Silencing of the Ca2+ Channel ORAI1 Improves the Multi-Systemic Phenotype of Tubular Aggregate Myopathy (TAM) and Stormorken Syndrome (STRMK) in Mice
Abstract
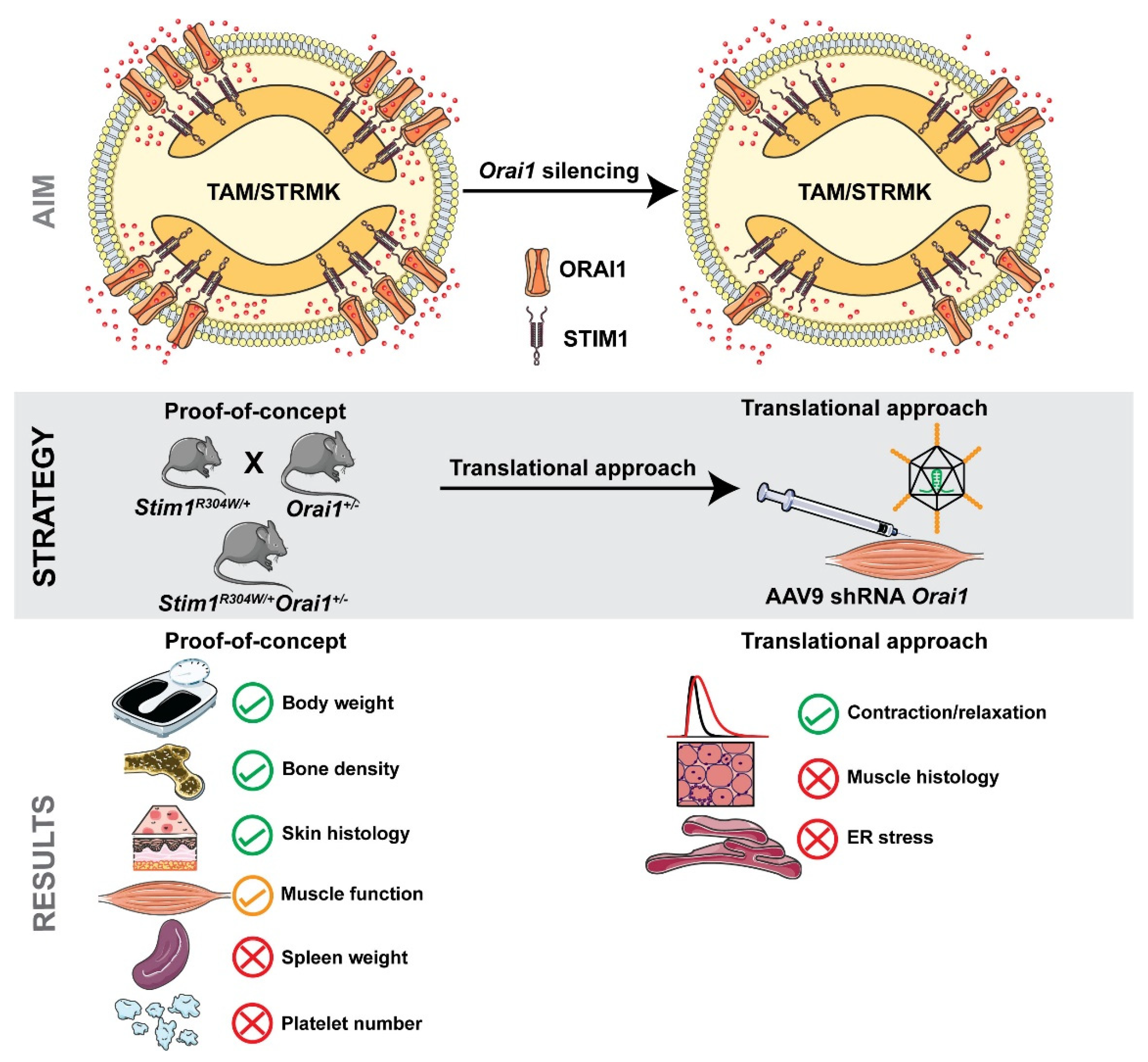
1. Introduction
2. Results
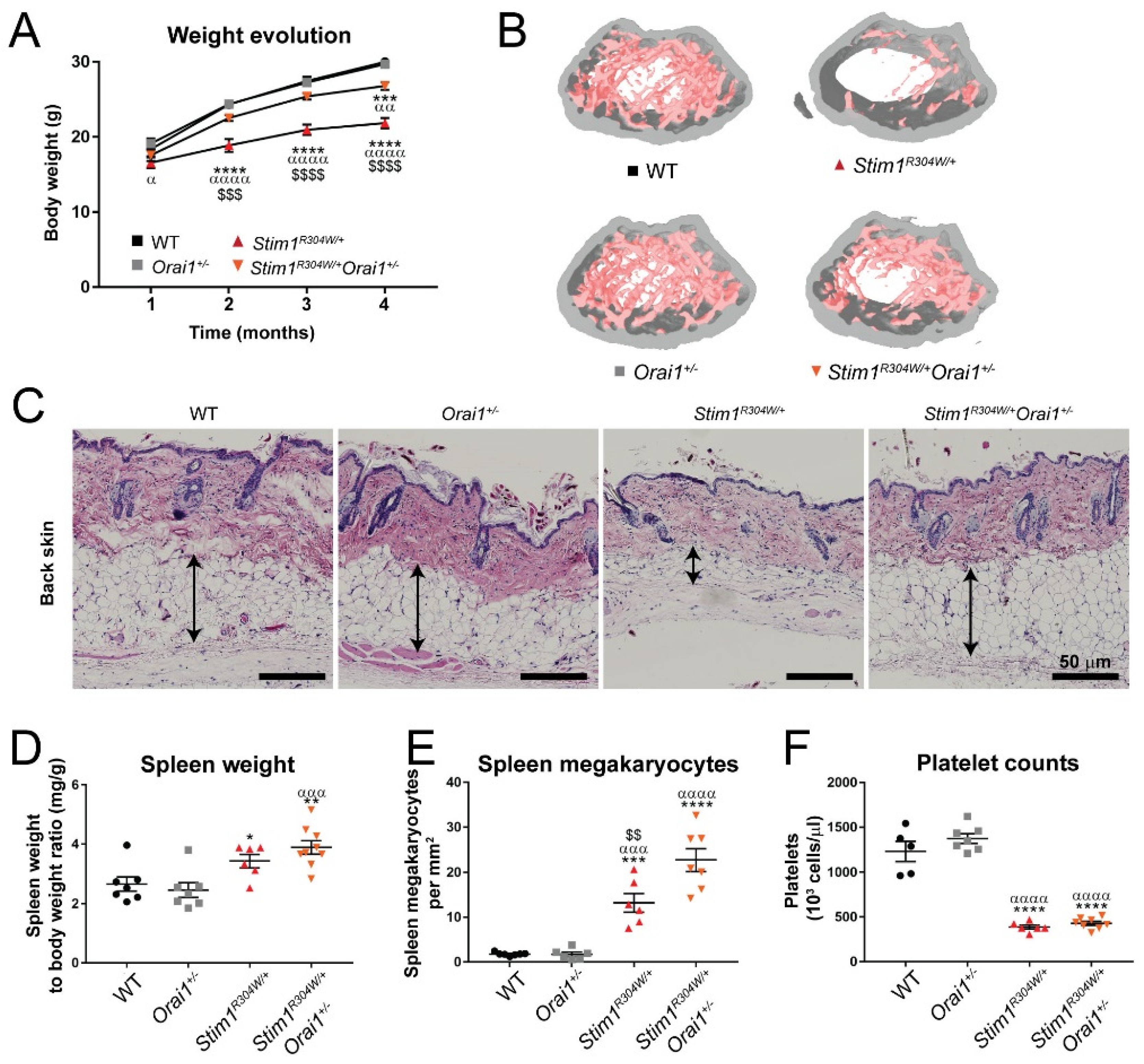
2.1. Normalized Birth Ratio, and Improved Body Size and Weight Gain of Stim1R304W/+Orai1+/− Mice
2.2. Improved Bone Architecture in Stim1R304W/+Orai1+/− Mice
2.3. Unchanged Skin, Spleen, and Platelet Phenotypes in Stim1R304W/+Orai1+/− Mice
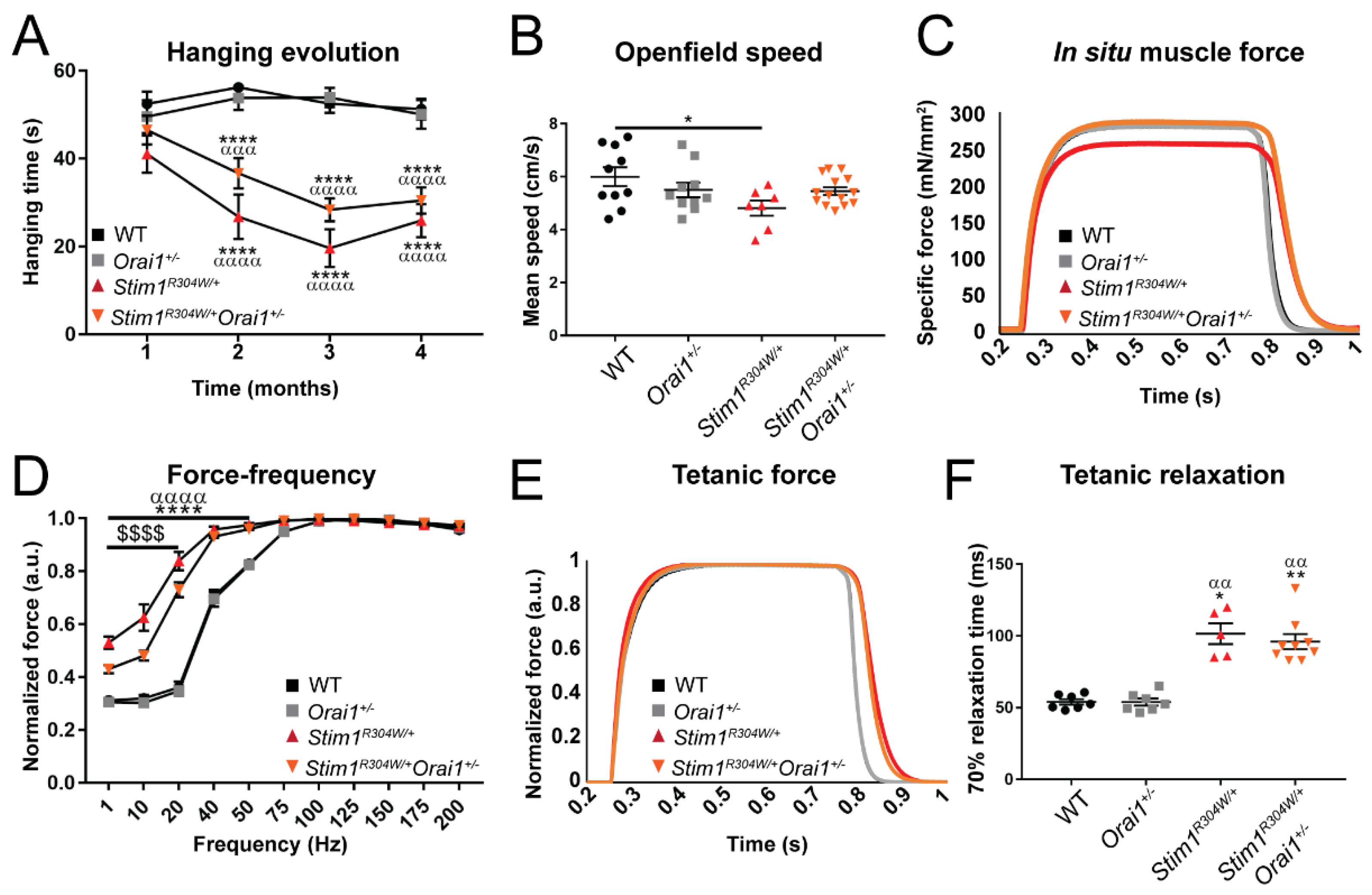
2.4. Improved Muscle Contraction Properties in Stim1R304W/+Orai1+/− Mice
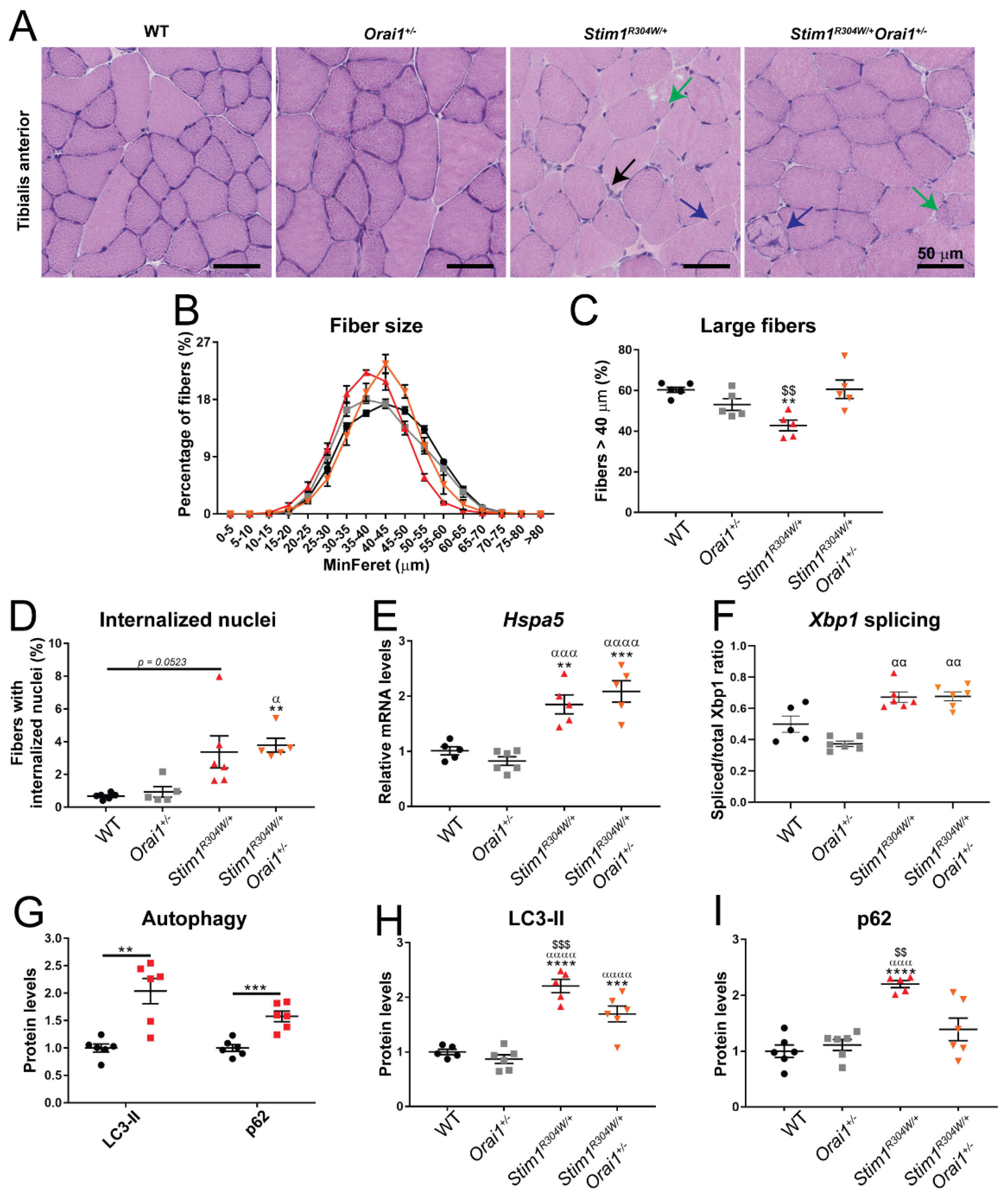
2.5. Normalized Muscle Fiber Size and Improved Autophagy Markers in Stim1R304W/+Orai1+/− Mice
2.6. shRNA-Driven Orai1 Silencing Partially Reverses the Muscle Phenotype of Stim1R304W/+ Mice
3. Discussion
3.1. ORAI1 as the Main Target to Treat the Multi-Systemic TAM/STRMK Phenotype
3.2. Orai1 Downregulation Improves Several but Not All Multi-Systemic TAM/STRMK Phenotype
3.3. shRNA-Mediated Silencing of Orai1 Partially Improved Muscle Function
3.4. Concluding Remarks
4. Materials and Methods
4.1. Animals
4.2. Hanging and Open Field Tests
4.3. In Situ Muscle Force
4.4. Micro-Computerized Bone Tomography (µCT)
4.5. Bleeding Test and Blood Counts
4.6. Muscle, Spleen, and Skin Histology
4.7. Gene Expression and Protein Studies
4.8. shRNA Cloning and AAV Production
4.9. shRNA Screening and Intramuscular AAV Injection
4.10. Study Randomization and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Zheng, L.; Li, G.Y.; Plevin, M.J.; Ikura, M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 2008, 135, 110–122. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef]
- Gattineni, J. Inherited disorders of calcium and phosphate metabolism. Curr. Opin. Pediatr. 2014, 26, 215–222. [Google Scholar] [CrossRef]
- Silva-Rojas, R.; Laporte, J.; Bohm, J. STIM1/ORAI1 loss-of-function and gain-of-function mutations inversely impact on SOCE and calcium homeostasis and cause multi-systemic mirror diseases. Front. Physiol. 2020, 11, 604941. [Google Scholar] [CrossRef]
- Picard, C.; McCarl, C.A.; Papolos, A.; Khalil, S.; Luthy, K.; Hivroz, C.; LeDeist, F.; Rieux-Laucat, F.; Rechavi, G.; Rao, A.; et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 2009, 360, 1971–1980. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Feske, S. Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 2015, 1356, 45–79. [Google Scholar] [CrossRef]
- Bohm, J.; Chevessier, F.; Maues De Paula, A.; Koch, C.; Attarian, S.; Feger, C.; Hantai, D.; Laforet, P.; Ghorab, K.; Vallat, J.M.; et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am. J. Hum. Genet. 2013, 92, 271–278. [Google Scholar] [CrossRef]
- Bohm, J.; Laporte, J. Gain-of-function mutations in STIM1 and ORAI1 causing tubular aggregate myopathy and Stormorken syndrome. Cell Calcium 2018, 76, 1–9. [Google Scholar] [CrossRef]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca2+ channels. Hum. Mol. Genet. 2015, 24, 637–648. [Google Scholar] [CrossRef]
- Misceo, D.; Holmgren, A.; Louch, W.E.; Holme, P.A.; Mizobuchi, M.; Morales, R.J.; De Paula, A.M.; Stray-Pedersen, A.; Lyle, R.; Dalhus, B.; et al. A dominant STIM1 mutation causes Stormorken syndrome. Hum. Mutat. 2014, 35, 556–564. [Google Scholar] [CrossRef]
- Morin, G.; Biancalana, V.; Echaniz-Laguna, A.; Noury, J.B.; Lornage, X.; Moggio, M.; Ripolone, M.; Violano, R.; Marcorelles, P.; Marechal, D.; et al. Tubular aggregate myopathy and Stormorken syndrome: Mutation spectrum and genotype/phenotype correlation. Hum. Mutat. 2020, 41, 17–37. [Google Scholar] [CrossRef]
- Morin, G.; Bruechle, N.O.; Singh, A.R.; Knopp, C.; Jedraszak, G.; Elbracht, M.; Bremond-Gignac, D.; Hartmann, K.; Sevestre, H.; Deutz, P.; et al. Gain-of-function mutation in STIM1 (P.R304W) is associated with Stormorken syndrome. Hum. Mutat. 2014, 35, 1221–1232. [Google Scholar] [CrossRef]
- Nesin, V.; Wiley, G.; Kousi, M.; Ong, E.C.; Lehmann, T.; Nicholl, D.J.; Suri, M.; Shahrizaila, N.; Katsanis, N.; Gaffney, P.M.; et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc. Natl. Acad. Sci. USA 2014, 111, 4197–4202. [Google Scholar] [CrossRef]
- Garibaldi, M.; Fattori, F.; Riva, B.; Labasse, C.; Brochier, G.; Ottaviani, P.; Sacconi, S.; Vizzaccaro, E.; Laschena, F.; Romero, N.B.; et al. A novel gain-of-function mutation in ORAI1 causes late-onset tubular aggregate myopathy and congenital miosis. Clin. Genet. 2017, 91, 780–786. [Google Scholar] [CrossRef]
- Harris, E.; Burki, U.; Marini-Bettolo, C.; Neri, M.; Scotton, C.; Hudson, J.; Bertoli, M.; Evangelista, T.; Vroling, B.; Polvikoski, T.; et al. Complex phenotypes associated with STIM1 mutations in both coiled coil and EF-hand domains. Neuromuscul. Disord. 2017, 27, 861–872. [Google Scholar] [CrossRef]
- Claeys, T.; Goosens, V.; Race, V.; Theys, T.; Thal, D.R.; Depuydt, C.E.; Claeys, K.G. Clinical and muscle MRI features in a family with tubular aggregate myopathy and novel STIM1 mutation. Neuromuscul. Disord. 2020, 30, 709–718. [Google Scholar] [CrossRef]
- Cordero-Sanchez, C.; Riva, B.; Reano, S.; Clemente, N.; Zaggia, I.; Ruffinatti, F.A.; Potenzieri, A.; Pirali, T.; Raffa, S.; Sangaletti, S.; et al. A luminal EF-hand mutation in STIM1 in mice causes the clinical hallmarks of tubular aggregate myopathy. Dis. Model. Mech. 2019, 13, dmm041111. [Google Scholar] [CrossRef]
- Oh-Hora, M.; Yamashita, M.; Hogan, P.G.; Sharma, S.; Lamperti, E.; Chung, W.; Prakriya, M.; Feske, S.; Rao, A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 2008, 9, 432–443. [Google Scholar] [CrossRef]
- Silva-Rojas, R.; Treves, S.; Jacobs, H.; Kessler, P.; Messaddeq, N.; Laporte, J.; Bohm, J. STIM1 over-activation generates a multi-systemic phenotype affecting the skeletal muscle, spleen, eye, skin, bones and immune system in mice. Hum. Mol. Genet 2019, 28, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Gwack, Y.; Srikanth, S.; Oh-Hora, M.; Hogan, P.G.; Lamperti, E.D.; Yamashita, M.; Gelinas, C.; Neems, D.S.; Sasaki, Y.; Feske, S.; et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell Biol. 2008, 28, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rojas, R.; Charles, A.L.; Djeddi, S.; Geny, B.; Laporte, J.; Bohm, J. Pathophysiological effects of overactive STIM1 on murine muscle function and structure. Cells 2021, 10, 1730. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Braun, A.; Varga-Szabo, D.; Beyersdorf, N.; Schneider, B.; Zeitlmann, L.; Hanke, P.; Schropp, P.; Muhlstedt, S.; Zorn, C.; et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J. Clin. Invest. 2007, 117, 3540–3550. [Google Scholar] [CrossRef]
- Gamage, T.H.; Gunnes, G.; Lee, R.H.; Louch, W.E.; Holmgren, A.; Bruton, J.D.; Lengle, E.; Kolstad, T.R.S.; Revold, T.; Amundsen, S.S.; et al. STIM1 R304W causes muscle degeneration and impaired platelet activation in mice. Cell Calcium 2018, 76, 87–100. [Google Scholar] [CrossRef]
- Chen, Y.; Ramachandran, A.; Zhang, Y.; Koshy, R.; George, A. The ER Ca2+ sensor STIM1 can activate osteoblast and odontoblast differentiation in mineralized tissues. Connect. Tissue Res. 2018, 59, 6–12. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Inoue, S.; Furuta, J.; Koguchi-Yoshioka, H.; Nakamura, Y.; Watanabe, R.; Okiyama, N.; Fujisawa, Y.; Enokizono, T.; Fukushima, H.; et al. Sweat retention anhidrosis associated with tubular aggregate myopathy. Br. J. Dermatol. 2019, 181, 1104–1106. [Google Scholar] [CrossRef]
- Markello, T.; Chen, D.; Kwan, J.Y.; Horkayne-Szakaly, I.; Morrison, A.; Simakova, O.; Maric, I.; Lozier, J.; Cullinane, A.R.; Kilo, T.; et al. York platelet syndrome is a CRAC channelopathy due to gain-of-function mutations in STIM1. Mol. Genet. Metab. 2015, 114, 474–482. [Google Scholar] [CrossRef]
- Schneider, M.F.; Chandler, W.K. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature 1973, 242, 244–246. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, D.; Nagaraj, R.Y.; Nosek, T.A.; Nishi, M.; Takeshima, H.; Cheng, H.; Ma, J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 2002, 4, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yoshida, M.; Brotto, L.; Takeshima, H.; Weisleder, N.; Hirata, Y.; Nosek, T.M.; Ma, J.; Brotto, M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol. Genom. 2005, 23, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.A.; Lin, Y.; Pessin, J.E. Skeletal muscle autophagy: A new metabolic regulator. Trends Endocrinol. Metab. 2013, 24, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Robinson, L.J.; Huang, C.L.; Sun, L.; Friedman, P.A.; Schlesinger, P.H.; Zaidi, M. Calcium and bone disease. Biofactors 2011, 37, 159–167. [Google Scholar] [CrossRef]
- Eapen, A.; Sundivakkam, P.; Song, Y.; Ravindran, S.; Ramachandran, A.; Tiruppathi, C.; George, A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J. Biol. Chem. 2010, 285, 36339–36351. [Google Scholar] [CrossRef]
- Van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Jardin, I.; Smani, T.; Rosado, J.A. Regulation of platelet function by Orai, STIM and TRP. Adv. Exp. Med. Biol. 2016, 898, 157–181. [Google Scholar] [CrossRef]
- Numaga-Tomita, T.; Putney, J.W. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J. Cell Sci. 2013, 126, 605–612. [Google Scholar] [CrossRef]
- Vandenberghe, M.; Raphael, M.; Lehen’kyi, V.; Gordienko, D.; Hastie, R.; Oddos, T.; Rao, A.; Hogan, P.G.; Skryma, R.; Prevarskaya, N. ORAI1 calcium channel orchestrates skin homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E4839–E4848. [Google Scholar] [CrossRef]
- Concepcion, A.R.; Vaeth, M.; Wagner, L.E., 2nd; Eckstein, M.; Hecht, L.; Yang, J.; Crottes, D.; Seidl, M.; Shin, H.P.; Weidinger, C.; et al. Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J. Clin. Invest. 2016, 126, 4303–4318. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. 1), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Bohm, J.; Bulla, M.; Urquhart, J.E.; Malfatti, E.; Williams, S.G.; O’Sullivan, J.; Szlauer, A.; Koch, C.; Baranello, G.; Mora, M.; et al. ORAI1 Mutations with distinct channel gating defects in tubular aggregate myopathy. Hum. Mutat. 2017, 38, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Del Re, V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Bohm, J.; Lornage, X.; Chevessier, F.; Birck, C.; Zanotti, S.; Cudia, P.; Bulla, M.; Granger, F.; Bui, M.T.; Sartori, M.; et al. CASQ1 mutations impair calsequestrin polymerization and cause tubular aggregate myopathy. Acta Neuropathol. 2018, 135, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Goonasekera, S.A.; Davis, J.; Kwong, J.Q.; Accornero, F.; Wei-LaPierre, L.; Sargent, M.A.; Dirksen, R.T.; Molkentin, J.D. Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum. Mol. Genet. 2014, 23, 3706–3715. [Google Scholar] [CrossRef]
- McCarl, C.A.; Khalil, S.; Ma, J.; Oh-hora, M.; Yamashita, M.; Roether, J.; Kawasaki, T.; Jairaman, A.; Sasaki, Y.; Prakriya, M.; et al. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J. Immunol. 2010, 185, 5845–5858. [Google Scholar] [CrossRef]
- Braun, A.; Varga-Szabo, D.; Kleinschnitz, C.; Pleines, I.; Bender, M.; Austinat, M.; Bosl, M.; Stoll, G.; Nieswandt, B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 2009, 113, 2056–2063. [Google Scholar] [CrossRef]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016, 30, 4109–4119. [Google Scholar] [CrossRef]
- Berlansky, S.; Humer, C.; Sallinger, M.; Frischauf, I. More than just simple interaction between STIM and Orai proteins: CRAC channel function enabled by a network of interactions with regulatory proteins. Int. J. Mol. Sci. 2021, 22, 471. [Google Scholar] [CrossRef]
- Seim, I.; Ma, S.; Gladyshev, V.N. Gene expression signatures of human cell and tissue longevity. NPJ Aging Mech. Dis. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Castets, P.; Frank, S.; Sinnreich, M.; Ruegg, M.A. “Get the balance right”: Pathological significance of autophagy perturbation in neuromuscular disorders. J. Neuromuscul. Dis. 2016, 3, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Fetalvero, K.M.; Yu, Y.; Goetschkes, M.; Liang, G.; Valdez, R.A.; Gould, T.; Triantafellow, E.; Bergling, S.; Loureiro, J.; Eash, J.; et al. Defective autophagy and mTORC1 signaling in myotubularin null mice. Mol. Cell Biol. 2013, 33, 98–110. [Google Scholar] [CrossRef]
- Kuno, A.; Hosoda, R.; Sebori, R.; Hayashi, T.; Sakuragi, H.; Tanabe, M.; Horio, Y. Resveratrol ameliorates mitophagy disturbance and improves cardiac pathophysiology of dystrophin-deficient mdx mice. Sci. Rep. 2018, 8, 15555. [Google Scholar] [CrossRef]
- Begam, M.; Abro, V.M.; Mueller, A.L.; Roche, J.A. Sodium 4-phenylbutyrate reduces myofiber damage in a mouse model of Duchenne muscular dystrophy. Appl. Physiol. Nutr. Metab. 2016, 41, 1108–1111. [Google Scholar] [CrossRef]
- Lee, C.S.; Hanna, A.D.; Wang, H.; Dagnino-Acosta, A.; Joshi, A.D.; Knoblauch, M.; Xia, Y.; Georgiou, D.K.; Xu, J.; Long, C.; et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat. Commun. 2017, 8, 14659. [Google Scholar] [CrossRef]
- Avila, G.; Dirksen, R.T. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J. Gen. Physiol. 2001, 118, 277–290. [Google Scholar] [CrossRef]
- Dirksen, R.T.; Avila, G. Altered ryanodine receptor function in central core disease: Leaky or uncoupled Ca2+ release channels? Trends Cardiovasc. Med. 2002, 12, 189–197. [Google Scholar] [CrossRef]
- Kraeva, N.; Zvaritch, E.; Rossi, A.E.; Goonasekera, S.A.; Zaid, H.; Frodis, W.; Kraev, A.; Dirksen, R.T.; Maclennan, D.H.; Riazi, S. Novel excitation-contraction uncoupled RYR1 mutations in patients with central core disease. Neuromuscul. Disord. 2013, 23, 120–132. [Google Scholar] [CrossRef][Green Version]
- Riva, B.; Pessolano, E.; Quaglia, E.; Cordero-Sanchez, C.; Bhela, I.P.; Topf, A.; Serafini, M.; Cox, D.; Harris, E.; Garibaldi, M.; et al. STIM1 and ORAI1 mutations leading to tubular aggregate myopathies are sensitive to the store-operated Ca2+-entry modulators CIC-37 and CIC-39. Cell Calcium 2022, 105, 102605. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, D.; Murray, F.; Parekh, A.B. Store-operated Ca2+ channels: Mechanism, function, pharmacology, and therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 629–654. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Zheng, X.F.; Garces Suarez, F.; Raftery, J.M.; Quinlan, K.G.; Yang, N.; North, K.N.; Houweling, P.J. Evidence based selection of commonly used RT-qPCR reference genes for the analysis of mouse skeletal muscle. PLoS ONE 2014, 9, e88653. [Google Scholar] [CrossRef]
- Gonzalez, V.; Paneda, C.; Martinez, T.; Guerra, A.; Monteiro, S.; Vargas, B.; Bleau, A.M.; Ruz, V.; Jimenez, A.I. Development of a RNAi therapeutic for the treatment of allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 2018, 59, 5567. [Google Scholar]
- Jimenez, A.I.; Martinez, T.; Gonzalez, V.; Martinez-Garcia, C.; Paneda, C. Development of a RNAi therapeutic for the treatment of allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 2015, 56, 4883. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Rojas, R.; Pérez-Guàrdia, L.; Lafabrie, E.; Moulaert, D.; Laporte, J.; Böhm, J. Silencing of the Ca2+ Channel ORAI1 Improves the Multi-Systemic Phenotype of Tubular Aggregate Myopathy (TAM) and Stormorken Syndrome (STRMK) in Mice. Int. J. Mol. Sci. 2022, 23, 6968. https://doi.org/10.3390/ijms23136968
Silva-Rojas R, Pérez-Guàrdia L, Lafabrie E, Moulaert D, Laporte J, Böhm J. Silencing of the Ca2+ Channel ORAI1 Improves the Multi-Systemic Phenotype of Tubular Aggregate Myopathy (TAM) and Stormorken Syndrome (STRMK) in Mice. International Journal of Molecular Sciences. 2022; 23(13):6968. https://doi.org/10.3390/ijms23136968
Chicago/Turabian StyleSilva-Rojas, Roberto, Laura Pérez-Guàrdia, Emma Lafabrie, David Moulaert, Jocelyn Laporte, and Johann Böhm. 2022. "Silencing of the Ca2+ Channel ORAI1 Improves the Multi-Systemic Phenotype of Tubular Aggregate Myopathy (TAM) and Stormorken Syndrome (STRMK) in Mice" International Journal of Molecular Sciences 23, no. 13: 6968. https://doi.org/10.3390/ijms23136968
APA StyleSilva-Rojas, R., Pérez-Guàrdia, L., Lafabrie, E., Moulaert, D., Laporte, J., & Böhm, J. (2022). Silencing of the Ca2+ Channel ORAI1 Improves the Multi-Systemic Phenotype of Tubular Aggregate Myopathy (TAM) and Stormorken Syndrome (STRMK) in Mice. International Journal of Molecular Sciences, 23(13), 6968. https://doi.org/10.3390/ijms23136968





