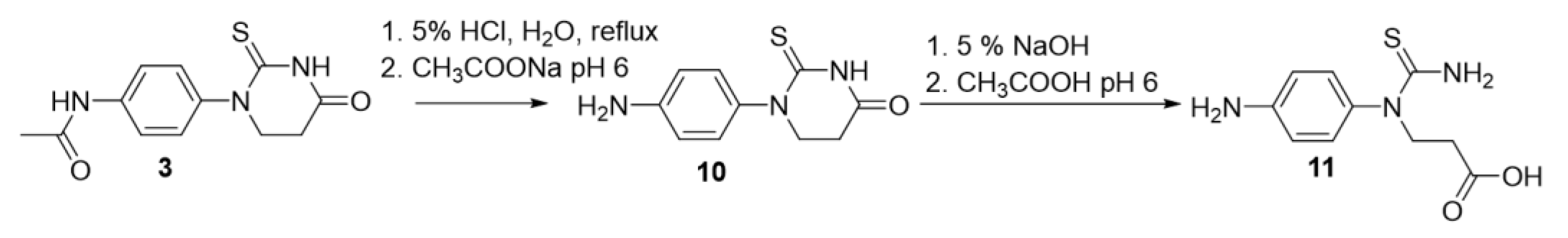
2.1. Synthesis of Novel Aminothiazoles
2.2. Study of Cytotoxic Activity of Compounds 3–11
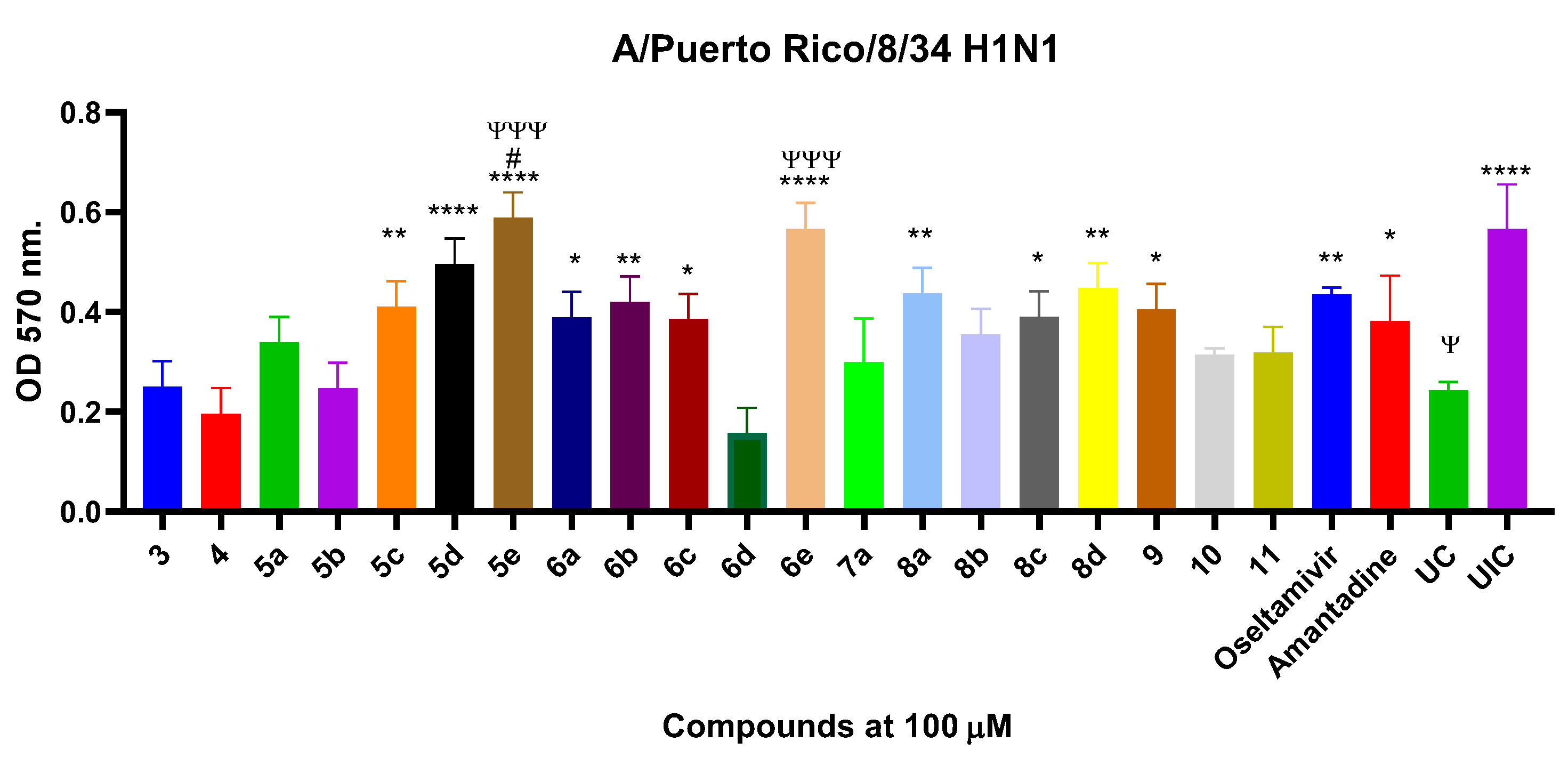
2.3. Study of Antiviral Activity of Compounds 3–11
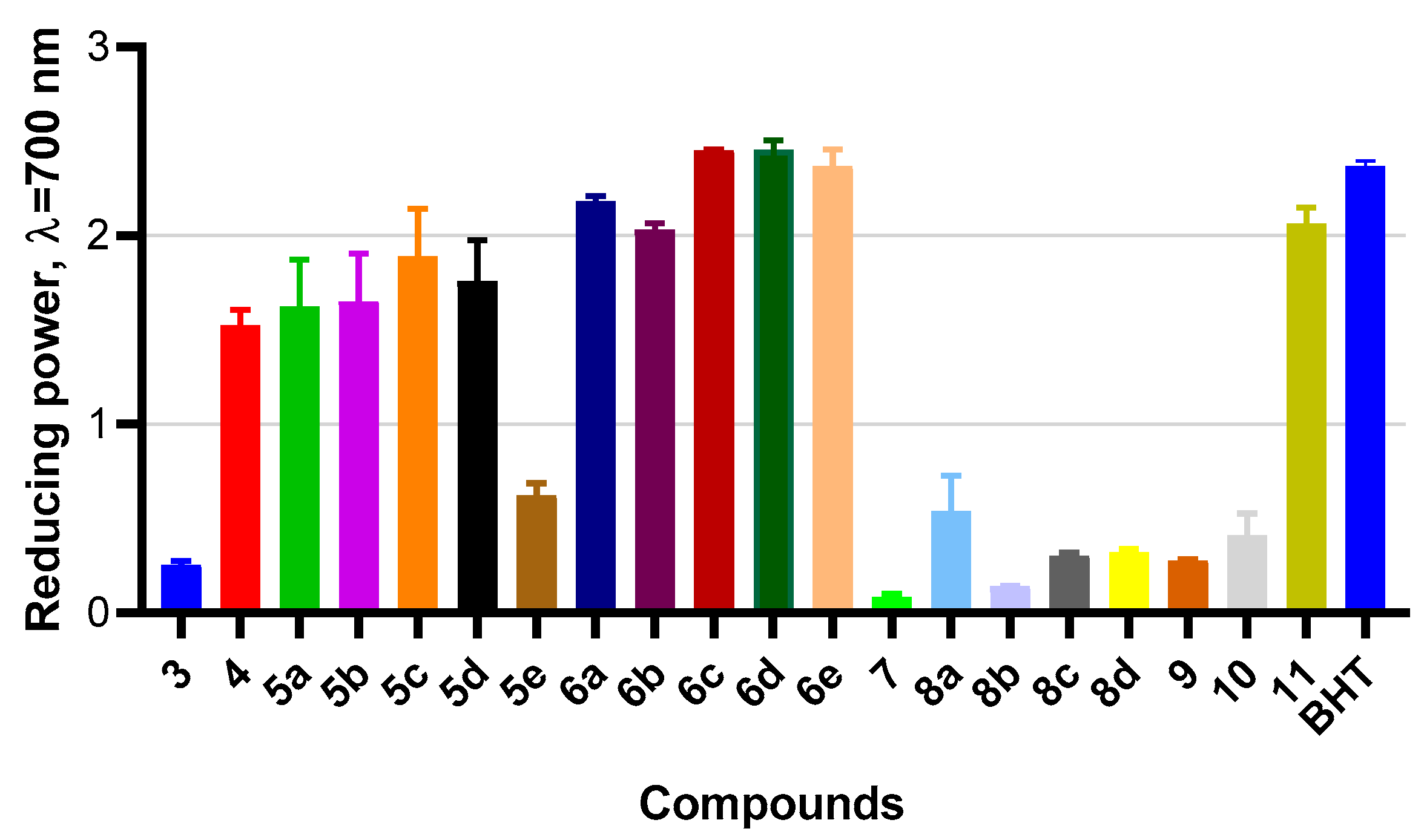
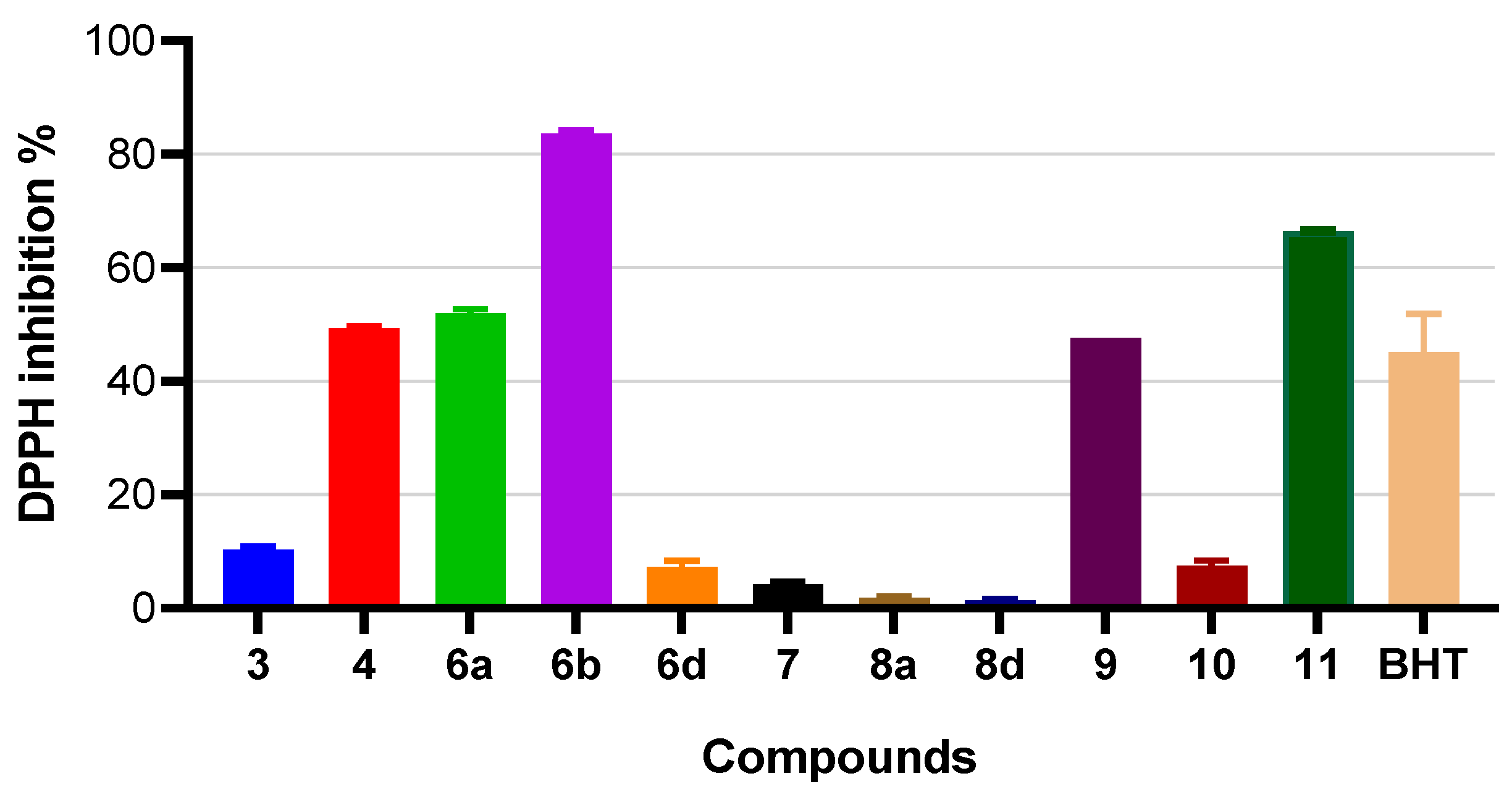
2.4. Measurement of Antioxidant Activities
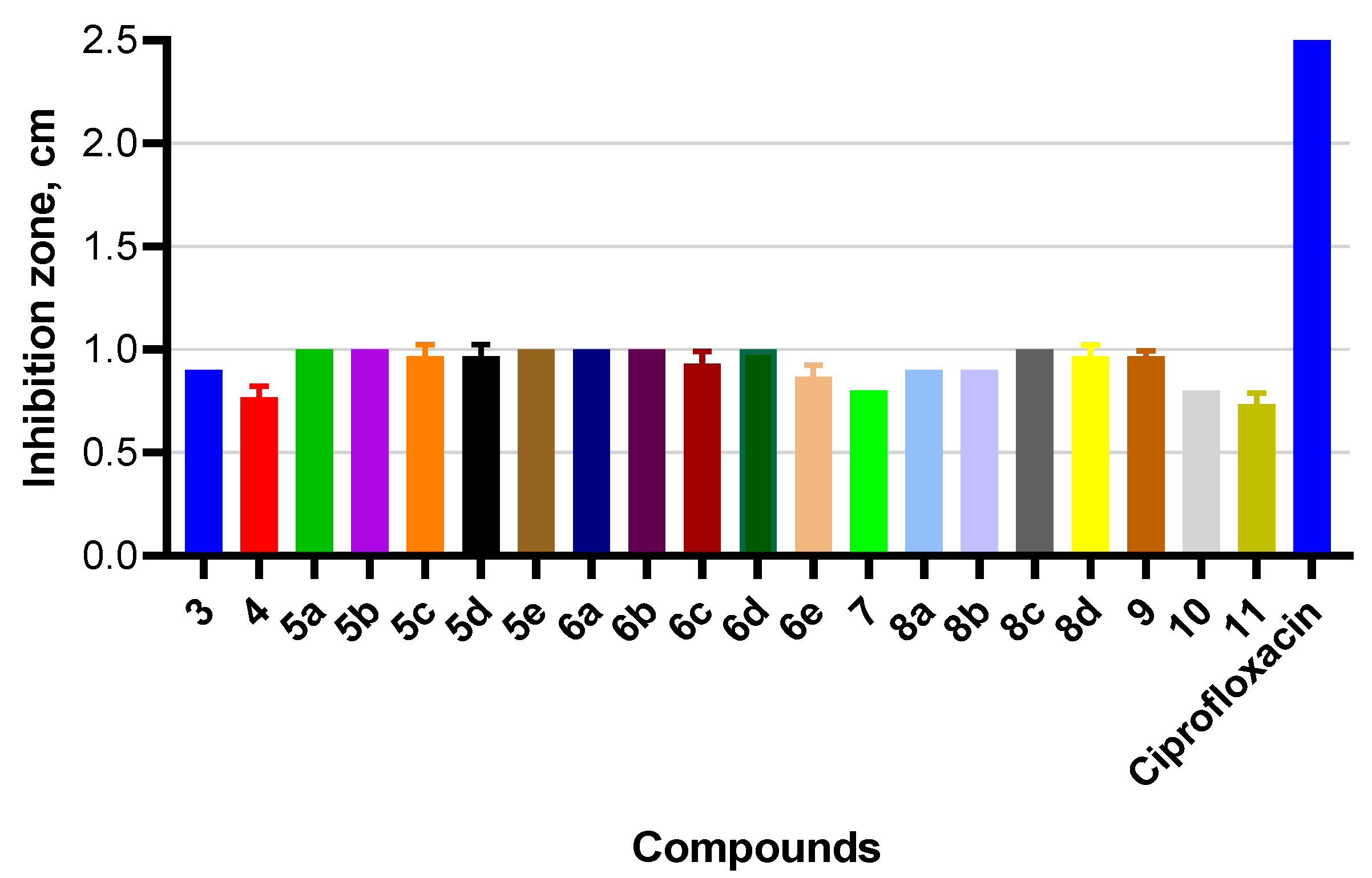
2.5. Evaluation of Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Synthesis of Novel Compounds
4.2. Cell lines and Culture Conditions
4.3. Cytotoxicity Assay
4.4. Viral Infection Assay
4.5. Measurement of Antioxidant Activities
4.5.1. Ferric ion (Fe3+) Reducing Antioxidant Power (Fe3 – Fe2+ Transformation Assay)
4.5.2. Ferric Reducing Antioxidant Power Assay (FRAP)
4.5.3. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
4.6. Evaluation of Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galstyan, A.S.; Martiryan, A.I.; Grigoryan, K.R.; Ghazaryan, A.G.; Samvelyan, M.A.; Ghochikyan, T.V.; Nenajdenko, V.G. Synthesis of Carvone-Derived 1,2,3-Triazoles Study of Their Antioxidant Properties and Interaction with Bovine Serum Albumin. Molecules 2018, 23, 2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakan, H. Preparation, structure elucidation, and antioxidant activity of new bis(thiosemicarbazone) derivatives. Turk. J. Chem. 2020, 44, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Carradori, S.; Petzer, A.; Guglielmi, P.; D’Ascenzio, M.; Chimenti, P.; Bagetta, D.; Alcaro, S.; Zengin, G.; Petzer, J.P.; et al. 4-(3-Nitrophenyl)thiazol-2-ylhydrazone derivatives as antioxidants and selective hMAO-B inhibitors: Synthesis, biological activity and computational analysis. J. Enzym. Inhib. Med. Chem. 2019, 34, 597–612. [Google Scholar] [CrossRef] [Green Version]
- Rogolino, D.; Gatti, A.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Buschini, A.; Montalbano, S.; Feretti, D.; et al. Thiosemicarbazone scaffold for the design of antifungal and antiaflatoxigenic agents: Evaluation of ligands and related copper complexes. Sci. Rep. 2017, 7, 11214. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.M.; Parrino, B.; Carbone, D.; Schillaci, D.; Giovannetti, E.; Cirrincione, G.; Diana, P. Thiazoles, Their Benzofused Systems, and Thiazolidinone Derivatives: Versatile and Promising Tools to Combat Antibiotic Resistance. J. Med. Chem. 2020, 63, 7923–7956. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.; Reddy, P.V.N.; Monteleone, D.; Mayhoub, A.S.; Cushman, M.; Hammac, G.K.; Seleem, M.N. Antibacterial Characterization of Novel Synthetic Thiazole Compounds against Methicillin-Resistant Staphylococcus pseudintermedius. PLoS ONE 2015, 10, e0130385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroni, C.; Pepe, A.; Kim, Y.S.; Lee, S.; Guerrini, A.; Parenti, M.D.; Tesei, A.; Zamagni, A.; Cortesi, M.; Zaffaroni, N.; et al. 1,4-Substituted Triazoles as Nonsteroidal Anti-Androgens for Prostate Cancer Treatment. J. Med. Chem. 2017, 60, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.M.; Gonzaga, D.T.G.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R.S. Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules 2020, 10, 1197. [Google Scholar] [CrossRef]
- Geronikaki, A.; Hadjipavlou-Litina, D.; Zablotskaya, A.; Segal, I. Organosilicon-containing thiazole derivatives as potential lipoxygenase inhibitors and anti inflammatory agents. Bioinorgan. Chem. Appl. 2007, 2007, 092145. [Google Scholar] [CrossRef]
- Almasirad, A.; Mousavi, Z.; Tajik, M.; Assarzadeh, M.J.; Shafiee, A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. DARU J. Pharm. Sci. 2014, 22, 22. [Google Scholar] [CrossRef] [Green Version]
- Huuskonen, M.T.; Tuo, Q.-Z.; Loppi, S.; Dhungana, H.; Korhonen, P.; McInnes, L.E.; Donnelly, P.S.; Grubman, A.; Wojciechowski, S.; Lejavova, K.; et al. The Copper bis(thiosemicarbazone) Complex CuII(atsm) Is Protective Against Cerebral Ischemia Through Modulation of the Inflammatory Milieu. Neurotherapeutics 2017, 14, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, L.; Desai, A.; Shampur, M.N.; Perumal, Y.; Sriram, D.; Vasanthapuram, R. N-methylisatin-beta-thiosemicarbazone derivative (SCH 16) is an inhibitor of Japanese encephalitis virus infection in vitro and in vivo. Virol. J. 2008, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadden, K.; Fletcher, P.S.; Rossi, F.; Kantharaju Umashankara, M.; Pirrone, V.; Rajagopal, S.; Gopi, H.N.; Krebs, F.C.; Martín-García, J.; Shattock, R.J.; et al. Antiviral Breadth and Combination Potential of Peptide Triazole HIV-1 Entry Inhibitors. Antimicrob. Agents Chemother. 2011, 56, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulou, M.V.; Trunz, B.B.; Bloomer, W.D.; McKenzie, C.; Wilkinson, S.R.; Prasittichai, C.; Brun, R.; Kaiser, M.; Torreele, E. Novel 3-Nitro-1H-1,2,4-triazole-Based Aliphatic and Aromatic Amines as Anti-Chagasic Agents. J. Med. Chem. 2011, 54, 8214–8223. [Google Scholar] [CrossRef] [Green Version]
- Borisenko, V.; Koll, A.; Kolmakov, E.; Rjasnyi, A. Hydrogen bonds of 2-aminothiazoles in intermolecular complexes (1:1 and 1:2) with proton acceptors in solutions. J. Mol. Struct. 2006, 783, 101–115. [Google Scholar] [CrossRef]
- Maj, J.; Rogóż, Z.; Skuza, G.; Kołodziejczyk, K. Antidepressant effects of pramipexole, a novel dopamine receptor agonist. J. Neural Transm. 1997, 104, 525–533. [Google Scholar] [CrossRef]
- Milne, G.W.A. Ashgate Handbook of Autineoplastic Agents, 1st ed.; Routledge: London, UK, 2000; p. 190. [Google Scholar]
- De Souza, M.V.N.; De Almeida, M.V. Drugs: Anti-HIV: Past, present and future directions. Quimica Nova 2003, 26, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Chhabria, M.T.; Patel, S.; Modi, P.; Brahmkshatriya, P.S. Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [CrossRef]
- Knadler, M.P.; Bergstrom, R.F.; Callaghan, J.T.; Rubin, A. Nizatidine, an H2-blocker. Its metabolism and disposition in man. Drug Metab. Dispos. Biol. Fate Chem. 1986, 14, 175–182. [Google Scholar]
- Dawood, K.M.; Raslan, M.A.; Abbas, A.A.; Mohamed, B.E.; Abdellattif, M.H.; Nafie, M.S.; Hassan, M.K. Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis with Molecular Docking Approaches. Front. Chem. 2021, 9, 694870. [Google Scholar] [CrossRef]
- Lednicer, D.; Mitscher, L.A.; Georg, G.I. The Organic Chemistry of Drug Synthesis; Wiley: Hoboken, NJ, USA, 1990; p. 304. [Google Scholar]
- Hassan, A.A.; Mohamed, N.K.; Aly, A.A.; Tawfeek, H.N.; Bräse, S.; Nieger, M. Synthesis and structure confirmation of 2,4-disubstituted thiazole and 2,3,4-trisubstituted thiazole as thiazolium bromide salts. Mon. Für Chem. Chem. Mon. 2020, 151, 1143–1152. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Beuchet, P.; Varache-Lembège, M.; Neveu, A.; Léger, J.-M.; Vercauteren, J.; Larrouture, S.; Deffieux, G.; Nuhrich, A. New 2-sulfonamidothiazoles substituted at C-4: Synthesis of polyoxygenated aryl derivatives and in vitro evaluation of antifungal activity. Eur. J. Med. Chem. 1999, 34, 773–779. [Google Scholar] [CrossRef]
- Takakura, H. Molecular Design of D-Luciferin-Based Bioluminescence and 1,2-Dioxetane-Based Chemiluminescence Substrates for Altered Output Wavelength and Detecting Various Molecules. Molecules 2021, 26, 1618. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Patel, S.; Sharma, A.; Das, A.; Meshram, P.; Shard, A. A perspective on environmentally benign protocols of thiazole synthesis. Chem. Heterocycl. Compd. 2020, 56, 455–463. [Google Scholar] [CrossRef]
- Quijano-Rubio, A.; Yeh, H.-W.; Park, J.; Lee, H.; Langan, R.A.; Boyken, S.E.; Lajoie, M.J.; Cao, L.; Chow, C.M.; Miranda, M.C.; et al. De novo design of modular and tunable protein biosensors. Nature 2021, 591, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef]
- Jain, S.; Pattnaik, S.; Pathak, K.; Kumar, S.; Pathak, D.; Jain, S.; Vaidya, A. Anticancer Potential of Thiazole Derivatives: A Retrospective Review. Mini-Rev. Med. Chem. 2018, 18, 640–655. [Google Scholar] [CrossRef]
- Ohsumi, K.; Hatanaka, T.; Fujita, K.; Nakagawa, R.; Fukuda, Y.; Nihei, Y.; Suga, Y.; Morinaga, Y.; Akiyama, Y.; Tsuji, T. Syntheses and antitumor activity of cis-restricted combretastatins: 5-Membered heterocyclic analogues. Bioorganic Med. Chem. Lett. 1998, 8, 3153–3158. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Q.; Xu, J.; Wu, Y.; Wang, Y.; Zuo, D.; Guan, Q.; Bao, K.; Wang, J.; Wu, Y.; et al. Synthesis and bioevaluation of N,4-diaryl-1,3-thiazole-2-amines as tubulin inhibitors with potent antiproliferative activity. PLoS ONE 2017, 12, e0174006. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Suh, H.-J.; Chung, M.-S.; Cho, Y.-H.; Kim, J.-W.; Kim, D.-H.; Han, K.-W.; Kim, C.-J. Estimated daily intakes of butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) andtert-butyl hydroquinone (TBHQ) antioxidants in Korea. Food Addit. Contam. 2005, 22, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Giacomini, D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef]
- Yamashita, N.; Taga, C.; Ozawa, M.; Kanno, Y.; Sanada, N.; Kizu, R. Camalexin, an indole phytoalexin, inhibits cell proliferation, migration, and mammosphere formation in breast cancer cells via the aryl hydrocarbon receptor. J. Nat. Med. 2022, 76, 110–118. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing AG: Manhattan, NY, USA, 2018; p. 269. [Google Scholar]
- Rutkauskas, K.; Jakienė, E.; Beresnevičius, Z.J. Products of reaction of p-phenylenediamine with unsaturated carboxylic acids and their biological activity. Cheminė Technol. 2003, 2, 68–73. [Google Scholar]
- Xue, J.; Chambers, B.S.; Hensley, S.E.; López, C.B. Propagation and Characterization of Influenza Virus Stocks That Lack High Levels of Defective Viral Genomes and Hemagglutinin Mutations. Front. Microbiol. 2016, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Crameri, M.; Lanz, C.; Yángüez, E. Propagation and Titration of Influenza Viruses. Methods Mol. Biol. 2018, 1836, 59–88. [Google Scholar] [CrossRef]
- Koksal, E.; Gulcin, I. Antioxidant Activity of Cauliflower (Brassica oleracea L.). Turk. J. Agric. For. 2008, 32, 65–78. [Google Scholar]
- Bursal, E.; Gülçin, I. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1996, 239, 15–27. [Google Scholar] [CrossRef]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. On the in vitro antioxidative properties of melatonin. J. Pineal Res. 2002, 33, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Oktay, M.; Küfrevioğlu, O.I.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Jaishree, V.; Ramdas, N.; Sachin, J.; Ramesh, B. In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc. 2012, 16, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Sztanke, M.; Sztanke, K. Biologically important hydrazide-containing fused azaisocytosines as antioxidant agents. Redox Rep. 2017, 22, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Salar, U.; Khan, K.M.; Chigurupati, S.; Taha, M.; Wadood, A.; Vijayabalan, S.; Ghufran, M.; Perveen, S. New Hybrid Hydrazinyl Thiazole Substituted Chromones: As Potential α-Amylase Inhibitors and Radical (DPPH & ABTS) Scavengers. Sci. Rep. 2017, 7, 16980. [Google Scholar] [CrossRef]
- Zaki, Y.H.; Al-Gendey, M.S.; Abdelhamid, A.O. A facile synthesis, and antimicrobial and anticancer activities of some pyridines, thioamides, thiazole, urea, quinazoline, β-naphthyl carbamate, and pyrano[2,3-d]thiazole derivatives. Chem. Central J. 2018, 12, 70. [Google Scholar] [CrossRef] [Green Version]



| Entry | Solvent | Temperature, °C | Reaction Time, h | Yield, % |
|---|---|---|---|---|
| A | Water | Reflux | 5 | 7 |
| B | Toluene | 24 | 22 | |
| C | Dioxane | 48 | ||
| D | 2-propanol | 55 | ||
| E | THF | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minickaitė, R.; Grybaitė, B.; Vaickelionienė, R.; Kavaliauskas, P.; Petraitis, V.; Petraitienė, R.; Tumosienė, I.; Jonuškienė, I.; Mickevičius, V. Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates. Int. J. Mol. Sci. 2022, 23, 7688. https://doi.org/10.3390/ijms23147688
Minickaitė R, Grybaitė B, Vaickelionienė R, Kavaliauskas P, Petraitis V, Petraitienė R, Tumosienė I, Jonuškienė I, Mickevičius V. Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates. International Journal of Molecular Sciences. 2022; 23(14):7688. https://doi.org/10.3390/ijms23147688
Chicago/Turabian StyleMinickaitė, Rūta, Birutė Grybaitė, Rita Vaickelionienė, Povilas Kavaliauskas, Vidmantas Petraitis, Rūta Petraitienė, Ingrida Tumosienė, Ilona Jonuškienė, and Vytautas Mickevičius. 2022. "Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates" International Journal of Molecular Sciences 23, no. 14: 7688. https://doi.org/10.3390/ijms23147688
APA StyleMinickaitė, R., Grybaitė, B., Vaickelionienė, R., Kavaliauskas, P., Petraitis, V., Petraitienė, R., Tumosienė, I., Jonuškienė, I., & Mickevičius, V. (2022). Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates. International Journal of Molecular Sciences, 23(14), 7688. https://doi.org/10.3390/ijms23147688







