Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions
Abstract
1. Introduction
2. Results
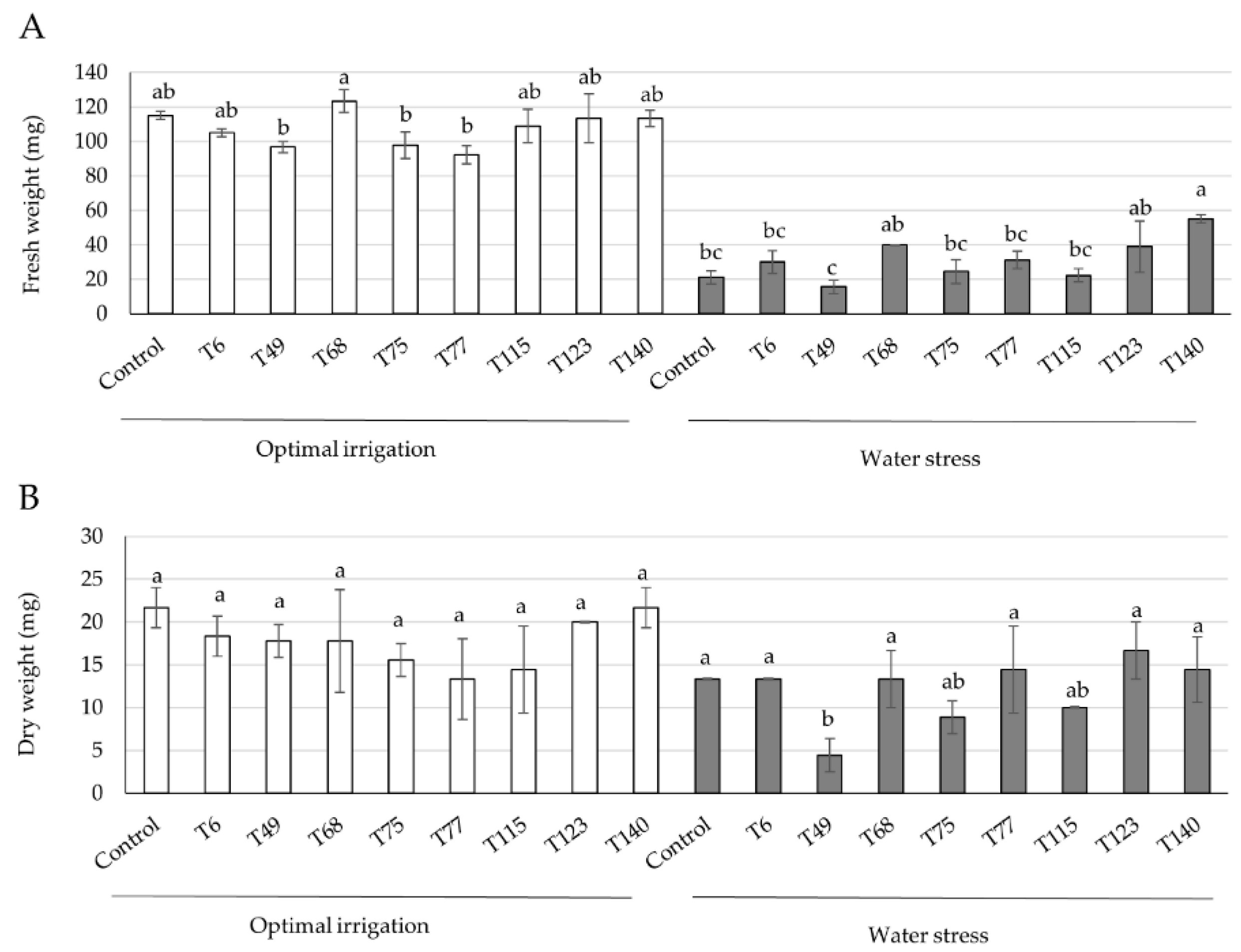
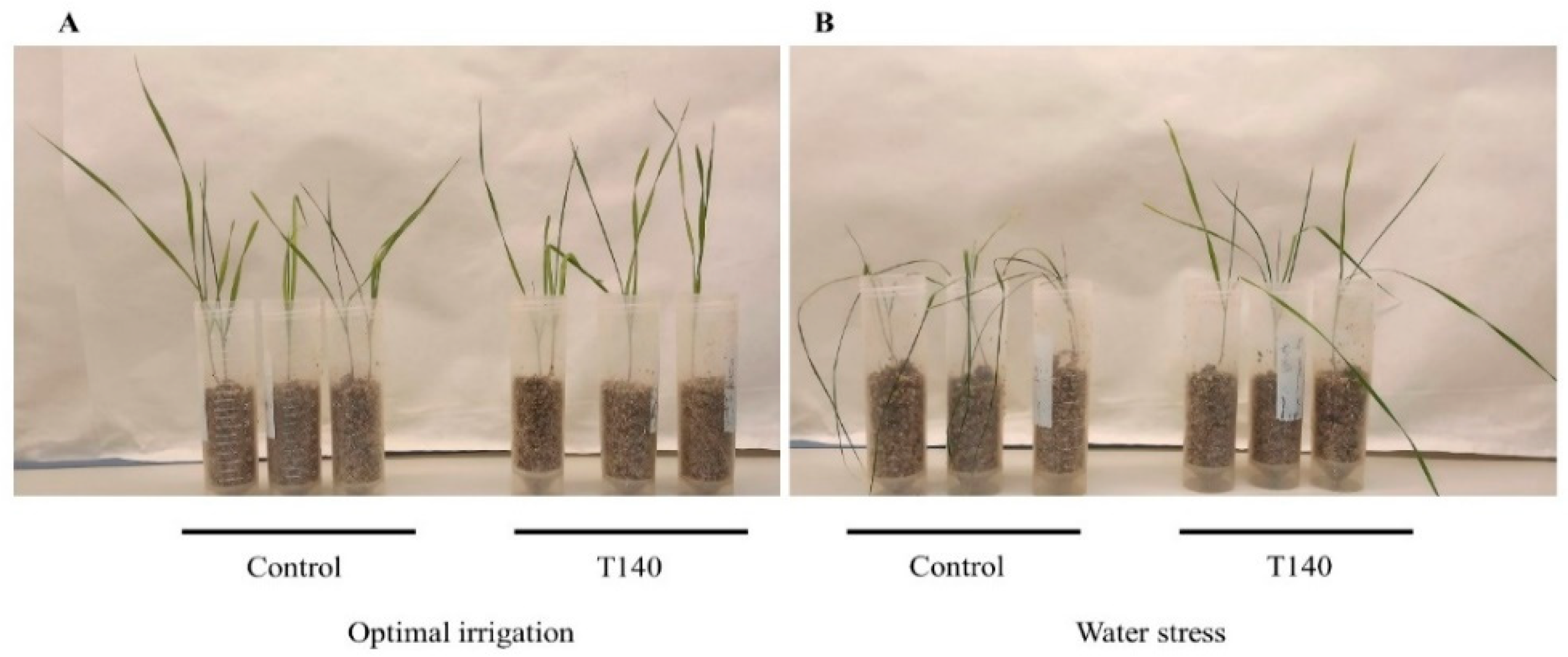
2.1. Trichoderma Strain Selection
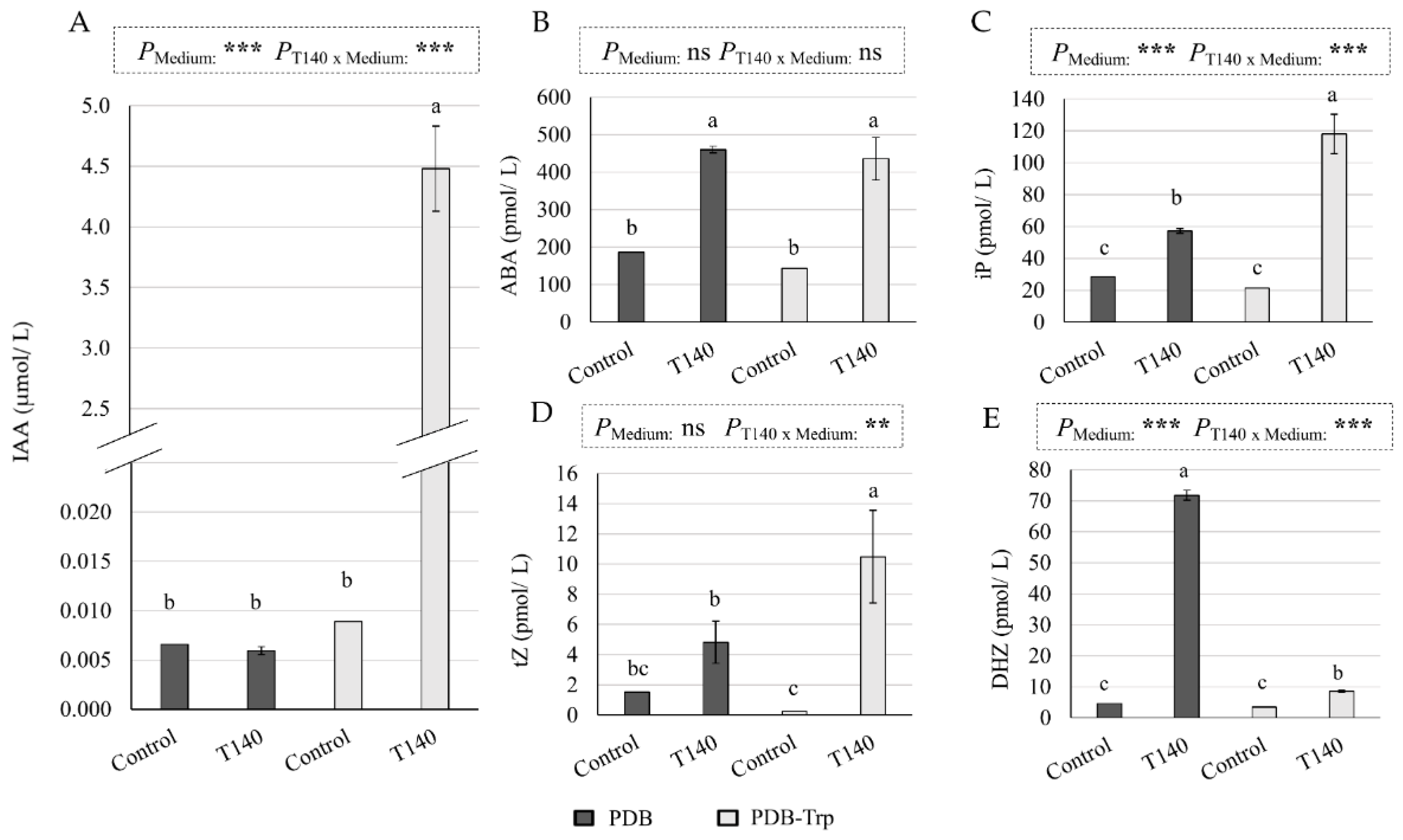
2.2. Phytohormone Production by Trichoderma Asperellum T140
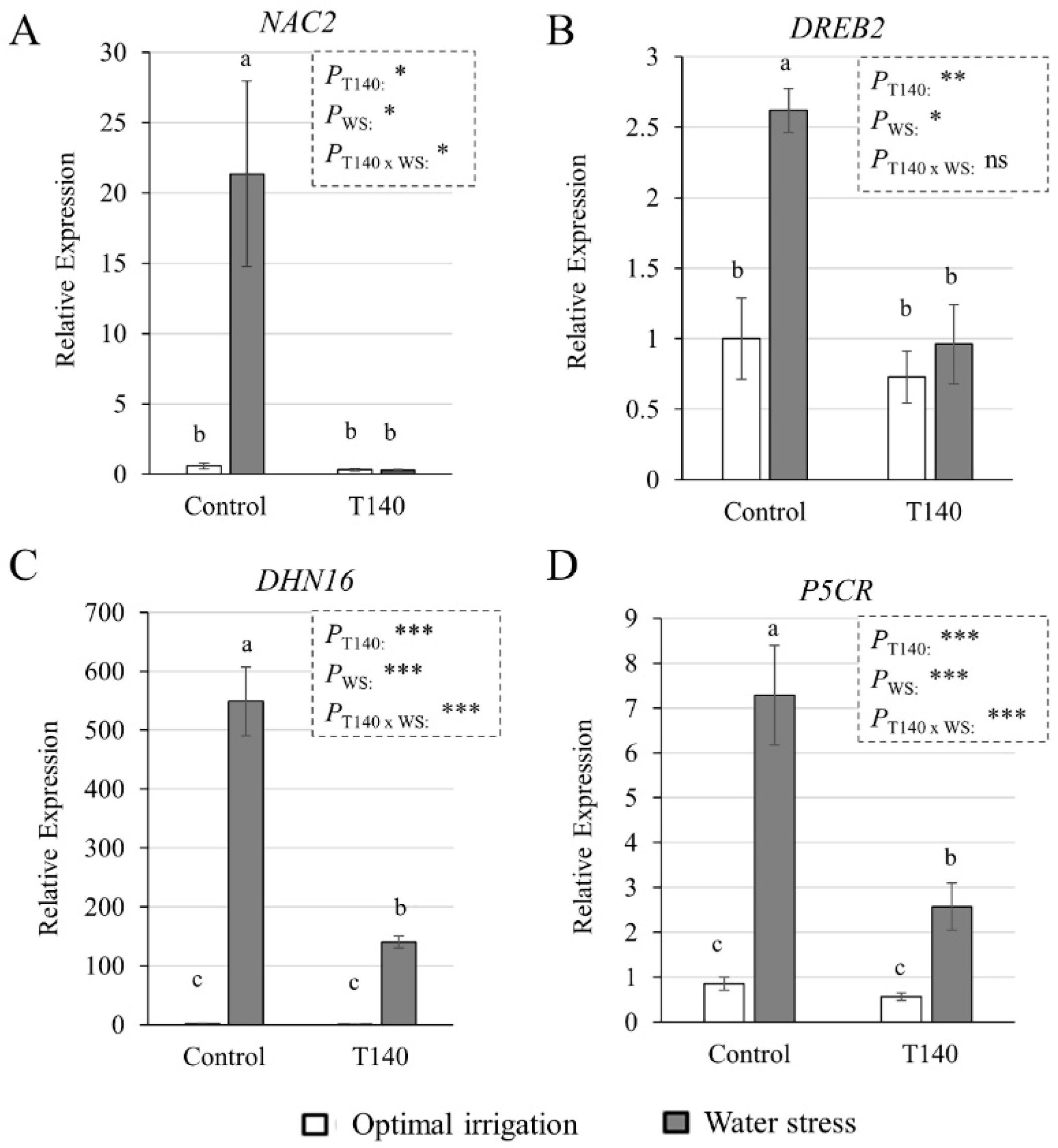
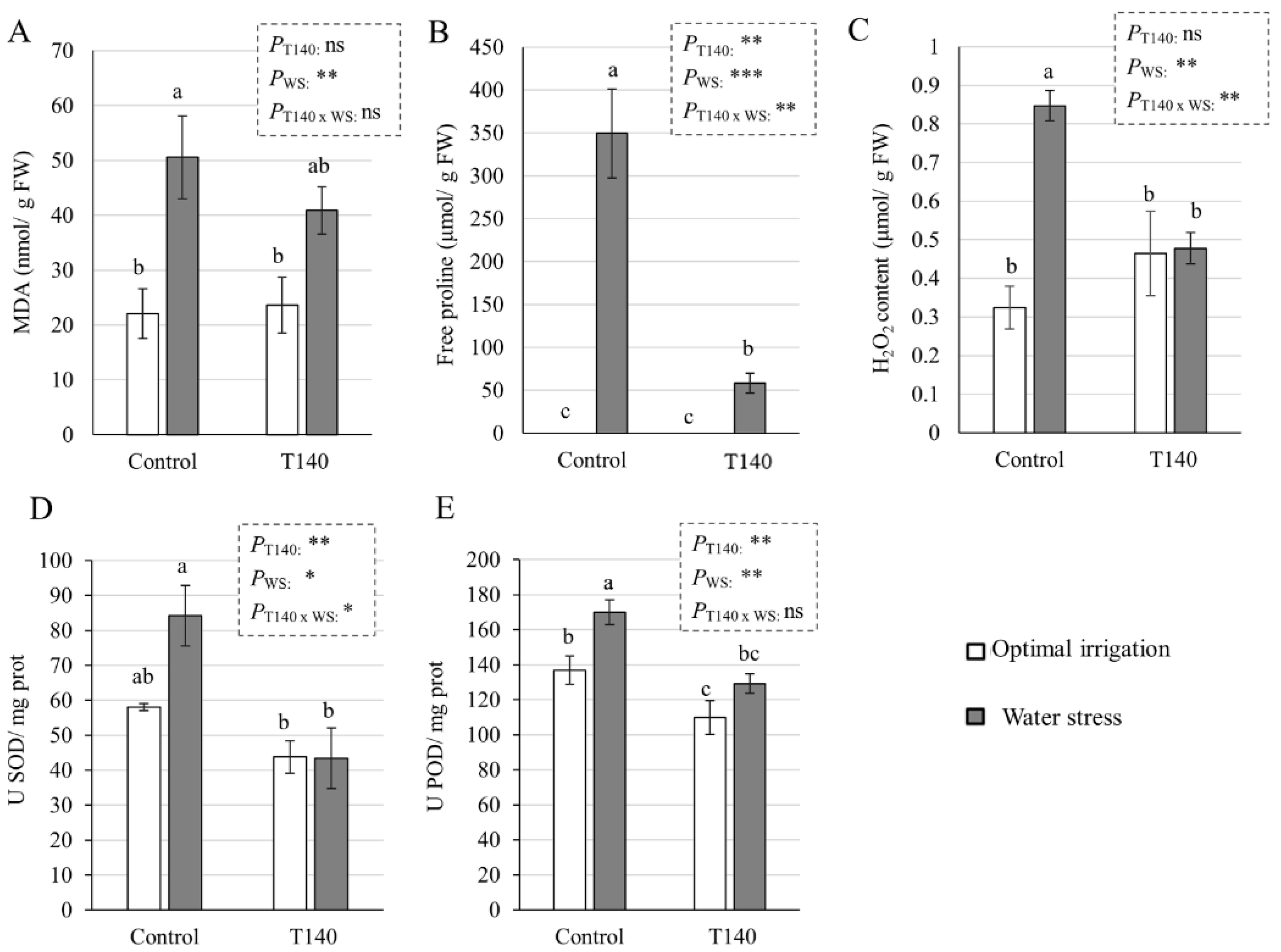
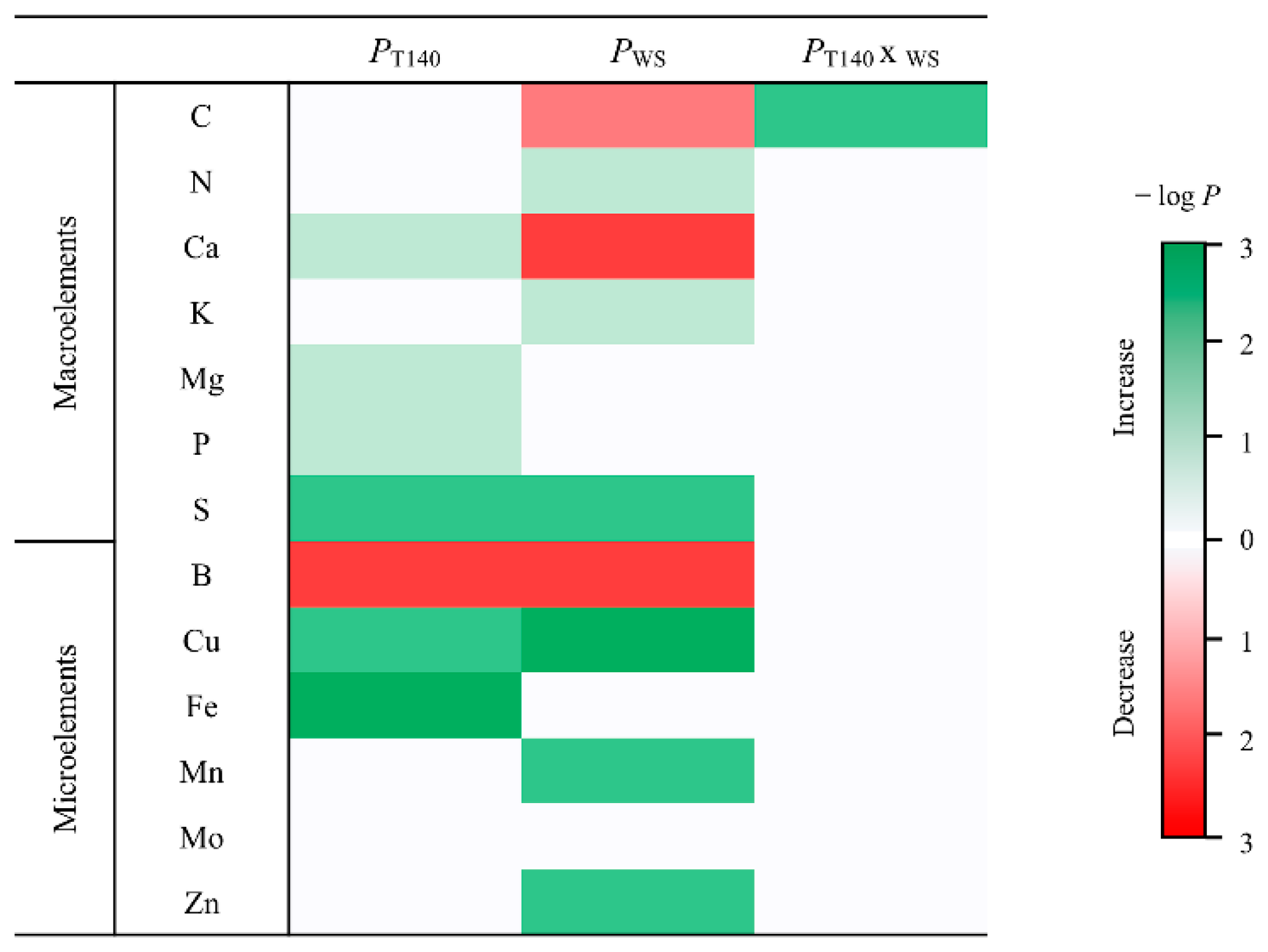
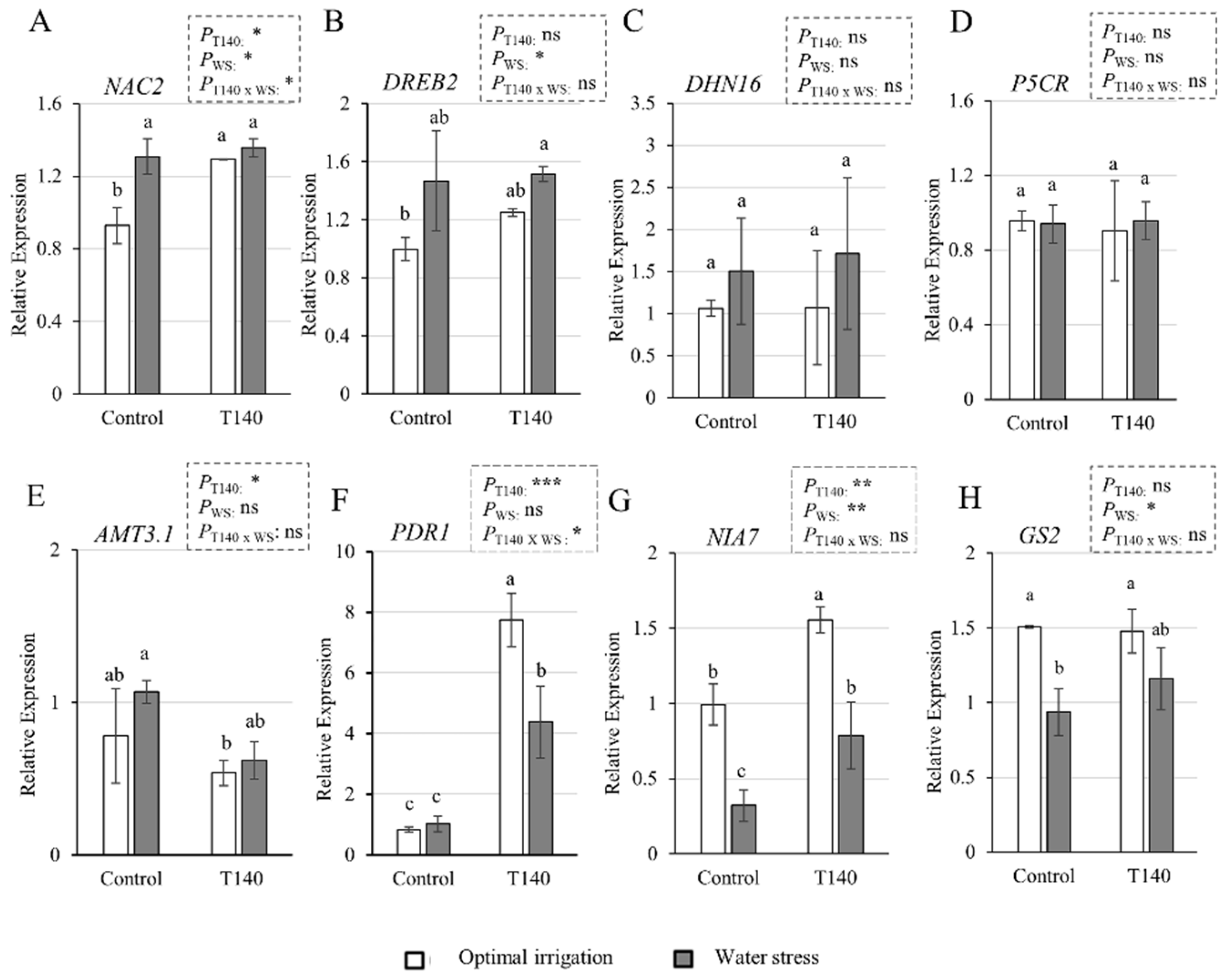
2.3. Molecular and Biochemical Changes in Wheat Seedlings
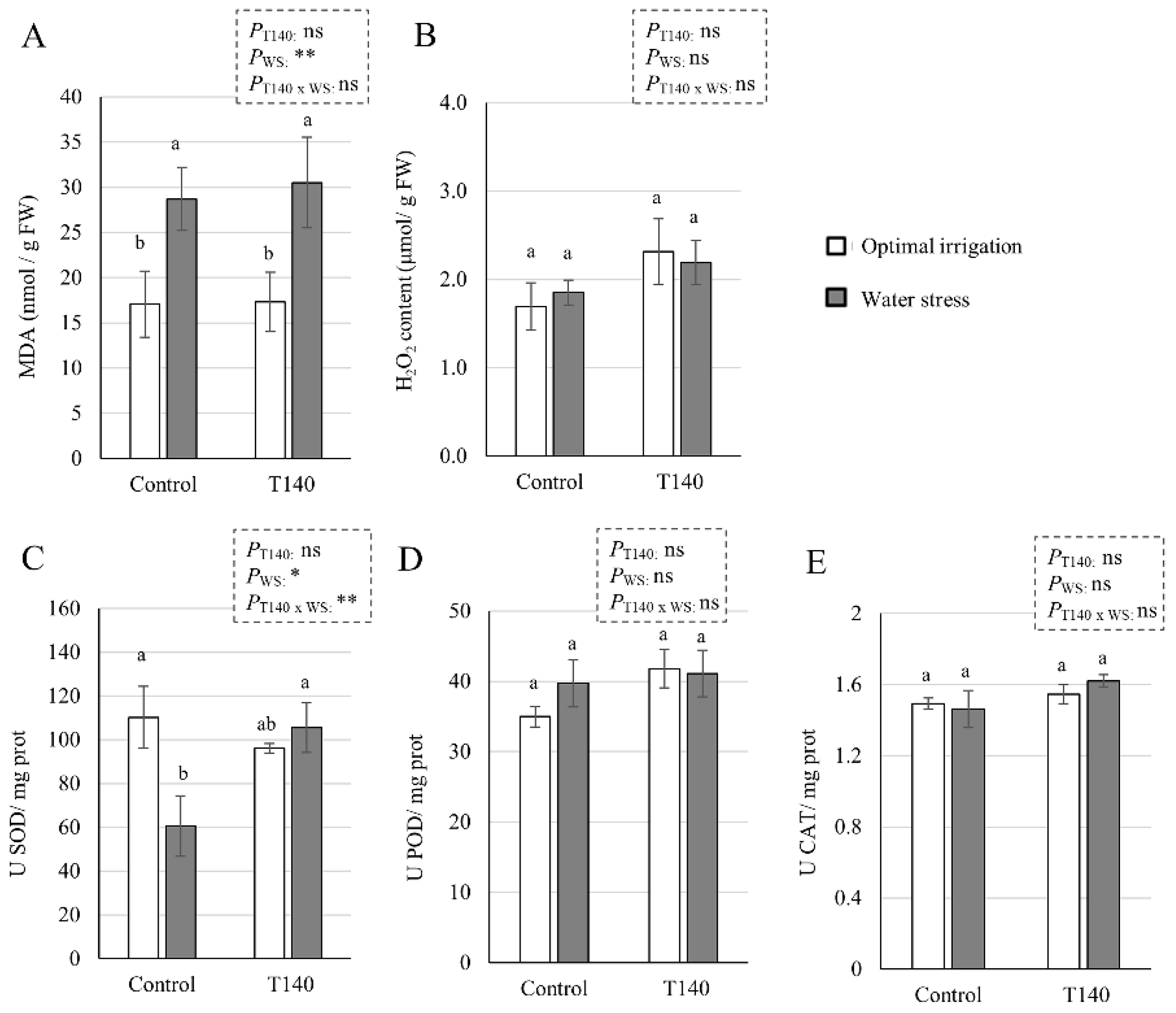
2.4. Greenhouse Trial up to Grain Production
3. Discussion
3.1. Trichoderma Strain Selection
3.2. Phytohormone Production by T. asperellum T140
3.3. Molecular and Biochemical Changes in Wheat Seedlings
3.4. Greenhouse Trial up to Grain Production
4. Materials and Methods
4.1. Trichoderma Strains
4.2. 1-aminocyclopropane-1-carboxylate Deaminase Activity of Trichoderma Strains
4.3. Determination of Phytohormone-like Compounds in Trichoderma
4.4. Trichoderma Assays on Wheat Seedlings under Different Irrigation Conditions
4.5. Greenhouse Assay with Trichoderma and Wheat Plants under Different Irrigation Conditions
4.6. Chemical Properties of Wheat Grains
4.7. Biochemical Analyses of Plants
4.7.1. Determination of Antioxidant Enzymatic Activities
4.7.2. Determination of H2O2 Content
4.7.3. Lipid Peroxidation
4.7.4. Determination of Free Proline Content
4.8. Real-Time Quantitative PCR (qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- FAOSTAT. Food and Agricultural Organization of United Nation, Roma. Available online: http://www.fao.org/faostat/ (accessed on 31 January 2022).
- Bento, V.A.; Ribeiro, A.F.S.; Russo, A.; Gouveia, C.M.; Cardoso, R.M.; Soares, P.M.M. The impact of climate change in wheat and barley yields in the Iberian Peninsula. Sci. Rep. 2021, 11, 15484. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain-Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the biochemical responses of wheat seedlings to soil drought after application of selective herbicide. Plants 2021, 10, 733. [Google Scholar] [CrossRef]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought response in wheat: Key-genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Illescas, M.; Rubio, M.B.; Hernández-Ruiz, V.; Morán-Diez, M.E.; Martínez de Alba, A.E.; Nicolás, C.; Monte, E.; Hermosa, R. Effect of inorganic N top dressing and Trichoderma harzianum seed-inoculation on crop yield and the shaping of root microbial communities of wheat plants cultivated under high basal N fertilization. Front. Plant Sci. 2020, 11, 575861. [Google Scholar] [CrossRef]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: A review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Zhang, J.; Zhao, Y.; She, M.; Ma, W. Genetic regulation of the traits contributing to wheat nitrogen use efficiency. Plant Sci. 2021, 303, 110759. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ‘omics to the field. Ann. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Monte, E.; Hermosa, R. The use of Trichoderma spp. to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W.J., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2022; pp. 401–429. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, A.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the plant heritable priming responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) enhances wheat seedlings tolerance to salt stress and resistance to Fusarium pseudograminearum. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef]
- Pedrero-Méndez, A.; Insuasti, H.C.; Neagu, T.; Illescas, M.; Rubio, M.B.; Monte, E.; Hermosa, R. Why is the correct selection of Trichoderma strains important? The case of wheat endophytic strains of T. harzianum and T. simmonsii. J. Fungi 2021, 7, 1087. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz-Tubaña, B.; De Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Khang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic fungi produce gibberellins and indolacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Ah-Yeong Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Bano, A.; Ullah, F.; Nosheen, A. Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ. 2012, 58, 181–185. [Google Scholar] [CrossRef]
- Nagar, S.; Singh, V.P.; Arora, A.; Dhakar, R.; Ramakrishnan, S. Assessment of terminal heat tolerance ability of wheat genotypes based on physiological traits using multivariate analysis. Acta Physiol. Plant 2015, 37, 257. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Mostofa, M.G.; Akter, M.M.; Srivastava, A.K.; Sayed, M.A.; Hasan, M.S.; Tran, L.S.P. Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: Oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 2017, 187, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.B.; Hermosa, R.; Vicente, R.; Gómez-Acosta, F.A.; Morcuende, R.; Monte, E.; Bettiol, W. The combination of Trichoderma harzianum and chemical fertilization leads to the deregulation of phytohormone networking, preventing the adaptative responses of tomato plants to salt stress. Front. Plant Sci. 2017, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2015, 166, 171–182. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant tolerance to drought stress in the presence of supporting bacteria and fungi: An efficient strategy in horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- Lupini, A.; Preiti, G.; Badagliacca, G.; Abenavoli, M.R.; Sunseri, F.; Monti, M.; Bacchi, M. Nitrogen use efficiency in durum wheat under different nitrogen and water regimes in the Mediterranean Basin. Front. Plant Sci. 2021, 11, 607226. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.J.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.L.; Cirillo, V.; Maggio, A. Biostimulant activity of Azotobacter chroococcum and Trichoderma harzianum in durum wheat under water and nitrogen deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Rawat, L.; Bisht, T.S.; Kukreti, A.; Prasad, M. Bioprospecting drought tolerant Trichoderma harzianum isolates promote growth and delay the onset of drought responses in wheat (Triticum aestivum L.). Mol. Soil Biol. 2016, 7, 1–15. [Google Scholar] [CrossRef][Green Version]
- Kimball, B.A.; Morris, C.F.; Pinter Jr, P.J.; Wall, G.W.; Hunsaker, D.J.; Adamsen, F.J.; LaMorte, R.L.; Leavitt, S.W.; Thompson, T.L.; Matthias, A.D.; et al. Elevated CO2, drought, and soil nitrogen effects on wheat grain quality. New Phytol. 2001, 150, 295–303. [Google Scholar] [CrossRef]
- Houshmand, S.; Arzani, A.; Mirmohammadi-Maibody, S.A.M. Effects of salinity and drought stress on grain quality of durum wheat. Commun. Soil Sci. Plant Anal. 2014, 45, 297–308. [Google Scholar] [CrossRef]
- Duan, J.; Tian, H.; Gao, Y. Expression of nitrogen transporter genes in roots of winter wheat (Triticum aestivum L.) in response to soil drought with contrasting nitrogen supplies. Crop Pasture Sci. 2016, 67, 128–136. [Google Scholar] [CrossRef]
- Crouzet, J.; Trombik, T.; Fraysse, A.S.; Boutry, M. Organization and function of the plant pleiotropic drug resistance ABC transporter family. FEBS Lett. 2006, 580, 1123–1130. [Google Scholar] [CrossRef]
- Curci, P.L.; Aiese-Cigliano, R.; Zuluaga, D.L.; Janni, M.; Sanseverino, W.; Sonnante, G. Transcriptomic response of durum wheat to nitrogen starvation. Sci. Rep. 2017, 7, 1176. [Google Scholar] [CrossRef]
- Rubio, M.B.; Martínez de Alba, A.E.; Nicolás, C.; Monte, E.; Hermosa, R. Early root transcriptomic changes in wheat seedlings colonized by Trichoderma harzianum under different inorganic nitrogen supplies. Front. Microbiol. 2019, 10, 2444. [Google Scholar] [CrossRef]
- Jallouli, S.; Ayadi, S.; Landi, S.; Capasso, G.; Santini, G.; Chamekh, Z.; Zouari, I.; Ben Azaiez, F.E.; Trifa, Y.; Esposito, S. Physiological and molecular osmotic stress responses in three durum wheat (Triticum turgidum ssp. durum) genotypes. Agronomy 2019, 9, 550. [Google Scholar] [CrossRef]
- Hurali, D.T.; Bhurta, R.; Tyagi, S.; Sathee, L.; Sandeep, A.B.; Singh, D.; Mallick, N.; Vinod; Jha, S.K. Analysis of NIA and GSNOR family genes and nitric oxide homeostasis in response to wheat-leaf rust interaction. Sci. Rep. 2022, 12, 803. [Google Scholar] [CrossRef]
- Tang, X.; Peng, Y.; Li, Z.; Guo, H.; Xia, X.; Li, B.; Yin, W. The regulation of nitrate reductases in response to abiotic stress in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1202. [Google Scholar] [CrossRef] [PubMed]
- Amoah, J.N.; Ko, C.S.; Yoon, J.S.; Weon, S.Y. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.). J. Plant Interact. 2019, 14, 492–505. [Google Scholar] [CrossRef]
- Hermosa, M.R.; Keck, E.; Chamorro, I.; Rubio, B.; Sanz, L.; Vizcaíno, J.A.; Grondona, I.; Monte, E. Genetic diversity shown in Trichoderma biocontrol isolates. Mycol. Res. 2004, 108, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.B.; Quijada, N.M.; Pérez, E.; Domínguez, S.; Monte, E.; Hermosa, R. Identifying beneficial qualities of Trichoderma parareesei for plants. Appl. Env. Microbiol. 2014, 80, 1864–1873. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidants systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Nieto-Taladriz, M.T.; Aparicio, N.; Chairi, F.; Vergara-Diaz, O.; Araus, J.L. The plant-transpiration response to Vapor Pressure Deficit (VPD) in durum wheat is associated with differential yield performance and specific expression of genes involved in primary metabolism and water transport. Front. Plant Sci. 2019, 9, 1994. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Prot. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
| Control | T140 | |||
|---|---|---|---|---|
| OI | WS | OI | WS | |
| DW (g) | 9.2 ± 1.2 a | 6.6 ± 1.6 c | 9.1 ± 0.7 a | 7.6 ± 0.9 b |
| GYP (g) | 2.8 ± 0.4 a | 2.1 ± 0.7 b | 2.8 ± 0.5 a | 2.6 ± 0.6 a |
| GNP | 87.8 ± 16.2 a | 63.9 ± 19.5 b | 84.0 ± 13.5 a | 79.7 ± 19.0 a |
| SNP | 3.9 ± 0.8 a | 3.0 ± 1.1 b | 4.3 ± 0.6 a | 3.7 ± 0.4 a |
| GY (g) | 101.99 | 74.14 | 101.74 | 91.99 |
| GN | 3160 | 2300 | 3024 | 2868 |
| SN | 139 | 109 | 154 | 133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illescas, M.; Morán-Diez, M.E.; Martínez de Alba, Á.E.; Hermosa, R.; Monte, E. Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. Int. J. Mol. Sci. 2022, 23, 6782. https://doi.org/10.3390/ijms23126782
Illescas M, Morán-Diez ME, Martínez de Alba ÁE, Hermosa R, Monte E. Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. International Journal of Molecular Sciences. 2022; 23(12):6782. https://doi.org/10.3390/ijms23126782
Chicago/Turabian StyleIllescas, María, María E. Morán-Diez, Ángel Emilio Martínez de Alba, Rosa Hermosa, and Enrique Monte. 2022. "Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions" International Journal of Molecular Sciences 23, no. 12: 6782. https://doi.org/10.3390/ijms23126782
APA StyleIllescas, M., Morán-Diez, M. E., Martínez de Alba, Á. E., Hermosa, R., & Monte, E. (2022). Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. International Journal of Molecular Sciences, 23(12), 6782. https://doi.org/10.3390/ijms23126782










