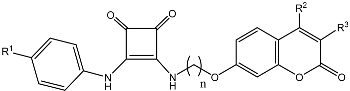
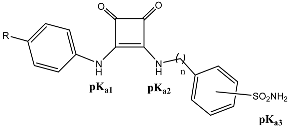
Abstract
(1) Background: carbonic anhydrases (CAs) are attractive targets for the development of new anticancer therapies; in particular, CAs IX and XII isoforms are overexpressed in numerous tumors. (2) Methods: following the tail approach, we have appended a hydrophobic aromatic tail to a pharmacophore responsible for the CA inhibition (aryl sulfonamide, coumarin). As a linker, we have used squaramides, featured with strong hydrogen bond acceptor and donor capacities. (3) Results: Starting from easily accessible dimethyl squarate, the title compounds were successfully obtained as crystalline solids, avoiding the use of chromatographic purifications. Interesting and valuable SARs could be obtained upon modification of the length of the hydrocarbon chain, position of the sulfonamido moiety, distance of the aryl sulfonamide scaffold to the squaramide, stereoelectronic effects on the aromatic ring, as well as the number and type of substituents on C-3 and C-4 positions of the coumarin. (4) Conclusions: For sulfonamides, the best profile was achieved for the m-substituted derivative 11 (Ki = 29.4, 9.15 nM, CA IX and XII, respectively), with improved selectivity compared to acetazolamide, a standard drug. Coumarin derivatives afforded an outstanding selectivity (Ki > 10,000 nM for CA I, II); the lead compound (16c) was a strong CA IX and XII inhibitor (Ki = 19.2, 7.23 nM, respectively). Docking simulations revealed the key ligand-enzyme interactions.
1. Introduction
Squaric acid (3,4-dihydroxycyclobut-3-ene-1,2-dione), also called quadratic acid due to its shape—close to a perfect square—is a four-membered ring system that exhibits some astonishing properties, like strong acidity, and strong hydrogen bonding; the latter feature is responsible for its high melting point temperature and low water solubility [1]. Squaric acid can be easily transformed into squarate esters upon reaction with alcohols; squarates can be further transformed into mixed squaramate (reaction with one equivalent of an amine), and also into symmetrical and non-symmetrical squaramides by condensation with one (2.0 mol. equiv.) or two amines (1.0 mol. equiv. each) [1]. The most remarkable physical property of squaramides is their capacity of participating in bidirectional hydrogen bonding interactions (if prepared from primary amines), as acceptors (carbonyl groups), and as donors (NH groups) [2]. Moreover, it has been found that the intrinsic aromatic character of the cyclobut-3-ene-1,2-dione system is directly correlated with the strength of the hydrogen bonding in squaramides [2].
Squaramides are currently gaining great attention and have found a myriad of applications [3] in diverse fields like: molecular sensors [4], including ion pair receptors [5,6], metal-organic frameworks (MOFs, due to their self-assembly properties) [7], organocatalysis [8], including Henry [9], Mannich [10], and aldol [11] reactions, asymmetric fluorinations [12], and cycloadditions/cascade reactions [13] to furnish carbo- and heterocycles, and also within medicinal chemistry [14]. Interestingly, squaramides are currently considered as vinylogous amides with increased conformational restriction and have been used in the bio-isosteric replacement of frequent connectors in drug design, like (thio)ureas, guanidines, and cyanoguanidines [15]. In this context, the squaramido motif has been incorporated, for instance, into RNA polymerase [16], histone deacetylase (HDAC) [17] and cholinesterase inhibitors [18], anti-chagasic [19] and anti-tuberculosis agents [20], fluorescent ligands for cell imaging [21], some of which also providing antiproliferative properties [22], photocages for photoactivated chemotherapy (PACT) [23], or into radiopharmaceuticals that conjugate chelators of radiometals and vectors with interest in radio-imaging and chemotherapy [24].
We herein envisioned the possibility of using squaramides as an interesting tether in the design of novel carbonic anhydrase (CA, EC 4.2.1.1) inhibitors, an area that has not been exploited so far. CAs are a superfamily of ubiquitous metalloenzymes in both, prokaryotic and eukaryotic organisms (in most of the cases, with Zn2+ as the prosthetic group) [25] that catalyze a simple, yet essential process, that is the reversible hydration of CO2 to furnish HCO3− plus a proton [26]. The spontaneous and non-catalyzed reaction is not fast enough to cover the physiological demand; high levels of CO2 have deleterious effects in organisms, and pH homeostasis is essential in numerous biochemical pathways and processes [27], like electrolyte secretion, lipogenesis, gluconeogenesis, or ureagenesis [28]. Remarkably, CA-mediated biocatalysis of this reaction affords a significant rate increase (kcat 104–106) [29], making CAs one of the fastest enzymes identified so far.
There are currently eight gene families of CAs, identified by Greek letters (α, β, γ, δ, ζ, η, θ, ι), and distributed among all kingdoms of life [29]. Mammals only encode for α-CAs, and in the case of humans, 15 isoforms can be found in different tissues and with different functions [30]: hCAs I, II, III, VII, VIII, X, XI, XIII can be found in cytosol; hCA IV is a glycosylphosphatidylinositol (GPI)-anchored protein; hCA VA and VB are located in the mitochondrial matrix; hCA VI is secreted in saliva and milk; hCA IX, XII and XIV are transmembrane enzymes. The only isoforms devoid of a known activity are hCAs VIII, X and XI, the so-called CARPs (CA-related proteins) [31].
Due to the countless implications of CAs in biological processes, they are currently a validated drug target, with some molecules already marketed [32]; in fact, the development of activators [33,34] and inhibitors [28,35,36] of CAs is a hot topic in the medicinal chemistry area. There are several inhibition mechanisms [37], among which metal chelation (sulfonamides and their isosteric sulfamates, dithiocarbamates, hydroxamates), or entry blockade through prior CA-mediated transformations of the inhibitor (coumarins) are the most widely exploited.
CA inhibitors can be used for the development of anti-infective agents (CAs are responsible for the survival and virulence of certain pathogens) [38], for the treatment of glaucoma [39], cerebral ischemia [40], rheumatoid arthritis [41], obesity [42], neuropathic pain [43], epilepsy [44], or cancer [45]; in this context, it is important to highlight that CA IX and XII are overexpressed in numerous tumors, where they control the microenvironment pH (acidification of the extracellular medium) [46] and certain metabolic processes connected to tumor growth and metastasis [47]. Recent evidence also correlates CA inhibition with neuroprotective effects in Alzheimer’s disease [48].
2. Results and Discussion
2.1. Drug Design, Synthesis and Characterization
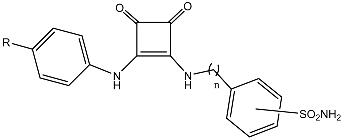
Encouraged by the remarkable properties of squaramides within the medicinal chemistry field, we decided to install it in a novel series of CA inhibitors and accomplish a comprehensive analysis of their bioactivities. Following the well-known tail approach [49], we envisioned the structures depicted in Figure 1. Aryl sulfonamides and coumarins were selected as the active pharmacophores for the inhibition of the metalloenzymes. Sulfonamides and isosteric sulfamates are reported to inhibit CAs by chelating the Zn2+ ion from the catalytic active site [50], whereas coumarins behave as suicide inhibitors [51]; esterase activity of CAs provokes a hydrolysis of the lactam skeleton of the coumarin to give a 2-hydroxy cinnamic acid derivative which occludes the entry to the enzyme active site [52].
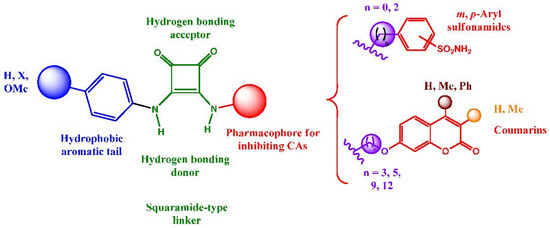
Figure 1.
Structural design of novel squaramide-based inhibitors of CAs.
In order to favor the anchorage of the inhibitor to the CA, a hydrophobic tail was incorporated for binding the hydrophobic subsite of the enzyme. Appropriate elongation might allow interaction of the ligand, not only with the catalytic site, but also with the middle and outer rims of the enzyme; modification of the electronic effects of the substituents located on the aromatic residue might also modulate the inhibitory properties. Structural modifications on the pharmacophores were also considered. And last, but not least, the squaramide tether, behaving as a vinylogous amide, or urea linkage [15] can act both, as a strong hydrogen bonding donor and acceptor and might contribute to the interaction with the hydrophilic subsite of the enzyme. Moreover, the use of a conformationally-restricted linker, as a bioisostere of more conventional linkages, can minimize the entropy loss in the formation of the enzyme-inhibitor complex, and enhance its potency/selectivity; this is a well validated approach in the drug discovery strategies that can even hinder or retard the drug degradation upon metabolization [53].
The key synthetic intermediate for preparing the target compounds is 3,4-dimethoxycyclobut-3-ene-1,2-dione (dimethyl squarate, 2), which is accessible in a multigram scale by treatment of commercially available squaric acid 1 with methanolic trimethyl orthoformate [54] under refluxing conditions (Scheme 1).
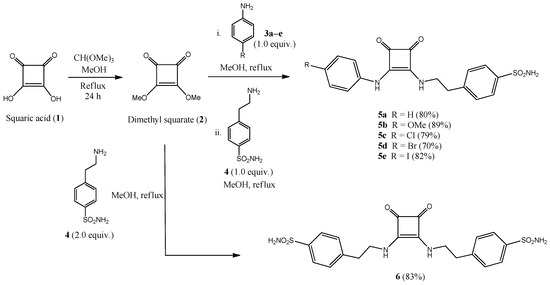
Scheme 1.
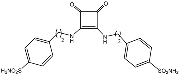
Preparation of sulfonamide-containing squaramides 5 and 6.
Sulfonamide-containing ureido/peptide-mimetics 5, 6, 8 and 11 were obtained in a one-pot, two-step methodology (Scheme 1 and Scheme 2) by sequential nucleophilic displacement of the two methoxy groups with amino-derivatives 3 (anilines) and 4, 7, 9 (sulfonamides); final compounds were obtained as highly crystalline derivatives just by filtration, without the need of chromatographic purifications.
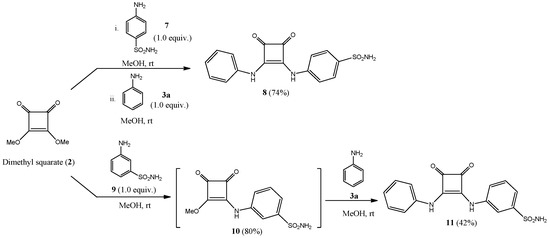
Scheme 2.
Preparation of sulfonamide-containing squaramides 8 and 11.
Reactions proceeded in higher rate and yields if the least nucleophilic amino compound was added in the first step. This is the reason for starting the synthesis with anilines 3 in Scheme 1, or with aminobenzenesulfonamides 7 and 9 in Scheme 2.
13C-NMR supported the proposed structures; thus, resonances at roughly 180–184 ppm (assigned to C=O) and at 164–169 ppm (C-3 and C-4) are in agreement with reported squaramides bearing N,N′-diaryl or N-alkyl-N′-aryl substituents [55].
Squaramides 5 derived from sulfonamide 4 were obtained under refluxing conditions for both steps, in a short period of time and high yields (70–89%). Dimeric derivative 6 was obtained in a similar fashion by using 2.0 mol. equivalents of sulfonamide 4 (83% yield).
Attempts to reproduce the aforementioned conditions for p- and m-substituted aminobenzenesulfonamides 7 and 9 proved to be unsuccessful, as desired compounds were obtained in a non-resolved mixture of compounds, including symmetrical dimeric species. In order to overcome such problems, and reduce the rate of side-products, both nucleophilic displacements on dimethyl squarate 2 were attempted at rt during significantly more prolonged reaction times (5 days for 8 and 17 days for 11).
In sulfonamides 5, 6, 8 and 11 several key structural motifs were modified in order to get valuable structure-activity relationships concerning their inhibitory properties against CAs: on the one hand, the stereoelectronic effects of the appended substituents on the aromatic scaffold (unsubstituted, electron-donating and electron-withdrawing, compounds 5a–e); unfortunately, the p-fluorophenyl derivative could not be obtained in a pure form; on the other hand, the distance between the arylsulfonamido moiety and the squaramide (5a vs. 8); and finally, the position of the sulfonamido motif (8 vs. 11).
We also envisioned the possibility of exploring a different pharmacophore for targeting CAs; we therefore replaced the arylsulfonamido motif, which very frequently leads to moderate selectivities, with coumarins (2H-chromen-2-ones), a natural and privileged structure within medicinal chemistry [56]. The coumarin skeleton, decorated with different substituents on C-3 and C-4 positions (compounds 14), is easily accessible using the acid-catalyzed Pechmann condensation [57] between resorcinol 12 and the appropriate β-ketoesters 13 (Scheme 3). Williamson synthesis on the free phenolic hydroxyl group of 14 at C-7 with an excess of α,ω-dibromoalkanes, followed by nucleophilic displacement of the terminal bromine atom with NaN3 and Pd-catalyzed hydrogenolysis afforded amino-coumarins 15, which were anchored on the cyclobutene-1,2-dione motif (Scheme 3) after reaction of dimethyl squarate 2 with different anilines 3 (R1 = H, OMe, F, Cl, Br, I). This gave access to squaramido-containing coumarins 16 in moderate to excellent yields (26–93%). Regarding the squaramide moiety, similar spectroscopic data as those obtained for arylsulfonamides were observed.
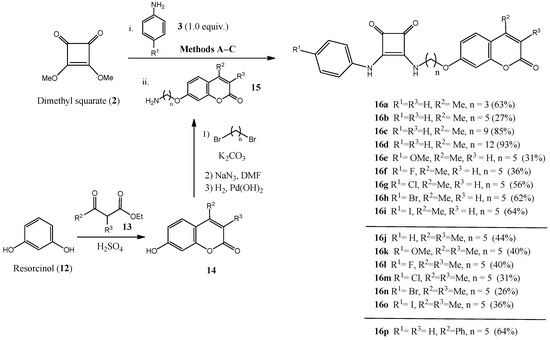
Scheme 3.
Preparation of coumarin-containing squaramides 16.
2.2. Biological Assessments
The vast panel of compounds reported herein, that is, sulfonamides 5, 6, 8 and 11, and coumarins 16, were tested in vitro against membrane-bound CAs IX and XII, with therapeutic interest against hypoxic tumors [58], and their activities were compared with off-target isoforms I and II (cytosolic) in order to calculate the selectivity index (S.I.). For that purpose, the stopped-flow CO2 hydration assay was used (Table 1). The drug acetazolamide (AAZ) was used as the reference compound. To the best of our knowledge, there is only one reported example of the use of squaramides in the inhibition of CAs; in this case, the squaramide motif was decorated with a bis-benzoxaborole fragment and turned out to be a moderate inhibitor of CAs [59].

Table 1.
Inhibition constants and selectivity indexes of sulfonamido-containing squaramides against hCAs I, II, IX and XII a.
Regarding sulfonamides, incorporation of substituents on the p-position of the phenyl ring (Table 1, derivatives 5b–e vs. 5a) yielded an impairment of the inhibition of off-target enzymes (Ki = 373–910 nM); nevertheless, this observation was translated into an improvement of selectivity just for p-bromophenyl derivative 5d, which turned out to be the strongest inhibitors of this first series against CA IX (Ki = 67.6 nM) and XII (Ki = 85.5 nM). Insertion of electron-donating groups (OMe, 5b) was particularly unfavorable for the inhibition of CA XII.
Dimeric sulfonamide 6, despite keeping a good inhibitory level against tumor-associated CAs, provoked a considerable improvement in the inhibition of the off-target enzymes, and thus, a sharp decrease in the selectivity (Table 1).
The shortening of the distance between the aryl sulfonamide and the squaramide moieties (8 vs. 5a) led to an increased inhibitory activity against CAs I and II, but also for CA IX and XII, affording a clear selectivity improvement, particularly for CA XII, reaching the low nanomolar range (Ki = 6.57 nM). Thus, S.I. (I/XII) were found to be 2.2 and 15 for compounds 5a and 8, respectively, and S.I. (II/XII), 7.4 and 10 for the same two compounds (Table 1).
Further SAR analysis revealed that m-regioisomers 10 (intermediate compound), and 11 kept within the low nM inhibition range for CA XII. Interestingly, the latter compound can be considered the lead compound of the sulfonamide series, as a clear impairment of activity against CA I and II was observed, improving the selectivity indexes of the reference drug AAZ (Table 1).
Table 2 depicts the same kind of data for squaramide-containing coumarins 16a–p; the first relevant conclusion that can be reached is that outstanding selectivities are observed for all compounds of this second series, as no relevant activities were detected for concentrations as high as 10 µM against CA I and II. This observation constitutes a significant difference with related sulfonamido counterparts.

Table 2.
Inhibition constants of coumarin-containing squaramides 16 against hCAs I, II, IX and XII a,b.
Compounds 16a–d, with an unsubstituted phenyl residue, provided information about the influence of the length of the hydrocarbon chain connecting the coumarin and squaramide moieties. Clearly, elongation led to an improvement of activity against tumor associated CAs, reaching the highest activities for n = 12 (16d) and 9 (16c) for CA IX and XII, respectively. Low nanomolar activities were achieved (Ki = 19.2, 18.1 nM for CA IX; 7.23, 7.91 nM for CA XII).
The second sub-series of compounds is comprised of derivatives 16e–i, where the hydrocarbon chain was kept unchanged (n = 5), and the stereoelectronic effects (electron withdrawing and donating effects) on the p-position of the phenyl ring were modified, and the effects, compared with the unsubstituted counterpart 16b. Data shown in Table 2 indicate that such substituents do not exert much influence on the bioactivities; just a moderate impairment of activity was observed for 16e (R = OMe), 16g (R = Cl), 16i (R = I).
The third set of compounds includes coumarins 16j–o, which were designed for analyzing the influence of a disubstituted pattern on C-3 and C-4 positions of the coumarin on the inhibition profile. The same hydrocarbon chain length (n = 5) and substitution pattern on the phenyl ring as previously indicated was settled. Comparison with unsubstituted 16b revealed (Table 2) an impairment of activity for CA IX and XII in all the members of the series, CA IX being more affected. Therefore, an increase in the steric hindrance was found to be detrimental on the activity.
The same conclusion can be reached by replacing the Me group on 16b with a Ph residue (16p); although being monosubstituted just on the C-4 position, such group is endowed with a high steric demand, that furnished a roughly 6.1-fold decreased activity for both enzymes when compared with 16b.
Some representative compounds (5a–e, 16b, 16e–i) were also tested as potential antiproliferative agents against a panel of six human tumor cell lines: A549 (non-small cell lung), HBL-100 (breast), HeLa (cervix), SW1573 (non-small cell lung), T-47D (breast), WiDr (colon). Unfortunately, no relevant activity was found for the tested compounds. Derivatives 16g–i were not soluble under the assay conditions; compounds 5a–e, 6, 16e and 16f had GI50 > 100 µM for all the cell lines. Compound 16b had GI50 = 94 ± 10 µM for line A549, and >100 µM for the rest.
Previous data were generated under normoxic conditions, when small concentrations of CAs are present; however, under hypoxic conditions, CA IX/XII are overexpressed and constitute a survival mechanism of the tumor cells. There are extensive data in literature indicating that under hypoxic conditions, the inhibition of such enzymes constitutes a potent antitumor mechanism [60,61,62,63,64].
Moreover, it is important to mention that a series of inhibitors of CA XII have also been found to inhibit the drug efflux transporter P-glycoprotein (P-gp) [65], one of the most common mechanisms for elimination of xenobiotics, and thus, responsible for the development of chemoresistance [66], one of the major challenges to be overcome in the development of new anticancer therapies.
2.3. Docking Simulations
The lead compounds in each series (m-benzenesulfonamide 11 and coumarin 16c) were subjected to docking simulations in order to predict the key interactions involved in their inhibition of CA XII, what can be of interest for future drug design. Squaramate 10, although not exhibiting an optimal selectivity profile, was also included in the calculations, as it was endowed with the strongest inhibition of CA XII within both sub-series. Sulfonamides are reported [37] to interact with CAs through its deprotonated form, and thus, as anionic species; accordingly, such observations were taken into consideration for the in silico study.
Figure 2 shows the predicted interactions of one of the most favorable poses for compound 10 binding the active site of CA XII; in this pose, the deprotonated sulfonamido motif exhibits interactions with the Zn2+ cation through the NH residue. Hydrogen bonding interactions between one of the oxygen atoms of the sulfonamido moiety and Thr 198, Thr 199 residues are also observed. This situation is similar to the interactions shown in crystal structures of arylsulfonamides-CA (II, IX) complexes [67]. Some other favourable poses also predict interaction, together with NH, of the SO2 residue and the Zn(II), due to the existence of a partial negative charge on the oxygen atoms.
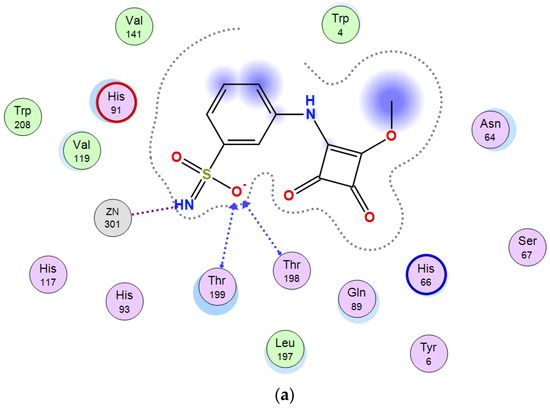
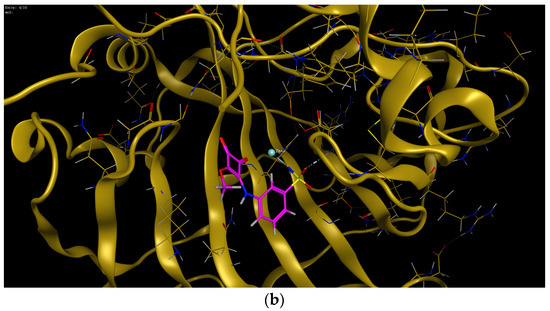
Figure 2.
Predicted binding mode of 10 and CA XII. (a) Two-dimensional view of main residues involved in the ligand-protein interactions. (b) Three-dimensional structure of CA XII showing the binding site.
When shifting to structurally related m-squaramide 11, the larger size of the appended phenyl residue compared to the methoxy group of 10, provokes its orientation towards the exit of the cavity. Similarly, to 10, interactions of the deprotonated sulfonamido group with Zn2+ (NH) and Thr 198, Thr 199 Leu 197 (oxygen atoms of the sulfonamido moiety) were observed in the most favorable poses (Figure 3).
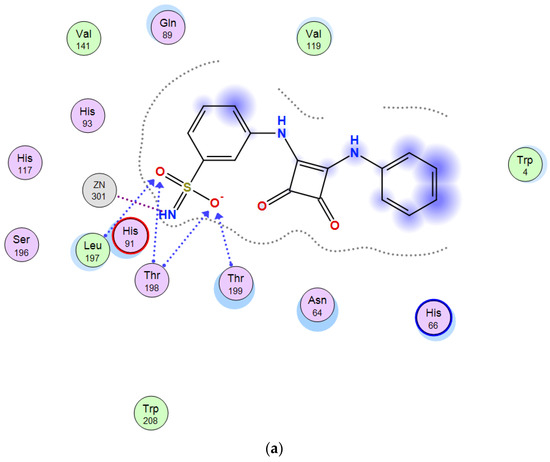
Figure 3.
Predicted binding mode of 11 and CA XII. (a) Two-dimensional view of main residues involved in the ligand-protein interactions. (b) Three-dimensional structure of CA XII showing the binding site.
As previously indicated, coumarins behave as prodrugs, as the lactone functionality is hydrolyzed by CAs to furnish the corresponding 2-hydroxycinnamic acid derivative [52], presumably with E-configuration (Scheme 4). This inhibitory mechanism was previously demonstrated both by X-ray crystallography and mass spectrometry [68].
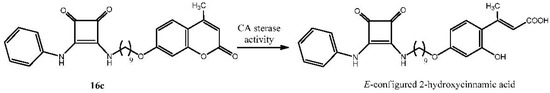
Scheme 4.
Mode of inhibition of squaramide 16c.
In fact, the binding energy of the non-hydrolyzed form shows a remarkable impairment compared to the cinnamic acid derivative binding the enzyme in the active site (Table 3).

Table 3.
Docking interaction energy of 16c-CA XII complex.
In this context, we have accomplished two different docking simulations for derivative 16c, one on the cavity of the enzyme active site (Figure 4), and another one in the entry to the cavity (Figure 5), in order to predict the most favorable binding region with the corresponding E-configured 2-hydroxycinnamic acid derivative.
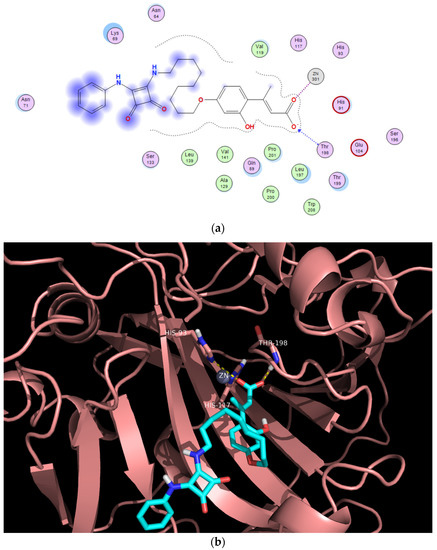
Figure 4.
Predicted binding mode of the open form of 16c and CA XII within the active site. (a) Two-dimensional view of main residues involved in the ligand-protein interactions. (b) Three-dimensional structure of CA XII showing the binding site.
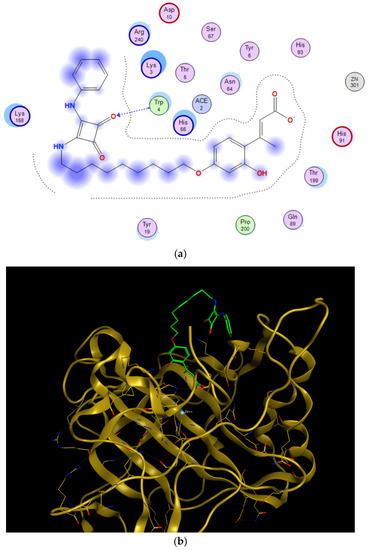
Figure 5.
Predicted binding mode of the open form of 16c and CA XII outside the active site. (a) Two-dimensional view of main residues involved in the ligand-protein interactions. (b) Three-dimensional structure of CA XII showing the binding outside the active site.
Regarding the simulation within the active site, it is the concomitant carboxylate moiety that interacts with the Zn2+ cation, whereas the bulky nonyl and squaramido moieties occlude the entry to the cavity of the catalytic site (Figure 4).
Nevertheless, simulations conducted at the entry of the cavity (Figure 5), where the carboxylate moiety cannot interact with the Zn(II) cation, led to a significant impairment in the binding energy (Table 3, roughly −10 kcal/mol vs. −6.9 kcal/mol). This would predict that the hydrolyzed coumarin system has the appropriate shape and size to be accommodated within the enzyme active site and interact with the Zn(II) through ionic interactions. A similar situation was recently reported for psoralen derivatives [69].
2.4. Prediction of Physicochemical Properties
A series of relevant molecular physicochemical properties, like logP, logS (Ali) [70], H-bond acceptors/donors, TPSA (SwissADME freeware suite) [71,72], or pKa (MolGpka) [73,74] were calculated for squaramides in order to predict their drug likeness properties (Table 4).

Table 4.
Predicted physicochemical properties for squaramides derivatives.
As it can be observed from Table 4, all compounds have the required lipophilicity, polar surface, number of H-bond acceptors/donors and molecular size so as to fulfill the Lipinsky rule of five, and therefore, to be considered to have drug likeness properties. Only derivatives 16d,h,I,n,o slightly exceeded the limitation of 500 g/mol for their molecular weights. Sulfonamido-containing 5, 6, 8, 10, 11 are expected to be either moderately soluble, or soluble in water; coumarin derivatives 16a,b are predicted to be moderately soluble in water, while the rest compounds of the series, with longer tethers, a disubstituted pattern on C-3/C-4 positions of the coumarin, or bearing a Ph ring at C-4 position, are expected to have poor water solubility.
With the exceptions of dimeric sulfonamide 6 and coumarin derivative 16d, the rest of the compounds of the series are predicted to have high gastrointestinal absorption, in contrast with the reference drug AAZ. This feature might ensure an appropriate bioavailability of the title compounds.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
TLCs (Merck 60 F254, gel thickness 0.25 mm) were performed using aluminum-coated sheets, using the appropriate eluant. Spots were visualized by UV light (λ = 254 nm), and by charring with 10% ethanolic vanillin containing 1% H2SO4, or with 3% ninhydrin in EtOH. Column chromatography purifications were performed using silica gel stationary phase (Merck 60, particle size 40–63 µm), eluting by gravity, or with a mild pressure, using the eluant indicated in the experimental section.
NMR spectra were registered in the Centro de Investigación, Tecnología e Innovación de la Universidad de Sevilla (CITIUS), using Bruker Avance III 300 and 500 spectrometers (300 and 500 MHz for 1H, 75.5 and 125.7 MHz for 13C), and DMSO-d6 as solvent (see Supplementary Material). Chemical shifts (δ) are expressed in ppm, and coupling constants (J), in Hz. Residual signals from the solvent are used as internal references [75]. Mass spectra were registered using a Q Exactive spectrometer, using Electrospray Ionization (ESI).
3.1.2. General Procedure for the Preparation of Squaramides 5a–e
A mixture of dimethyl squarate 2 (1.0 equiv.) and the corresponding aniline derivative 3 (1.0 equiv.) in MeOH (5 mL) was refluxed for 2 h. Then, 4-(2′-aminoethyl)benzenesulfonamide 4 (1.0 equiv.) was added, and refluxed for further 2 h. After that, the crude reaction mixture was filtered and the solid was washed with cold MeOH.
3-Phenylamino-4-[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (5a). Aniline (33 µL, 0.36 mmol) and 4-(2′-aminoethyl)benzenesulfonamide (72.6 mg, 0.36 mmol) were used. Compound 5a was obtained as a grey solid. Yield: 106.6 mg (80%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 9.69 (brs, 1H, NH-Ar), 7.78 (m, 2H, H-3″, H-5″), 7.70 (brs, 1H, NH-CH2), 7.47 (m, 2H, H-2″, H-6″), 7.40 (m, 2H, Ar-H), 7.36–7.25 (m, 2H, SONH2), 7.33 (m, 2H, Ar-H), 7.02 (tt, 1H, JAr-3,4 = JAr-4,5 = 7.5 Hz, JAr-2,4 = JAr-4,6 = 1.2 Hz, Ar-H4), 3.90 (brt, JH,H = 6.6 Hz, CH2N), 2.99 (t, 1H, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.0, 180.4 (C=O), 169.1, 163.7 (C-3, C-4), 142.7, 142.4 (C-1″, C-4″), 139.0 (Ar-Cipso), 129.3 (Ar-C), 125.8 (Ar-C), 122.7 (Ar-C), 118.1 (Ar-Cp), 44.6 (CH2-N), 36.6 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C18H17N3NaO4S ([M + Na]+): 394.0832, found: 394.0829.
3-(p-Methoxyphenyl)amino-4-[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (5b). p-Methoxyaniline (45.6 mg, 0.36 mmol) and 4-(2′-aminoethyl)benzenesulfonamide (72.6 mg, 0.36 mmol) were used. Compound 5b was obtained as a grey solid. Yield: 128.7 mg (89%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 9.57 (brs, 1H, NH-Ar), 7.77 (m, 2H, H-3″, H-5″), 7.55 (brs, 1H, NH-CH2), 7.46 (m, 2H, H-2″, H-6″), 7.30 (m, 4H, 2Ar-H, NH2), 6.90 (m, 2H, 2Ar-H), 3.87 (brq, JH,H = JNH,CH3 = 6.7 Hz, CH2-NH), 2.98 (t, 2H, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO- d6) δ 183.5, 180.6 (C=O), 168.6, 163.8 (C-3, C-4), 155.3 (Ar-C-OMe), 142.7, 142.3 (C-1″, C-4″), 132.1 (Ar-Cipso), 129.3, 125.8 (C-2″, C-3″, C-5″, C-6″), 119.7 (Ar-Co), 114.5 (Ar-Cm), 55.3 (OMe), 44.5 (CH2-N), 36.6 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C19H19N3NaO5S ([M + Na]+): 424.0938, found: 424.0931.
3-(p-Chlorophenyl)amino-4-[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (5c). p-Chloroaniline (46.7 mg, 0.36 mmol) and 4-(2′-aminoethyl)benzenesulfonamide (72.6 mg, 0.36 mmol) were used. Compound 5c was obtained as a white solid. Yield: 115.6 mg (79%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 9.72 (brs, 1H, NH-Ar), 7.78 (m, 2H, H-3″, H-5″), 7.62 (brs, 1H, NH-CH2), 7.46 (m, 2H, H-2″, H-6″), 7.40–7.35 (m, 4H, 2Ar-H, NH2), 7.30 (m, 2H, 2Ar-H), 3.87 (brq, JH,H = JNH,CH3 = 6.5 Hz, CH2-NH), 2.97 (t, 2H, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.1, 180.3 (C=O), 169.1, 163.2 (C-3, C-4), 142.5, 142.3 (C-1″, C-4″), 137.9 (Ar-Cipso), 129.3, 129.1, 126.5, 125.7 (Ar-C), 44.5 (CH2-N), 36.6 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C18H16ClN3NaO4S ([M + Na]+): 428.0442, found: 428.0435.
3-(p-Bromophenyl)amino-4-[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (5d). p-bromoaniline (61.8 mg, 0.36 mmol) and 4-(2′-aminoethyl)benzenesulfonamide (72.6 mg, 0.36 mmol) were used. Compound 5d was obtained as a white solid. Yield: 113.7 mg (70%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 9.73 (brs, 1H, NH-Ar), 7.77 (m, 2H, H-3″, H-5″), 7.64 (brs, 1H, NH-CH2), 7.49 (m, 2H, 2Ar-H), 7.46 (m, 2H, H-2″, H-6″), 7.34 (m, 2H, 2Ar-H), 7.31 (brs, 2H, CH2), 3.88 (brq, JH,H = JNH,CH3 = 6.8 Hz, CH2-NH), 2.98 (t, 2H, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.2, 180.4 (C=O), 169.2, 163.3 (C-3, C-4), 142.6, 142.4 (C-1″, C-4″), 138.4 (Ar-Cipso), 132.1, 129.3, 125.8, 120.1, 114.5 (Ar-C), 44.6 (CH2-N), 36.6 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C18H1679BrN3NaO4S ([M + Na]+): 471.9937, found: 471.9932; m/z calcd. for C18H1681BrN3NaO4S ([M + Na]+): 473.9917, found: 473.9908.
3-(p-Iodophenyl)amino-4-[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (5e). p-iodoaniline (81.2 mg, 0.36 mmol) and 4-(2′-aminoethyl)benzenesulfonamide (72.6 mg, 0.36 mmol) were used. Compound 5e was obtained as a yellowish solid. Yield: 147.6 mg (82%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 9.70 (brs, 1H, NH-Ar), 7.77 (m, 2H, H-3″, H-5″), 7.64 (m, 3H, 2Ar-H, NH-CH2), 7.46 (m, 2H, 2Ar-H), 7.30 (brs, 2H, NH2), 7.21 (m, 2H, 2Ar-H), 3.88 (brq, JH,H = JNH,CH3 = 6.8 Hz, CH2-NH), 2.98 (t, 2H, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.3, 180.4 (C=O), 169.2, 163.3 (C-3, C-4), 142.6, 142.4 (C-1″, C-4″), 138.8, 137.9, 129.3, 125.8 (Ar-C), 120.4 (Ar-Co), 86.1 (Ar-C-I), 44.6 (CH2-N), 36.6 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C18H16IN3NaO4S ([M + Na]+): 519.9798, found: 519.9794.
3,4-Bis[2′-(4″-sulfonamidophenyl)ethylamino]cyclobut-3-ene-1,2-dione (6). A solution of dimethyl squarate 2 (54.4 mg, 0.38 mmol) and 4-(2′-aminoethyl)benzenesulfonamide 4 (154.9 mg, 0.77 mmol, 2.0 equiv.) in MeOH (15 mL) was refluxed for 2 h. Filtration afforded 6 as a white solid. Yield: 150.5 mg (83%). Mp > 250 °C. 1H-NMR (500 MHz, DMSO-d6) δ 7.75 (m, 4H, H-3″, H-5″), 7.42 (m, 4H, H-2″, H-6″), 7.29 (m, 4H, NH2), 3.75 (brs, 4H, CH2-NH), 2.91 (brt, 4H, JH,H = 6.9 Hz, CH2-Ar) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 182.6 (C=O), 167.7 (C-3, C-4), 142.8, 142.3 (C-1″, C-4″), 129.3, 125.8 (C-2″, C-3″, C-5″, C-6″), 44.2 (CH2-N), 36.7 (CH2-Ar) ppm; HRESI-MS m/z calcd. for C20H22N4NaO6S2 ([M + Na]+): 501.0873, found: 501.0868.
3-Phenylamino-4-[4′-(sulfonamidophenyl)]aminocyclobut-3-ene-1,2-dione (8). To a solution of dimethyl squarate 2 (83 mg, 0.58 mmol) in MeOH (7 mL) was added 4-aminobenzenosulfonamide 7 (100 mg, 0.58 mmol, 1.0 equiv.), and the corresponding mixture was stirred at rt for 5 days; formation of a precipitate was observed. Then, aniline (53 µL, 0.58 mmol, 1.0 equiv.) was added and the mixture was stirred at rt for further 48 h. The corresponding solid was filtrated and washed with cold MeOH to give compound 8 as a white solid. Yield: 148 mg, 74%. Mp > 250 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 10.15 (brs, 1H, NH), 10.02 (brs, 1H, NH), 7.80 (m, 2H, Ar-H), 7.61 (m, 2H, Ar-H), 7.50 (m, 2H, Ar-H), 7.40 (m, 2H, Ar-H), 7.29 (brs, 2H, SO2NH2), 7.11 (t, 1H, JH,H = 7.2 Hz, H-4, Ph) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 182.2, 181.5 (C=O), 166.4, 165.1 (C-3, C-4), 141.4, 138.3, 138.2, 129.4, 127.3, 123.6, 118.7, 118.2 ppm; HRESI-MS m/z calcd. for C16H13N3NaO4S ([M+Na]+): 366.0519, found: 366.0511.
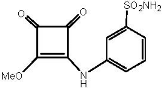
3-Methoxy-4-[3′-(sulfonamidophenyl)]aminocyclobut-3-ene-1,2-dione (10) and 3-phenyl-4-[3′-(sulfonamidophenyl)]aminocyclobut-3-ene-1,2-dione (11). To a solution of dimethyl squarate 2 (83 mg, 0.58 mmol) in MeOH (7 mL) was added 3-aminobenzenosulfonamide 9 (100 mg, 0.58 mmol, 1.0 equiv.), and the corresponding mixture was stirred at rt for 5 days. Then, aniline (53 µL, 0.58 mmol) was added, and the mixture was stirred for 48 h. The precipitate was filtered and washed with cold MeOH to give 3-methoxy derivative 10 as a light yellow solid (131 mg, 80% yield). The solid was suspended again in MeOH (7 mL) and aniline (55 µL, 0.60 mmol) was added and the mixture was kept stirring at rt for 96 h. After that, 1H-NMR showed a 1:2 mixture of 10 and 11. Aniline (12 µL, 0.13 mmol) was added and the reaction was kept stirring for a further 6 days. The precipitate was filtrated and washed with cold MeOH to give 11 as a white solid (67 mg, 42% yield).
Data for 10: Rf 0.2 (1:1 cyclohexane-EtOAc); 1H-NMR (300 MHz, DMSO-d6) δ 10.98 (brs, 1H, NH), 7.81 (m, 1H, Ar-H), 7.56 (m, 3H, Ar-H), 7.41 (brs, 2H, SONH2), 4.39 (brs, 3H, OMe) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 187.8, 184.4 (C=O), 179.1, 169.2 (C-2, C-3), 145.1, 138.4, 130.0, 122.5, 121.0, 116.7 (Ar-C), 60.6 (OCH3) ppm; HRESI-MS m/z calcd. for C11H10N2NaO5S ([M + Na]+): 305.0203, found: 305.0197.
Data for 11: Mp: 227–229 °C (dec.). Rf 0.2 (1:1 cyclohexane-EtOAc); 1H-NMR (300 MHz, DMSO-d6) δ 10.10 (brs, 1H, NH), 9.87 (brs, 1H, NH), 7.81 (m, 2H, Ar-H), 7.61–7.37 (m, 8H, Ar-H, SONH2), 7.11 (t, 1H, JH,H = 7.3 Hz, H-4, Ph) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 182.1, 181.5 (C=O), 166.0, 165.3 (C-3, C-4), 145.2, 139.0, 138.3, 130.3, 129.4, 123.5, 121.4, 120.1, 118.6, 115.3 (Ar-C) ppm; HRESI-MS m/z calcd. for C16H13N3NaO4S ([M + Na]+): 366.0519, found: 366.0514.
3.1.3. General Procedures for the Preparation of Coumarin Derivatives 16a–p
Method A. A solution of dimethyl squarate 2 (1.0 equiv.) and the corresponding aniline 3 (1.0 equiv.) in MeOH (5 mL) was heated in a Fisher-Porter tube at 100 °C for 2 h. Then, the corresponding amino-coumarin 15 (1.0 equiv.) was added and the mixture was heated in the Fisher-Porter tube at 100 °C for further 24 h. A precipitate was formed, which was filtered and washed with cold MeOH.
Method B. A solution of dimethyl squarate 2 (1.0 equiv.) and the corresponding aniline 3 (1.0 equiv.) in MeOH (7 mL) was refluxed for 4 h. Then, the corresponding amino-coumarin 15 (1.0 equiv.) was added and the reaction was refluxed for further 12 h. A precipitate was formed, which was filtered and washed with cold MeOH.
Method C. A solution of dimethyl squarate 2 (1.0 equiv.) and the corresponding aniline 3 (1.0 equiv.) in MeOH (7 mL) was kept at rt for 24 h. Then, the corresponding amino-coumarin 15 (1.0 equiv.) was added and the reaction was kept at the same temperature for further 24 h. A precipitate was formed, which was filtered and washed with cold MeOH.
3-{[3′-((4″-Methyl-2″-oxo-2″H-chromen-7″-yl)oxy)propyl]amino}-4-phenylaminocyclobut-3-ene-1,2-dione (16a). Method C. Dimethyl squarate (92 mg, 0.65 mmol), aniline (59 µL, 0.65 mmol), and 15 (151 mg, 0.65 mmol, n = 3, R2 = Me, R3 = H) were used. Yield: 166 mg (63%, white solid). Mp > 250 °C. Rf 0.40 (1:1 cyclohexane-EtOAc). 1H-NMR (500 MHz, DMSO-d6) δ 9.62 (brs, 1H, NH-Ar), 7.67 (m, 2H, H-5″, NH-CH2), 7.49–7.32 (m, 4H, Ar-H), 7.07–6.95 (m, 3H, Ar-H), 6.20 (brs, 1H, H-3″), 4.19 (m, 2H, CH2O), 3.78 (m, 2H, CH2N), 2.38 (brs, 3H, CH3), 2.08 (m, 2H, CH2-CH2-O) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.9, 180.3 (C=O), 169.3, 163.6 (C-3, C-4), 161.5, 160.1 (C-2″, C-7″), 154.7, 153.3 (C-4″, C-8a″), 139.0, 129.3, 126.4, 122.5, 117.8, 112.4, 111.1 (Ar-C), 101.2 (C-8″), 65.5 (CH2O), 40.7 (CH2N), 29.9 (CH2-CH2-O), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C23H20N2NaO5 ([M + Na]+): 427.1264, found: 427.1256.
3-{[5′-((4″-Methyl-2″-oxo-2″H-chromen-7″-yl)oxy)pentyl]amino}-4-phenylaminocyclobut-3-ene-1,2-dione (16b). Method A. Dimethyl squarate (35 mg, 0.25 mmol), aniline (23 µL, 0.25 mmol), and 15 (65 mg, 0.25 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 29 mg (27%, light yellow solid). Mp > 250 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.59 (s, 1H, NH-Ar), 7.66–7.64 (m, 1H, NH), 7.65 (m, 2H, H-5″, NH-CH2), 7.44-7.30 (m, 4H, Ar-H), 7.04–6.93 (m, 3H, Ar-H), 6.19 (brs, 1H, H-3″), 4.10 (brt, 2H, JH,H = 6.1 Hz, OCH2), 3.64 (brq, 2H, JH,H = JCH2,NH = 6.8 Hz, NCH2), 2.38 (brs, 3H, CH3), 1.79 (brquint, 2H, JH,H = 7.0 Hz, CH2), 1.65 (quint, 2H, JH,H = 6.8 Hz, CH2), 1.50 (quint, 2H, JH,H = 6.2 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.0, 180.1 (C=O), 169.2, 163.6 (C-3, C-4), 161.7, 160.1 (C-2″, C-7″), 154.7, 153.4 (C-4″, C-8a″), 139.0, 129.4, 126.4, 122.5, 117.9, 113.00, 112.4, 111.0 (Ar-C), 101.0 (C-8″), 68.1 (OCH2), 43.5 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C25H24NaN2O5 ([M + Na]+): 455.1577, found: 455.1569.
3-{[9′-((4″-Methyl-2″-oxo-2″H-chromen-7″-yl)oxy)nonyl]amino}-4-phenylaminocyclobut-3-ene-1,2-dione (16c). Method C. Dimethyl squarate (58 mg, 0.41 mmol), aniline (37 µL, 0.41 mmol) and 15 (130 mg, 0.41 mg, n = 9, R2 = Me, R3 = H) were used. Yield: 170 mg (85%, white solid). Mp: 200–201 °C. Rf 0.30 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.59 (brs, 1H, NH-Ar), 7.68–7.64 (m, 2H, NH, H-5″), 7.44–7.42 (m, 2H, Ar-H), 7.35–7.30 (m, 2H, Ar-H), 7.01 (t, 1H, JH,H = 7.3 Hz, H-4, Ph), 6.95–6.92 (m, 2H, Ar-H), 6.19 (d, 1H, JH,CH3 = 1.1 Hz, H-3″), 4.06 (t, 2H, JH,H = 6.5 Hz, OCH2), 3.59 (brq, 2H, JH,H = JCH2,NH = 6.4 Hz, NCH2), 2.38 (d, 3H, JH,CH3 = 1.1 Hz, CH3), 1.75 (quint, 2H, JH,H = 6.9 Hz, CH2), 1.57 (quint, 2H, JH,H = 6.9 Hz, CH2), 1.39–1.35 (m, 2H, CH2), 1.31 (m, 8H, 4CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.9, 180.0 (C=O), 169.1, 163.3 (C-3, C-4), 161.6, 160.0 (C-2″, C-7″), 154.6, 153.3, 138.9, 129.2, 126.3, 122.4, 117.8, 112.8, 112.3, 110.9 (Ar-C), 101.0 (C-8″), 68.1 (OCH2), 43.5 (NCH2), 30.4, 28.7, 28.5, 28.4, 28.3, 25.7, 25.2 (CH2), 17.7 (CH3) ppm; HRESI-MS calcdm/z. for C29H32N2NaO5 ([M + Na]+): 511.2203, found: 511.2193.
3-{[12′-((4″-Methyl-2″-oxo-2″H-chromen-7″-yl)oxy)dodecyl]amino}-4-phenylaminocyclobut-3-ene-1,2-dione (16d). Method C. Dimethyl squarate (51 mg, 0.36 mmol), aniline (33 µL, 0.36 mmol) and 15 (130 mg, 0.36 mmol, n = 12, R2 = Me, R3 = H) were used. Yield: 178 mg (93%, light brown solid). Mp: 178–179 °C. Rf 0.40 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.48 (brs, 1H, NH-Ar), 7.71 (brs, 1H, NH), 7.66 (m, 1H, H-5″), 7.44 (m, 2H, Ar-H), 7.33 (m, 2H, Ar-H), 7.01 (t, 1H, JH,H = 7.3 Hz, H-4, Ph), 6.94–6.92 (m, 2H, Ar-H), 6.19 (d, 1H, JH,H = 1.0 Hz, H-3″), 4.05 (brt, 2H, JH,H = 6.5 Hz, OCH2), 3.59 (brt, 2H, JH,H = 6.4 Hz, NCH2), 2.39 (d, 3H, JH,H = 0.9 Hz, CH3), 1.72 (quint, 2H, JH,H = 6.6 Hz, CH2), 1.56 (quint, 2H, JH,H = 6.3 Hz, CH2), 1.40 (brquint, 2H, JH,H = 6.3 Hz, CH2), 1.26 (m, 14H, 7CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.0, 180.1 (C=O), 169.2 (C-3), 163.5 (C-4), 161.8 (C-7″), 160.1 (C-2′), 154.7 (C-9), 153.4 (C-4″), 139.1 (C-1 Ph), 129.3 (Ar-C, Ph), 126.4 (C-5″), 122.5, 117.9 (Ar-C, Ph), 113.0 (C-10), 112.4 (C-6″), 111.0 (C-3″), 101.1 (C-8″), 68.2 (OCH2), 43.6 (NCH2), 30.5 (CH2), 28.9 (CH2), 28.9 (×3) (CH2), 28.7 (CH2), 28.5 (CH2), 28.4 (CH2), 25.8 (CH2), 25.4 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C32H38N2NaO5 ([M + Na]+): 553.2673, found: 553.2662.
3-[(4′-Methoxyphenyl)amino]-4-{[5″-(4‴-methyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16e). Method A. Dimethyl squarate (31 mg, 0.22 mmol), 4-methoxyaniline (27 mg, 0.22 mmol) and 15 (57 mg, 0.22 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 32 mg (31%, white solid). Mp: 215–216 °C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 1H-NMR (300 MHz, DMSO-d6) δ 9.49 (s, 1H, NH-Ar), 7.66 (d, 1H, J5‴,6‴ = 8.4 Hz, H-5‴), 7.55 (brs, 1H, NH), 7.34 (m, 2H, Ar-H), 6.97–6.90 (m, 4H, Ar-H), 6.20 (brs, 1H, H-3‴), 4.10 (t, 2H, JH,H = 6.2 Hz, OCH2), 3.73 (s, 3H, OMe), 3.63 (brq, 2H, JH,H = 6.7 Hz, NCH2), 2.39 (brs, 3H, CH3), 1.79 (quint, 2H, JH,H = 7.0 Hz, CH2), 1.65 (quint, 2H, JH,H = 7.3 Hz, CH2), 1.50 (quint, 2H, JH,H = 6.8 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.4, 180.3 (C=O), 168.8, 163.6 (C-3, C-4), 161.7, 160.1 (C-2‴, C-7‴), 155.2, 154.7, 153.4, 132.2, 126.4, 119.5, 114.5, 113.0, 112.4, 111.0 (Ar-C), 101.1 (C-8‴), 68.1 (OCH2), 55.3 (OMe), 43.5 (NCH2), 30.3 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C26H26N2NaO6 ([M + Na]+): 485.1683, found: 485.1685.
3-[(4′-Fluorophenyl)amino]-4-{[5″-(4‴-methyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16f). Method A. Dimethyl squarate (50 mg, 0.35 mmol), 4-fluoroaniline (33 µL, 0.35 mmol) and 15 (92 mg, 0.35 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 57 mg (36%, white solid). Mp: 224–226 °C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.61 (s, 1H, NH-Ar), 7.65 (d, 1H, J5‴,6‴ = 9.0 Hz, H-5‴), 7.66–7.61 (brs, 1H, NH), 7.42 (m, 2H, Ar-H), 7.17 (m, 2H, Ar-H), 6.96-6.93 (m, 2H, Ar-H), 6.19 (brd, 1H, JH,CH3 = 0.7 Hz, H-3‴), 4.10 (t, 2H, JH,H = 6.0 Hz, OCH2), 3.63 (brq, 2H, JH,H = JCH2,NH = 6.8 Hz, NCH2), 2.38 (brs, 3H, CH3), 1.79 (quint, 2H, JH,H = 6.8 Hz, CH2), 1.65 (quint, 2H, JH,H = 6.9 Hz, CH2), 1.49 (quint, 2H, JH,H = 6.7 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.8, 180.2 (C=O), 169.1, 163.4 (C-3, C-4), 161.7, 160.1 (C-2‴, C-7‴), 158.0 (d, 1JC,F = 239.1 Hz, C-4′), 154.7, 153.4 (Ar-C), 135.5 (d, 4JC,F = 2.3 Hz, C-1′), 126.4 (Ar-C), 119.7 (brd, 3JC,F = 6.2 Hz, C-2′), 115.9 (d, 2JC,F = 22.6 Hz, C-3′), 113.0, 112.4, 111.0 (Ar-C), 101.1 (C-8‴), 68.1 (OCH2), 43.6 (NCH2), 30.3 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C25H23FN2NaO5 ([M + Na]+): 473.1483, found: 473.1468.
3-[(4′-Chlorophenyl)amino]-4-{[5″-(4‴-methyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16g). Method A. Dimethyl squarate (50 mg, 0.35 mmol), 4-chloroaniline (45 mg, 0.35 mmol) and 15 (92 mg, 0.35 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 92 mg (56%, light yellow solid). Mp: 225–227 °C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.68 (s, 1H, NH-Ar), 7.65 (d, 1H, J5‴,6‴ = 8.8 Hz, H-5‴), 7.67–7.62 (brs, 1H, NH), 7.45-7.36 (m, 4H, Ar-H), 6.96-6.93 (m, 2H, Ar-H), 6.19 (brd, 1H, JH,CH3 = 1.0 Hz, H-3‴), 4.10 (t, 2H, JH,H = 6.3 Hz, OCH2), 3.64 (q, 2H, JH,H = 6.3 Hz, NCH2), 2.38 (brd, 3H, JH,H = 0.8 Hz CH3), 1.79 (quint, 2H, JH,H = 7.1 Hz, CH2), 1.66 (quint, 2H, JH,H = 7.2 Hz, CH2), 1.51 (m, 2H, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.1, 180.1 (C=O), 169.3, 163.1 (C-3, C-4), 161.7, 160.1 (C-2‴, C-7‴), 154.7, 153.4, 138.1, 129.2, 129.1, 126.4, 119.6, 113.0, 112.4, 111.0 (Ar-C), 101.1 (C-8‴), 68.1 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C25H23ClN2NaO5 ([M + Na]+): 489.1188, found: 489.1186.
3-[(4′-Bromophenyl)amino]-4-{[5″-(4‴-methyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16h). Method A. Dimethyl squarate (50 mg, 0.35 mmol), 4-bromoaniline (61 mg, 0.35 mmol) and 15 (92 mg, 0.35 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 111 mg (62%, light yellow solid). Mp: 248–249 °C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.67 (s, 1H, NH-Ar), 7.67–7.63 ( brs, 1H, NH), 7.64 (d, 1H, J5‴,6‴ = 8.7 Hz, H-5‴), 7.49 (m, 2H, Ar-H), 7.37 (m, 2H, Ar-H), 6.96–6.92 (m, 2H, Ar-H), 6.19 (brd, 1H, JH,H = 1.0 Hz, H-3‴), 4.10 (t, 2H, JH,H = 6.4 Hz, OCH2), 3.63 (brq, 2H, JH,H = 7.1 Hz, NCH2), 2.38 (brd, 3H, JH,H = 0.8 Hz CH3), 1.78 (quint, 2H, JH,H = 6.8 Hz, CH2), 1.65 (quint, 2H, JH,H = 7.3 Hz, CH2), 1.49 (m, 2H, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.2, 180.1 (C=O), 169.3, 163.0 (C-3, C-4), 161.7, 160.1 (C-2‴, C-7‴), 154.7, 153.4, 138.5, 132.1, 126.4, 120.0, 114.3, 113.0, 112.4, 111.0 (Ar-C), 101.1 (C-8‴), 68.1 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C25H2379BrN2NaO5 ([M + Na]+): 533.0683, found: 533.0672.
3-[(4′-Iodophenyl)amino]-4-{[5″-(4‴-methyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16i). Method A. Dimethyl squarate (50 mg, 0.35 mmol), 4-yodoaniline (77 mg, 0.35 mmol) and 15 (92 mg, 0.35 mmol, n = 5, R2 = Me, R3 = H) were used. Yield: 125 mg (64%, light yellow solid). Mp > 250 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.67 (s, 1H, NH-Ar), 7.65 (m, 4H, H-5‴,NH, 2Ar-H), 7.25 (m, 2H, Ar-H), 6.97–6.92 (m, 2H, Ar-H), 6.20 (brd, 1H, JH,H = 1.1 Hz, H-3‴), 4.10 (t, 2H, JH,H = 6.4 Hz, OCH2), 3.64 (brq, 2H, JH,H = 6.2 Hz, NCH2), 2.39 (brd, 3H, JH,H = 1.0 Hz, CH3), 1.79 (quint, 2H, JH,H = 7.2 Hz, CH2), 1.65 (quint, 2H, JH,H = 7.3 Hz, CH2), 1.50 (m, 2H, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.2, 180.1 (C=O), 169.3, 163.0 (C-3, C-4), 161.7, 160.1 (C-2‴, C-7‴), 154.7, 153.4, 138.9, 137.8, 126.4, 120.3, 113.0, 112.4, 111.0 (Ar-C), 101.1 (C-8‴), 85.9 (C-4′), 68.1 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 18.1 (CH3) ppm; HRESI-MS m/z calcd. for C25H23IN2NaO5 ([M + Na]+): 581.0544, found: 581.0543.
3-{[5′-((3″,4″-Dimethyl-2″-oxo-2″H-chromen-7″-yl)oxy)pentyl]amino}-4-phenylaminocyclobut-3-ene-1,2-dione (16j). Method B. Dimethyl squarate (51 mg, 0.36 mmol), aniline (33 µL, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 71 mg (44%, light yellow solid). Mp > 250 °C. Rf 0.40 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.60 (s, 1H, NH-Ar), 7.67–7.63 (m, 1H, NH), 7.66 (d, 1H, J5″,6″ = 8.5 Hz, H-5″), 7.44–7.30 (m, 4H, Ar-H), 7.01 (t, 1H, JH,H = 7.3 Hz, H-4, Ph), 6.94–6.91 (m, 2H, Ar-H), 4.09 (brt, 2H, JH,H = 6.1 Hz, OCH2), 3.63 (brs, 2H, NCH2), 2.35 (brs, 3H, CH3), 2.07 (brs, 3H, CH3), 1.78 (brquint, 2H, JH,H = 7.0 Hz, CH2), 1.64 (brquint, 2H, JH,H = 7.0 Hz, CH2), 1.51 (brquint, 2H, JH,H = 7.0 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.0, 180.1 (C=O), 169.2, 163.5 (C-3, C-4), 161.2, 160.7 (C-2″, C-7″), 153.0, 146.8, 139.0, 129.3, 126.1, 122.5, 117.9, 117.8, 113.5, 112.2 (Ar-C), 100.8 (C-8″), 68.0 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.4 (CH2), 14.9 (CH3), 12.9 (CH3) ppm; HRESI-MS m/z calcd. for C26H26N2NaO5 ([M + Na]+): 469.1734, found: 469.1727.
3-{[5′-(3″,4″-Dimethyl-2″-oxo-2″H-chromen-7″-yl)oxypentyl]amino}-4-(4‴-methoxyphenylamino)cyclobut-3-ene-1,2-dione (16k). Method B. Dimethyl squarate (51 mg, 0.36 mmol), 4-methoxyaniline (45 mg, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 69 mg (40%, light green solid). Mp > 250 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.49 (brs, 1H, NH-Ar), 7.65–7.33 (m, 4H, NH, H-5″, 2Ar-H), 6.92 (m, 4H, Ar-H), 4.08 (brs, 2H, NCH2), 3.72–3.61 (m, 5H, OCH2, OMe), 2.35 (brs, 3H, CH3), 2.06 (brs, 3H, CH3), 1.77 (m, 2H, CH2), 1.63 (m, 2H, CH2), 1.50 (m, 2H, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.4, 180.3 (C=O), 168.8, 163.5 (C-3, C-4), 161.2, 160.6 (C-2″, C-7″), 155.1, 153.0, 146.8, 132.2, 126.0, 120.0, 119.5, 117.7, 114.5, 113.5, 112.2 (Ar-C), 100.8 (C-8″), 67.9 (OCH2), 55.2 (OCH3), 43.5 (NCH2), 30.3 (CH2), 28.0 (CH2), 22.4 (CH2), 14.8 (CH3), 12.8 (CH3) ppm; HRESI-MS m/z calcd. for C27H28N2NaO6 ([M + Na]+): 499.1840, found: 499.1832.
3-{[5′-(3″,4″-Dimethyl-2″-oxo-2″H-chromen-7″-yl)oxypentyl]amino}-4-(4‴-fluorophenylamino)cyclobut-3-ene-1,2-dione (16l). Method B. Dimethyl squarate (51 mg, 0.36 mmol), 4-fluoroaniline (35 µL, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 67 mg (40%, yellow solid). Mp > 250 °C. Rf 0.30 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.61 (s, 1H, NH-Ar), 7.70–7.61 (brs, 1H, NH), 7.66 (brd, 1H, J5″,6″= 8.3 Hz, H-5″), 7.43 (m, 2H, Ar-H), 7.22–7.15 (m, 2H, Ar-H), 6.94 (m, 2H, Ar-H), 4.09 (brt, 2H, JH,H = 6.5 Hz, OCH2), 3.62 (brs, 2H, NCH2), 2.36 (brs, 3H, CH3), 2.07 (brs, 3H, CH3), 1.79 (brquint, 2H, JH,H = 6.7 Hz, CH2), 1.64 (brquint, 2H, JH,H = 7.1 Hz, CH2), 1.50 (m, 2H, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 183.8, 180.2 (C=O), 169.1, 163.4 (C-3, C-4), 161.2, 160.6 (C-2″, C-7″), 158.0 (d, 1JC,F =238.2 Hz, C-4‴), 153.0, 146.8 (Ar-C), 135.5 (d, 4JC,F= 2.7 Hz, C-1‴), 126.1 (Ar-C), 119.7 (brd, 3JC,F = 6.2 Hz, C-2‴), 117.7 (Ar-C), 115.9, (d, 2JC,F = 22.6 Hz, C-3‴), 113.5, 112.2 Ar-C), 100.8 (C-8″), 67.9 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 14.8 (CH3), 12.8 (CH3) ppm; HRESI-MS m/z calcd. for C26H25FN2NaO5 ([M + Na]+): 487.1640, found: 487.1630.
3-[(4′-Chlorophenyl)amino]-4-{[5″-(3‴,4‴-dimethyl-2‴-oxo-2‴H-chromen-7‴-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16m). Method B. Dimethyl squarate (51 mg, 0.36 mmol), 4-chloroaniline (46 mg, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 54 mg (31%, yellow solid). Mp > 250 °C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.68 (s, 1H, NH-Ar), 7.69–7.63 (m, 1H, NH), 7.65 (d, 1H, J5‴,6‴ = 9.4 Hz, H-5‴), 7.44-7.33 (m, 4H, Ar-H), 6.92–6.90 (m, 2H, Ar-H), 4.08 (t, 2H, JH,H = 6.3 Hz, OCH2), 3.63 (brq, 2H, JH,H = 6.9 Hz, NCH2), 2.34 (brs, 3H, CH3), 2.06 (brs, 3H, CH3), 1.78 (quint, 2H, JH,H = 7.1 Hz, CH2), 1.65 (quint, 2H, JH,H = 6.8 Hz, CH2), 1.49 (quint, 2H, JH,H = 7.2 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.1, 180.1 (C=O), 169.3, 163.1 (C-3, C-4), 161.2, 160.6 (C-2‴, C-7‴), 153.0, 146.8, 138.1, 129.1, 129.0, 126.1, 119.5, 117.7, 113.5, 112.2 (Ar-C), 100.8 (C-8‴), 67.9 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 14.8 (CH3), 12.8 (CH3) ppm; HRESI-MS m/z calcd. for C26H25ClN2NaO5 ([M + Na]+): 503.1344, found: 503.1338.
3-[(4′-Bromophenyl)amino]-4-{[5″-(3‴,4‴-dimethyl-2‴-oxo-2‴H-chromen-7″-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16n). Method B. Dimethyl squarate (51 mg, 0.36 mmol), 4-bromoaniline (62 mg, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 49 mg (26%, yellow solid). Mp >250 ⁰C. Rf 0.10 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.87 (s, 1H, NH-Ar), 7.70–7.64 (m, 1H, NH), 7.65 (d, 1H, J5‴,6‴ = 8.8 Hz, H-5‴), 7.54–7.35 (m, 4H, Ar-H), 6.93–6.91 (m, 2H, Ar-H), 4.08 (brt, 2H, JH,H = 6.0 Hz, OCH2), 3.63 (m, 2H, NCH2), 2.35 (brs, 3H, CH3), 2.07 (brs, 3H, CH3), 1.78 (brquint, 2H, JH,H = 6.3 Hz, CH2), 1.65 (brquint, 2H, JH,H = 6.3 Hz, CH2), 1.49 (brquint, 2H, JH,H = 7.0 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.1, 180.1 (C=O), 169.3, 163.0 (C-3, C-4), 161.2, 160.6 (C-2‴, C-7‴), 153.0, 146.8, 138.4, 132.0, 126.0, 119.0, 117.7, 114.3, 113.4, 112.2 (Ar-C), 100.8 (C-8‴), 67.9 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 14.8 (CH3), 12.8 (CH3) ppm; HRESI-MS m/z calcd. for C26H2579BrN2NaO5 ([M + Na]+): 547.0839, found: 547.0826; m/z calcd. for C26H2581BrN2NaO5 ([M + Na]+): 549.0819, found: 549.0805.
3-{[5′-(3″,4″-Dimethyl-2″-oxo-2″H-chromen-7″-yl)oxypentyl]amino}-4-(4‴-iodophenylamino)cyclobut-3-ene-1,2-dione (16o). Method B. Dimethyl squarate (51 mg, 0.36 mmol), 4-iodoaniline (79 mg, 0.36 mmol), and 15 (100 mg, 0.36 mmol, n = 5, R2 = R3 = Me) were used. Yield: 74 mg (36%, yellow solid). Mp > 250 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.64 (s, 1H, NH-Ar), 7.70–7.62 (m, 4H, NH, H-5″, 2Ar-H), 7.27–7.22 (m, 2H, Ar-H), 6.93–6.90 (m, 2H, Ar-H), 4.07 (brt, 2H, JH,H = 6.1 Hz, OCH2), 3.62 (brs, 2H, NCH2), 2.34 (brs, 3H, CH3), 2.06 (brs, 3H, CH3), 1.77 (brquint, 2H, JH,H = 6.5 Hz, CH2), 1.62 (brquint, 2H, JH,H = 6.5 Hz, CH2), 1.49 (quint, 2H, JH,H = 6.7 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.2, 180.1 (C=O), 169.3, 163.0 (C-3, C-4), 161.2, 160.6 (C-2″, C-7″), 153.0, 146.8, 138.9, 137.9, 137.8, 126.1, 120.3, 117.7, 113.5, 112.2 (Ar-C), 100.8 (C-8″), 85.9 (C-4‴), 67.9 (OCH2), 43.6 (NCH2), 30.2 (CH2), 28.0 (CH2), 22.3 (CH2), 14.9 (CH3), 12.9 (CH3) ppm; HRESI-MS m/z calcd. for C26H25IN2NaO5 ([M + Na]+): 595.0700, found: 595.0690.
3-Phenylamino-4-{[5′-(4″-Phenyl-2″-oxo-2″H-chromen-7″-yl)oxypentyl]amino}cyclobut-3-ene-1,2-dione (16p). Method C. Dimethyl squarate (59 mg, 0.41 mmol), aniline (38 µL, 0.41 mmol), and 15 (132.6 mg, 0.41 mmol, n = 5, R2 = Ph, R3 = H) were used. Yield: 130 mg (64%, yellow solid). Mp: 209–210 °C. Rf 0.20 (1:1 cyclohexane-EtOAc). 1H-NMR (300 MHz, DMSO-d6) δ 9.61 (brs, 1H, NH-Ar), 7.67 (brs, 1H, NH), 7.58–7.49 (m, 5H, H-5″, 4Ar-H), 7.42 (m, 2H, Ar-H), 7.35–7.29 (m, 3H, Ar-H), 7.08 (d, 1H, J6″,8″ = 2.4 Hz, H-8″), 7.01 (brt, 1H, JH,H = 7.3 Hz, H-4 Ph), 6.91 (dd, 1H, J6″,8″ = 2.4 Hz, J5″,6″ = 8.9 Hz H-6″), 6.22 (brs, 1H, H-3″), 4.11 (t, 2H, JH,H = 6.3 Hz, OCH2), 3.64 (brq, 2H, JH,H = JNH,CH3 = 6.7 Hz, NCH2), 1.79 (quint, 2H, JH,H = 6.9 Hz, CH2), 1.65 (quint, 2H, JH,H = 7.3 Hz, CH2), 1.50 (quint, 2H, JH,H = 6.5 Hz, CH2) ppm; 13C-NMR (125.7 MHz, DMSO-d6) δ 184.0, 180.1 (C=O), 169.2, 163.5 (C-3, C-4), 161.9, 160.0 (C-2″, C-7″), 155.5, 155.2, 139.0, 135.0, 129.6, 129.3, 128.8, 128.4, 127.8, 122.5, 117.9, 112.8, 111.7, 111.2 (Ar-C), 101.6 (C-8″), 68.2 (OCH2), 43.6 (NCH2), 30.3 (CH2), 28.0 (CH2), 22.3 (CH2) ppm; HRESI-MS m/z calcd. for C30H26N2NaO5 [M + Na]+): 517.1734, found: 517.1730.
3.2. CA Inhibition Assays
The inhibitory properties of title compounds against CAs were determined using the stopped-flow CO2 hydrase assay, as previously reported [40,41]. All enzymes employed were recombinant, and obtained in-house as reported [40,41,42], with concentrations in the assay ranging from 5–12 nM.
3.3. Antiproliferative Assays
Minor modifications of the US National Cancer Institute (NCI) protocol were used [76].
3.4. Docking Simulations
Previously reported methodology was used [77]. For sulfonamides, deprotonated species were considered.
4. Conclusions
A vast series of novel CA inhibitors have been accessed in a straightforward fashion by combining a pharmacophore targeting CA inhibition (arylsulfonamide, coumarin) and an aromatic hydrophobic appendage through a squaramide-type tether.
Considering the inhibition of tumor-associated CAs IX and XII, sulfonamides exhibited considerably lower selectivity toward off-target enzymes (CAs I and II) than coumarin counterparts. Conjugating potency and selectivity, the best profile for p-substituted sulfonamides was found for compound 5d (p-bromophenyl derivative), with activities within the mid-nM range against CAs IX and XII (Ki = 67.6 and 85.5 nM, respectively), and good selectivity indexes (S.I. 6.8–14). Preparation of a dimer (6) strongly diminished selectivity. Shortening the distance between the squaramido and the aryl sulfonamido motifs furnished improved potency against targeted CAs; when this was accompanied by location of the sulfonamido functionality on the m-position (compound 11), the lead compound of the sub-series was achieved (low nM activities for CA XII (9.5 nM) and improved selectivities compared to AAZ; S.I. 10–63 vs. 0.48–44).
Coumarin derivatives showed in all cases outstanding selectivities (Ki > 10 µM for CA I, II). Elongation of the hydrocarbon chain connecting the coumarin skeleton provoked a clear improvement in activity against CAs IX and XII, reaching low nanomolar activities for n = 9, 12; Ki values against CA IX and CA XII were found to be 19.2 and 7.23 nM for compound 16c and 18.1, 7.91 nM for 16d, the lead derivatives of the series. Stereoelectronic effects on the appended phenyl residue had little influence on the inhibitory profiles; moreover, increase in the steric hindrance on C-3 and/or C-4 positions of the coumarin moiety had a detrimental effect.
The main interactions involving the most favorable poses of the lead compounds of the series within the catalytic active site of CA XII were predicted using docking simulations.
Although compounds prepared herein were not active against tumor cells (measured under normoxic conditions), it would be expected that in vivo treatment, where numerous tumor cells overexpress CA IX/XII isoforms as an efficient survival mechanism (hypoxic conditions), antiproliferative activities would be observed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23147685/s1.
Author Contributions
Conceptualization, Ó.L.; molecular docking and modelling, A.P. (Adrián Puerta), M.X.F., J.M.P.; data analysis, A.N., M.X.F., J.M.P., C.T.S.; synthesis, G.A., F.J.H.; biological assays: A.P. (Adrián Puerta), A.P. (Andrea Petrini), J.M.P.; writing—original draft preparation, Ó.L.; writing—review and editing, A.N., J.M.P., C.T.S., J.G.F.-B., Ó.L.; supervision, A.N., M.X.F., J.M.P., C.T.S., J.G.F.-B., Ó.L.; funding acquisition, J.M.P., C.T.S., J.G.F.-B., Ó.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry for Science and Innovation, MCIN/AEI/10.13039/501100011033 (grant number PID2020-116460RB-I00), the Junta de Andalucía (FQM-134), the Canary Islands Government (ACIISI/FEDER, UE, grant number ProID2020010101) and by the Italian Ministry for University and Research (MIUR), grant PRIN: prot. 2017XYBP2R (CTS).
Data Availability Statement
Not applicable.
Acknowledgments
A.P. (Adrián Puerta) thanks the EU Social Fund (FSE) and the Canary Islands ACIISI for a predoctoral grant TESIS2020010055. We would also like to thank the Servicio de Resonancia Magnética Nuclear, CITIUS (University of Seville) for the performance of NMR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wurm, F.R.; Klok, H.-A. Be squared: Expanding the horizon of squaric acid-mediated conjugations. Chem. Soc. Rev. 2013, 42, 8220–8236. [Google Scholar] [CrossRef] [PubMed]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.A.; Kumawat, L.K.; Mao, N.; Stephens, J.C.; Elmes, R.B.P. The versatility of squaramides: From supramolecular chemistry to chemical biology. Chemistry 2019, 5, 1398–1485. [Google Scholar] [CrossRef]
- Kumawat, L.K.; Abogunrin, A.A.; Kickham, M.; Pardeshi, J.; Fenelon, O.; Schroeder, M.; Elmes, R.B.P. Squara-mide—naphthalimide conjugates as “turn-on” fluorescent sensors for bromide through an aggregation-disaggregation approach. Front. Chem. 2019, 7, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaleskaya, M.; Jagleniec, D.; Romański, J. Macrocyclic squaramides as ion pair receptors and fluorescent sensors selective towards sulfates. Dalton Trans. 2021, 50, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Zaleskaya, M.; Jagleniec, D.; Karbarz, M.; Dobrzycki, Ł.; Romański, J. Squaramide based ion pair receptors possessing ferrocene as a signaling unit. Inorg. Chem. Front. 2020, 7, 972–983. [Google Scholar] [CrossRef]
- Jiang, M.; Li, P.; Wu, P.; Zhang, F.; Tian, X.; Deng, C.; Wang, J. A squaramide-based metal–organic framework as a luminescent sensor for the detection of lactose in aqueous solution and in milk. Chem. Commun. 2018, 54, 9131–9134. [Google Scholar] [CrossRef] [Green Version]
- Alemán, J.; Parra, A.; Jiang, H.; Jørgensen, K.A. Squaramides: Bridging from molecular recognition to bifunctional organoca-talysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P. “Push−pull π+/π−” (PPππ) systems in catalysis. ACS Catal. 2017, 7, 6430–6439. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.-Q.; Du, D.-M. Recent advances in squaramide-catalyzed asymmetric Mannich reactions. Adv. Synth. Catal. 2020, 362, 4487–4512. [Google Scholar] [CrossRef]
- Sonsona, I.G.; Vicenzi, A.; Guidotti, M.; Bisag, G.D.; Fochi, M.; Herrera, R.P.; Bernardi, L. Investigation of squaramide catalysts in the aldol reaction en route to funapide. Eur. J. Org. Chem. 2022, 2022, e202101254. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Mohammadi, S.; Divar, M.; Gimeno, M.C.; Herrera, R.P. Asymmetric fluorination reactions promoted by chiral hydrogen-bonding-based organocatalysts. Adv. Synth. Catal. 2020, 362, 5275–5300. [Google Scholar] [CrossRef]
- Biswas, A.; Ghosh, A.; Shankhdhar, R.; Chatterjee, I. Squaramide catalyzed asymmetric synthesis of five- and six-membered rings. Asian J. Org. Chem. 2021, 10, 1345–1376. [Google Scholar] [CrossRef]
- Chasák, J.; Šlachtová, V.; Urban, M.; Brulíková, L. Squaric acid analogues in medicinal chemistry. Eur. J. Med. Chem. 2021, 209, 112872. [Google Scholar] [CrossRef] [PubMed]
- Agnew-Francis, K.A.; Williams, C.M. Squaramides as bioisosteres in contemporary drug design. Chem. Rev. 2020, 120, 11616–11650. [Google Scholar] [CrossRef] [PubMed]
- Molodtsov, V.; Fleming, P.R.; Eyermann, C.J.; Ferguson, A.D.; Foulk, A.; McKinney, D.C.; Masse, C.E.; Buurman, E.T.; Mura-kami, K.S. X-ray crystal structures of Escherichia coli RNA polymerase with switch region binding inhibitors enable rational design of squaramides with an improved fraction unbound to human plasma protein. J. Med. Chem. 2015, 58, 3156–3171. [Google Scholar] [CrossRef] [Green Version]
- Fournier, J.-F.; Bhurruth-Alcor, Y.; Musicki, B.; Aubert, J.; Aurelly, M.; Bouix-Peter, C.; Bouquet, K.; Chantalat, L.; Delorme, M.; Drean, B.; et al. Squaramides as novel class I and IIB histone deacetylase inhibitors for topical treatment of cutaneous T-cell lymphoma. Bioorg. Med. Chem. Lett. 2018, 28, 2985–2992. [Google Scholar] [CrossRef]
- Svobodova, B.; Mezeiova, E.; Hepnarova, V.; Hrabinova, M.; Muckova, L.; Kobrlova, T.; Jun, D.; Soukup, O.; Jimeno, M.L.; Marco-Contelles, J.; et al. Exploring structure-activity relationship in tacrine-squaramide derivatives as potent cholin-esterase inhibitors. Biomolecules 2019, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Olmo, F.; Rotger, C.; Ramírez-Macías, I.; Martínez, L.; Marín, C.; Carreras, L.; Urbanová, K.; Vega, M.; Chaves-Lemaur, G.; Sampedro, Á.; et al. Synthesis and biological evaluation of N,N‘-squaramides with high in vivo efficacy and low toxicity: Toward a low-cost drug against chagas disease. J. Med. Chem. 2014, 57, 987–999. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Li, G.; Fu, L.; Zhang, D.; Lin, Z.; Huang, H.; Lu, Y. Design, synthesis and biological evaluation of diamino substituted cyclobut-3-ene-1,2-dione derivatives for the treatment of drug-resistant tuberculosis. Eur. J. Med. Chem. 2020, 206, 112538. [Google Scholar] [CrossRef]
- Biselli, S.; Alencastre, I.; Tropmann, K.; Erdmann, D.; Chen, M.; Littmann, T.; Maia, A.F.; Gómez-Lázaro, M.; Tanaka, M.; Ozawa, T.; et al. Fluorescent H2 receptor squaramide-type antagonists: Synthesis, characterization, and applications. ACS Med. Chem. Lett. 2020, 11, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreira, V.; Alegre-Requena, J.V.; Herrera, R.P.; Marzo, I.; Gimeno, M.C. Synthesis of luminescent squaramide monoesters: Cytotoxicity and cell imaging studies in HeLa cells. RSC Adv. 2016, 6, 14171–14177. [Google Scholar] [CrossRef] [Green Version]
- Morales, K.; Samper, K.G.; Peña, Q.; Hernando, J.; Lorenzo, J.; Rodríguez-Diéguez, A.; Capdevila, M.; Figueredo, M.; Palacios, Ó.; Bayón, P. Squaramide-based Pt(II) complexes as potential oxygen-regulated light-triggered photocages. Inorg. Chem. 2018, 57, 15517–15525. [Google Scholar] [CrossRef]
- Grus, T.; Lahnif, H.; Klasen, B.; Moon, E.-S.; Greifenstein, L.; Roesch, F. Squaric acid-based radiopharmaceuticals for tumor imaging and therapy. Bioconjug. Chem. 2021, 32, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, C.; Lim, S.W.; Adhikari, A.; Andring, J.T.; McKenna, R.; Ghim, C.-M.; Kim, C.U. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 2020, 11, 4557. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.D.; Pinard, M.; McKenna, R.; Silverman, D. Catalytic mechanism of α-class carbonic anhydrases: CO2 hydration and proton transfer. Subcell. Biochem. 2014, 75, 31–52. [Google Scholar] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Nocentini, A.; Supuran, C.T.; Capasso, C. An overview on the recently discovered iota-carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2021, 36, 1988–1995. [Google Scholar] [CrossRef]
- Nocentini, A.; Donald, W.A.; Supuran, C.T. Human carbonic anhydrases: Tissue distribution, physiological role, and druggability. In Carbonic Anhydrases-Biochemistry and Pharmacology of an Evergreen Pharmaceutical Target; Supuran, C.T., Nocentini, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 151–185. [Google Scholar]
- Aspatwar, A.; Tolvanen, M.E.E.; Parkkila, S. An update on carbonic anhydrase-related proteins VIII, X and XI. J. Enzym. Inhib. Med. Chem. 2013, 28, 1129–1142. [Google Scholar] [CrossRef]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Cuffaro, D.; Ciccone, L.; Orlandini, E.; Nencetti, S.; Nuti, E.; Rossello, A.; Supuran, C.T. Activation of carbonic anhydrases from human brain by amino alcohol oxime ethers: Towards human carbonic anhydrase VII selective activators. J. Enzym. Inhib. Med. Chem. 2021, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ- δ-, ζ- and η- class of carbonic anhydrases with amines and amino acids: A review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef]
- Supuran, C.T. Multitargeting approaches involving carbonic anhydrase inhibitors: Hybrid drugs against a variety of disorders. J. Enzym. Inhib. Med. Chem. 2021, 36, 1702–1714. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]
- Supuran, C.T.; Capasso, C. Antibacterial carbonic anhydrase inhibitors: An update on the recent literature. Expert Opin. Ther. Pat. 2020, 30, 963–982. [Google Scholar] [CrossRef]
- Mincione, F.; Nocentini, A.; Supuran, C.T. Advances in the discovery of novel agents for the treatment of glaucoma. Expert Opin. Drug Discov. 2021, 16, 1209–1225. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Carta, F.; Cozzi, A.; Ghelardini, C.; Supuran, C.T. Carbonic anhydrase inhibition for the management of cerebral ischemia: In vivo evaluation of sulfonamide and coumarin inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Berrino, E.; Milazzo, L.; Micheli, L.; Vullo, D.; Angeli, A.; Bozdag, M.; Nocentini, A.; Menicatti, M.; Bartolucci, G.; Mannelli, L.D.C.; et al. Synthesis and evaluation of carbonic anhydrase inhibitors with carbon monoxide releasing properties for the management of rheumatoid arthritis. J. Med. Chem. 2019, 62, 7233–7249. [Google Scholar] [CrossRef]
- Costa, G.; Carta, F.; Ambrosio, F.A.; Artese, A.; Ortuso, F.; Moraca, F.; Rocca, R.; Romeo, I.; Lupia, A.; Maruca, A.; et al. A computer-assisted discovery of novel potential anti-obesity compounds as selective carbonic anhydrase VA inhibitors. Eur. J. Med. Chem. 2019, 181, 111565. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Lucarini, E.; Di Cesare Mannelli, L.; Ghelardini, C.; D’Ambrosio, K.; Buonanno, M.; Monti, S.M.; De Simone, G.; Angeli, A.; Supuran, C.T.; et al. Sultam based carbonic anhydrase VII inhibitors for the management of neuropathic pain. Eur. J. Med. Chem. 2022, 227, 113956. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Cerri, C.; Nencetti, S.; Orlandini, E. Carbonic anhydrase inhibitors and epilepsy: State of the art and future per-spectives. Molecules 2021, 26, 6380. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.O.; Petreni, A.; Supuran, C.T.; El-Hamamsy, M.H. Discovery of new carbonic anhydrase IX inhibitors as anticancer agents by toning the hydrophobic and hydrophilic rims of the active site to encounter the dual-tail approach. Eur. J. Med. Chem. 2022, 232, 114190. [Google Scholar] [CrossRef]
- Lee, S.-H.; Griffiths, J.R. How and why are cancers acidic? Carbonic anhydrase IX and the homeostatic control of tumour ex-tracellular pH. Cancers 2020, 12, 1616. [Google Scholar] [CrossRef]
- Sarnella, A.; Ferrara, Y.; Auletta, L.; Albanese, S.; Cerchia, L.; Alterio, V.; De Simone, G.; Supuran, C.T.; Zannetti, A. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J. Exp. Clin. Cancer Res. 2022, 41, 122. [Google Scholar] [CrossRef]
- Lemon, N.; Canepa, E.; Ilies, M.A.; Fossati, S. Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer’s disease and stroke. Front. Aging Neurosci. 2021, 13, 772278. [Google Scholar] [CrossRef]
- Nocentini, A.; Angeli, A.; Carta, F.; Winum, J.-Y.; Zalubovskis, R.; Carradori, S.; Capasso, C.; Donald, W.A.; Supuran, C.T. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J. Enzym. Inhib. Med. Chem. 2021, 36, 561–580. [Google Scholar] [CrossRef]
- Supuran, C.T. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 671–686. [Google Scholar] [CrossRef]
- Supuran, C.T. Coumarin carbonic anhydrase inhibitors from natural sources. J. Enzym. Inhib. Med. Chem. 2020, 35, 1462–1470. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Supuran, C.T.; McKenna, R. Non-classical inhibition of carbonic anhydrase. Int. J. Mol. Sci. 2016, 17, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Song, Y.; Zhan, P.; Zhang, Q.; Liu, X. Conformational restriction: An effective tactic in ‘follow-on’-based drug discovery. Future Med. Chem. 2014, 6, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lu, Q.-B.; Honek, J.F. Squarate-based carbocyclic nucleosides: Syntheses, computational analyses and anticancer/antiviral evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sopeña, S.; Martin, E.; Escudero-Adán, E.C.; Kleij, A.W. Pushing the limits with squaramide-based organocatalysts in cyclic carbonate synthesis. ACS Catal. 2017, 7, 3532–3539. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambare, A.S.; Khan, F.A.K.; Zambare, S.P.; Shinde, S.D.; Sangshetti, J.N. Recent advances in the synthesis of coumarin de-rivatives via Pechmann condensation. Curr. Org. Chem. 2016, 20, 798–828. [Google Scholar] [CrossRef] [Green Version]
- Krasavin, M.; Kalinin, S.; Sharonova, T.; Supuran, C.T. Inhibitory activity against carbonic anhydrase IX and XII as a candidate selection criterion in the development of new anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 1555–1561. [Google Scholar] [CrossRef]
- Larcher, A.; Nocentini, A.; Supuran, C.T.; Winum, J.-Y.; van der Lee, A.; Vasseur, J.J.; Laurencin, D.; Smietana, M. Bis-benzoxaboroles: Design, synthesis, and biological evaluation as carbonic anhydrase inhibitors. ACS Med. Chem. Lett. 2019, 10, 1205–1210. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T. Experimental carbonic anhydrase inhibitors for the treatment of hypoxic tumors. J. Exp. Pharmacol. 2020, 12, 603–617. [Google Scholar] [CrossRef]
- Chafe, S.C.; Vizeacoumar, F.S.; Venkateswaran, G.; Nemirovsky, O.; Awrey, S.; Brown, W.S.; McDonald, P.C.; Carta, F.; Metcalfe, A.; Karasinska, J.M.; et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci. Adv. 2021, 7, eabj0364. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; dem Keller, U.A.; Leung, S.; Huntsman, D.; et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.C.; Chia, S.; Bedard, P.L.; Chu, Q.; Lyle, M.; Tang, L.; Singh, M.; Zhang, Z.; Supuran, C.T.; Renouf, D.J.; et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am. J. Clin. Oncol. 2020, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.-A.; Riganti, C. Carbonic anhydrase XII inhibitors overcome P-glycoprotein–mediated resistance to temozolomide in glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-mediated multidrug-resistant cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar] [PubMed]
- Nocentini, A.; Trallori, E.; Singh, S.; Lomelino, C.L.; Bartolucci, G.; Di Cesare Mannelli, L.; Ghelardini, C.; McKenna, R.; Gratteri, P.; Supuran, C.T. 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides selectively target the tumor-associated carbonic anhydrase isoforms IX and XII showing hypoxia-enhanced antiproliferative profiles. J. Med. Chem. 2018, 61, 10860–10874. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [Green Version]
- Meleddu, R.; Deplano, S.; Maccioni, E.; Ortuso, F.; Cottiglia, F.; Secci, D.; Onali, A.; Sanna, E.; Angeli, A.; Angius, R.; et al. Selective inhibition of carbonic anhydrase IX and XII by coumarin and psoralen derivatives. J. Enzym. Inhib. Med. Chem. 2021, 36, 685–692. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the General Solubility Equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.swissadme.ch/index.php (accessed on 26 June 2022).
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpka: A web server for small molecule pKa prediction using a graph-convolutional neural network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://xundrug.cn/molgpka (accessed on 26 June 2022).
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.L. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the Or-ganometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Elshaflu, H.; Todorović, T.R.; Nikolić, M.; Lolić, A.; Višnjevac, A.; Hagenow, S.; Padrón, J.M.; García-Sosa, A.T.; Djordjević, I.S.; Grubišić, S.; et al. Selenazolyl-hydrazones as novel selective MAO inhibitors with antiproliferative and an-tioxidant activities: Experimental and in-silico studies. Front. Chem. 2018, 6, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes-Aguilar, A.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Puerta, A.; Fernandes, M.X.; Padrón, J.M.; Petreni, A.; Nocentini, A.; et al. 2-Aminobenzoxazole-appended coumarins as potent and selective inhibitors of tumour-associated carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2022, 37, 168–177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).