New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
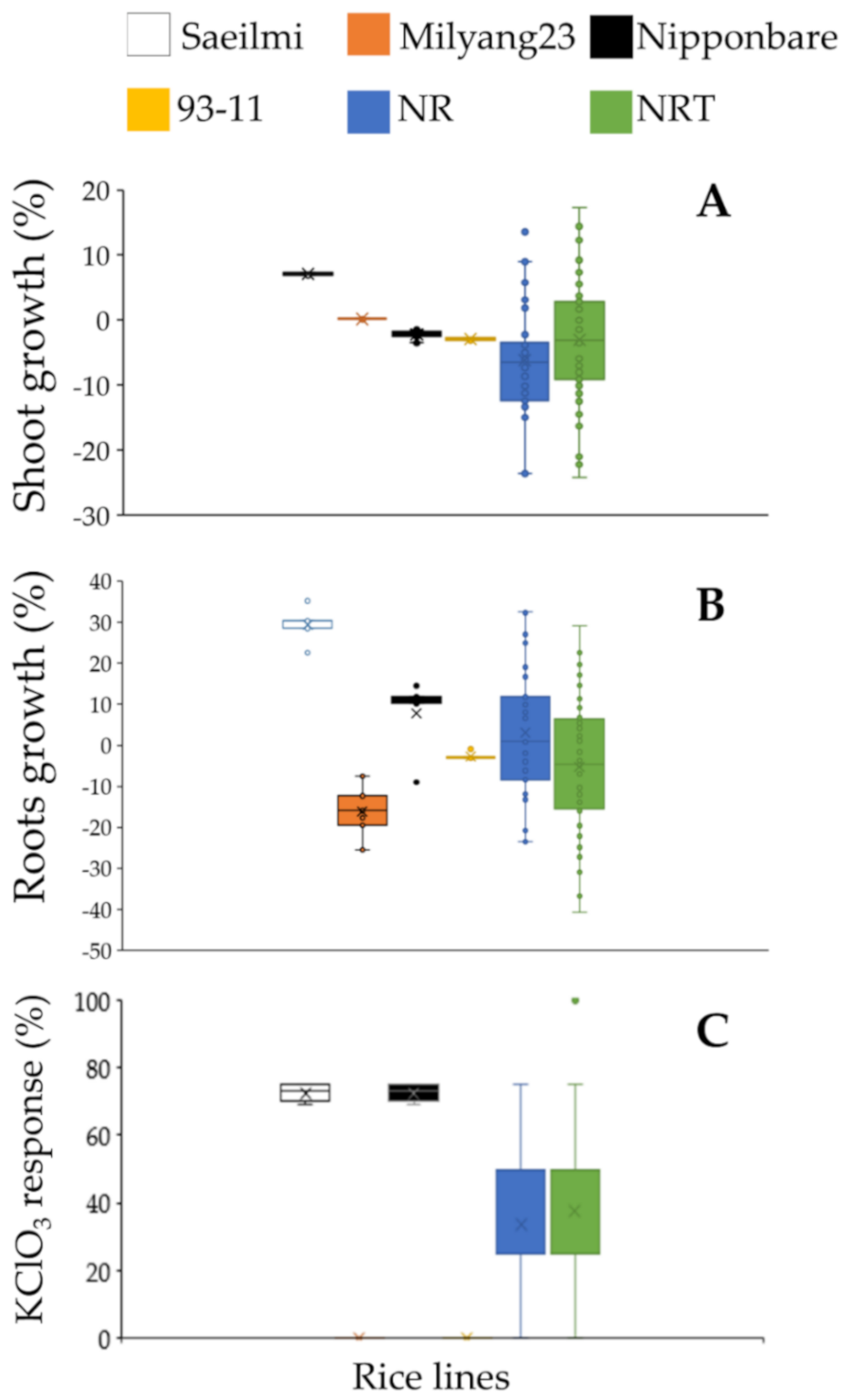
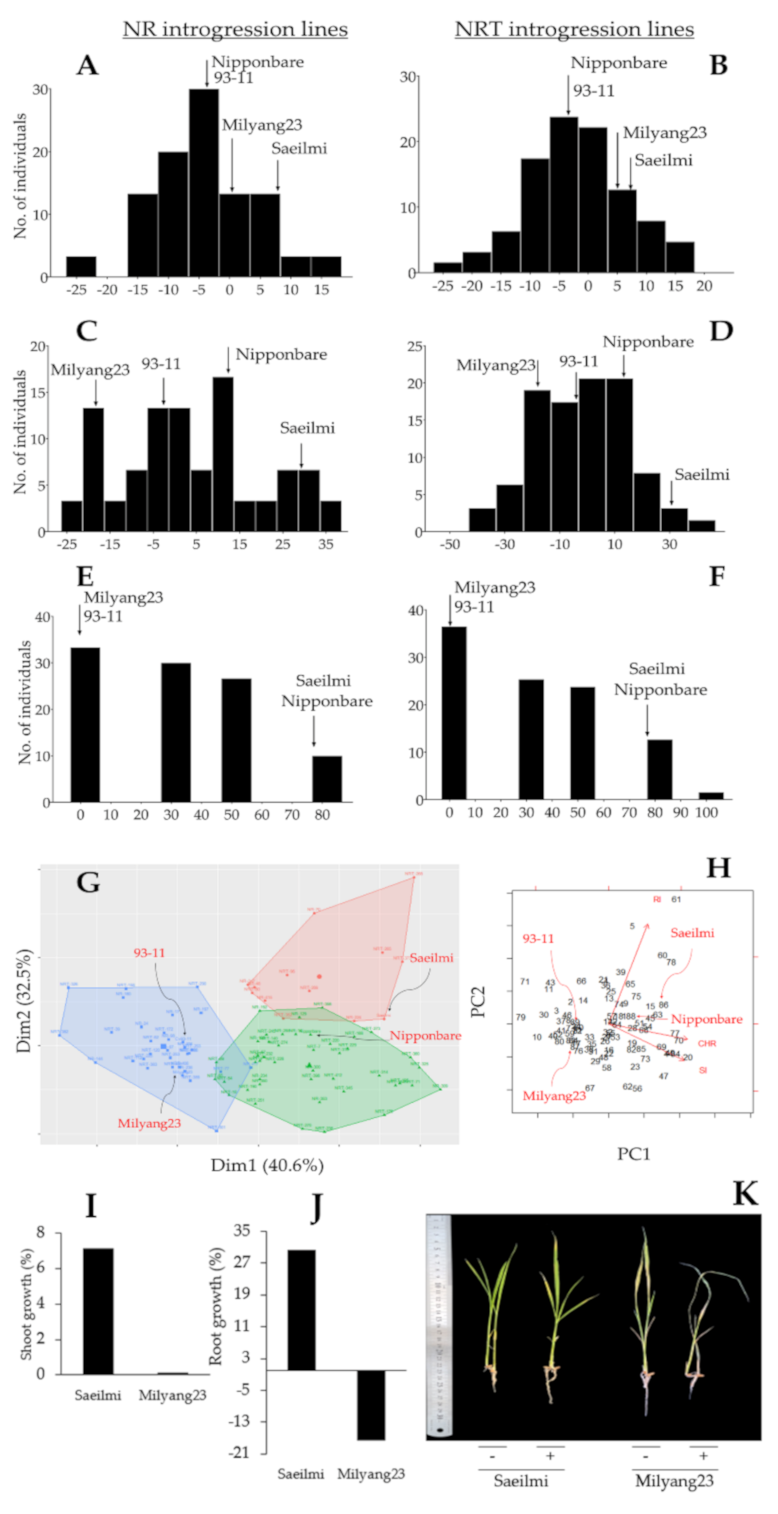
2.1. Distinctive Phenotypic Response between Parental Lines, and Identification of Introgression Lines
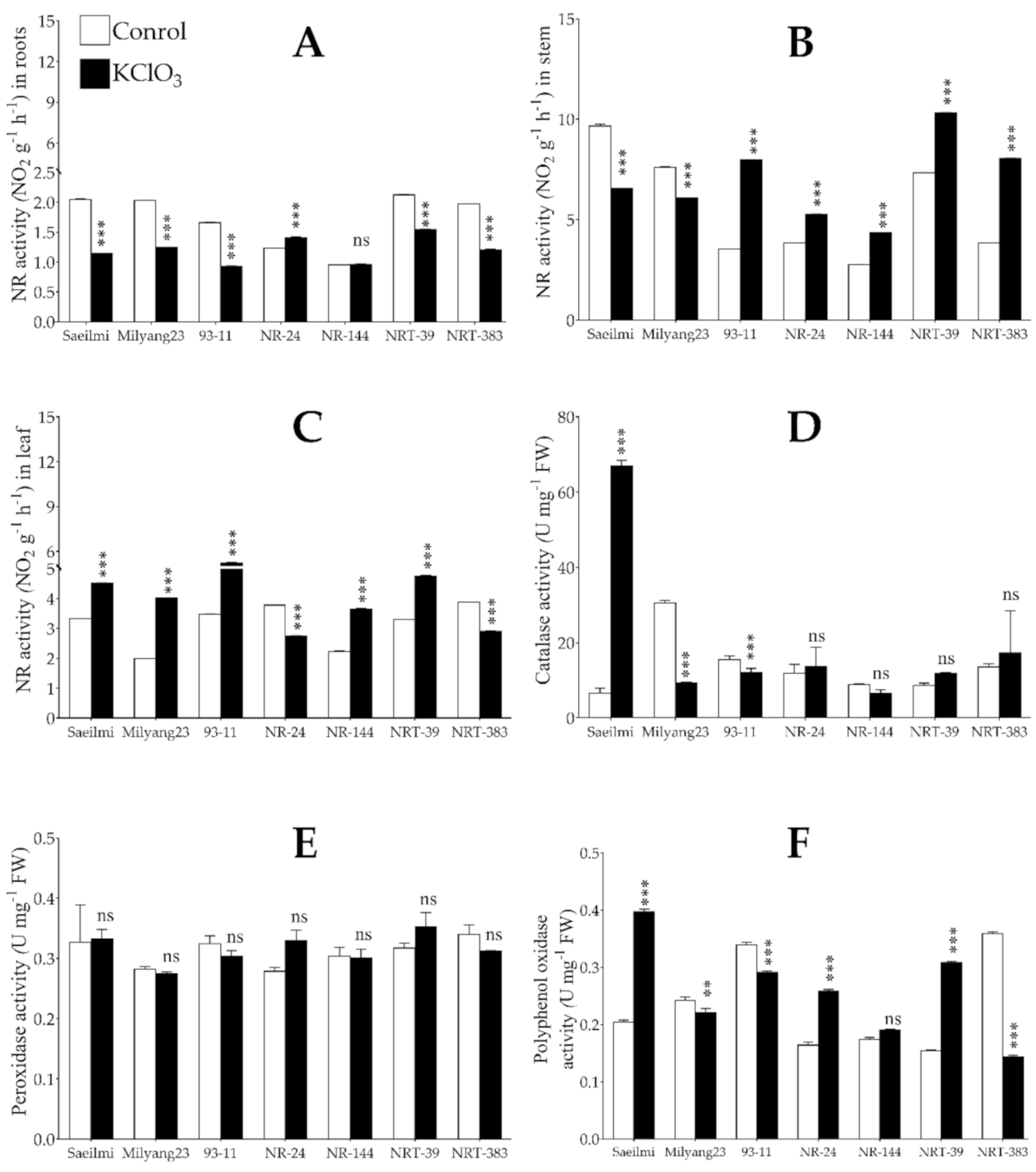
2.2. Exogenous Application of Potassium Chlorate Differentially Controlled the Activity of Nitrate Reductase in a Tissue-Specific Dependent Manner
2.3. Increased Chlorophyll and Carotenoids Content in Response to Potassium Chlorate
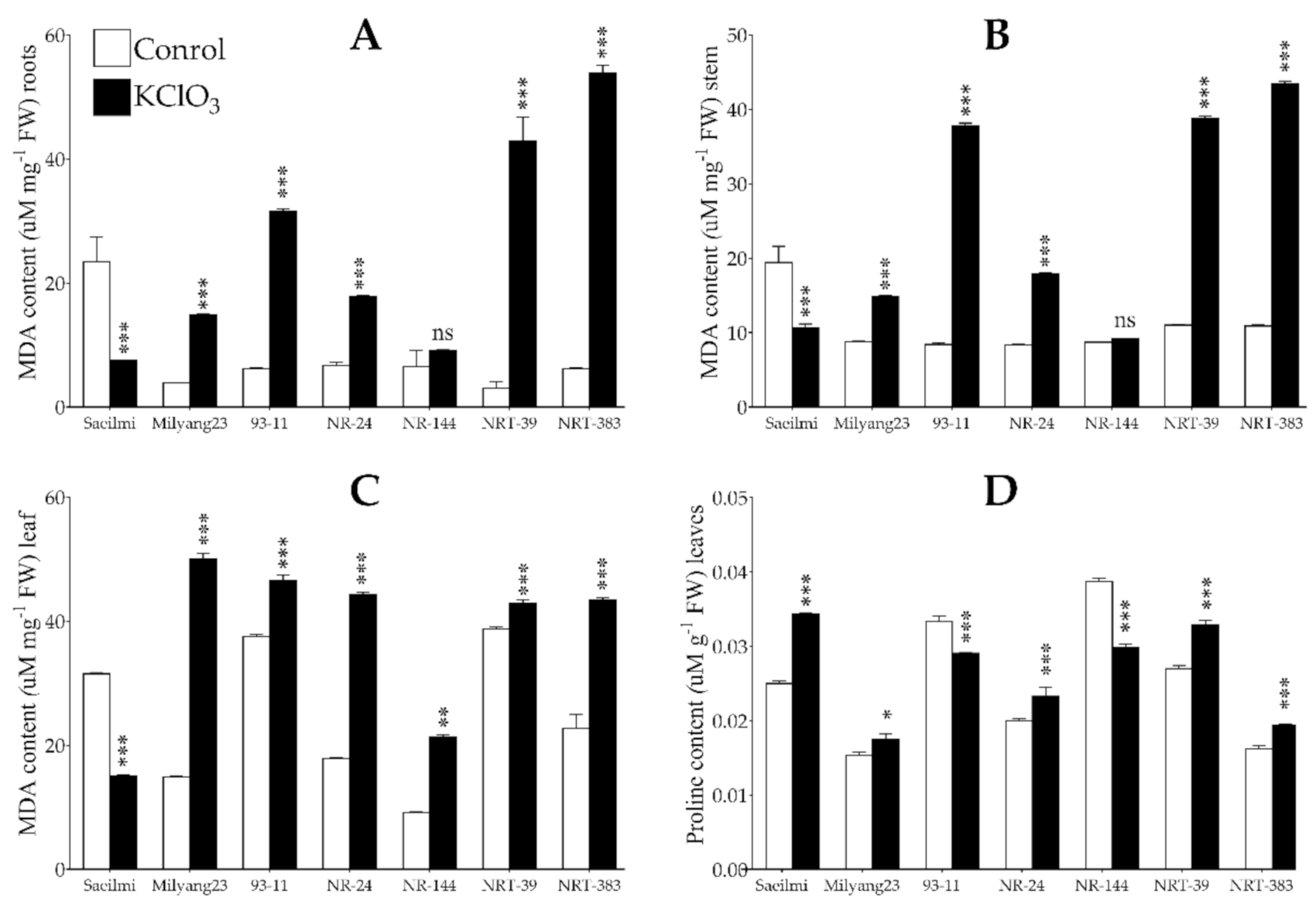
2.4. Potassium Chlorate Treatment Caused Lipid Peroxidation and Changes in Proline Content
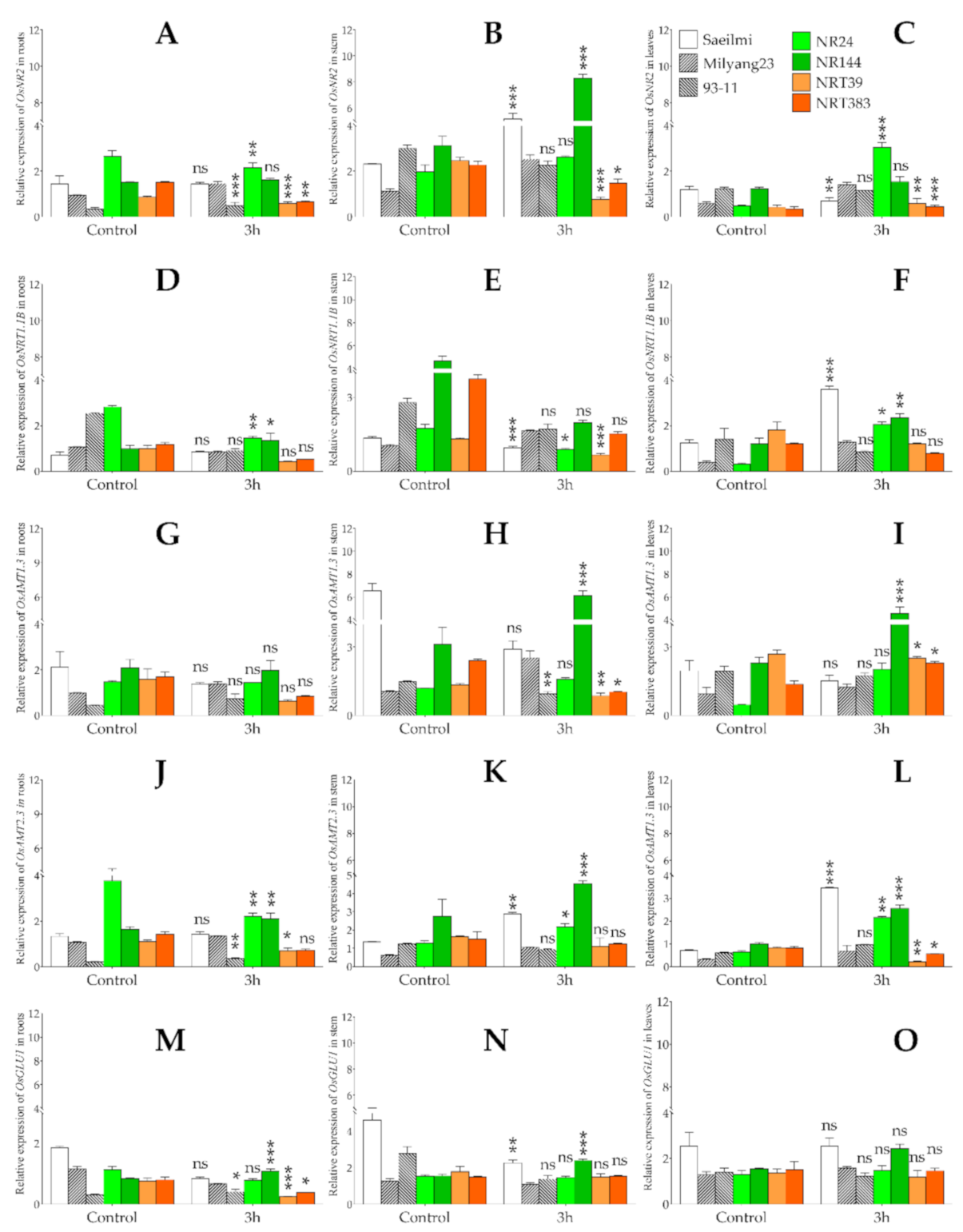
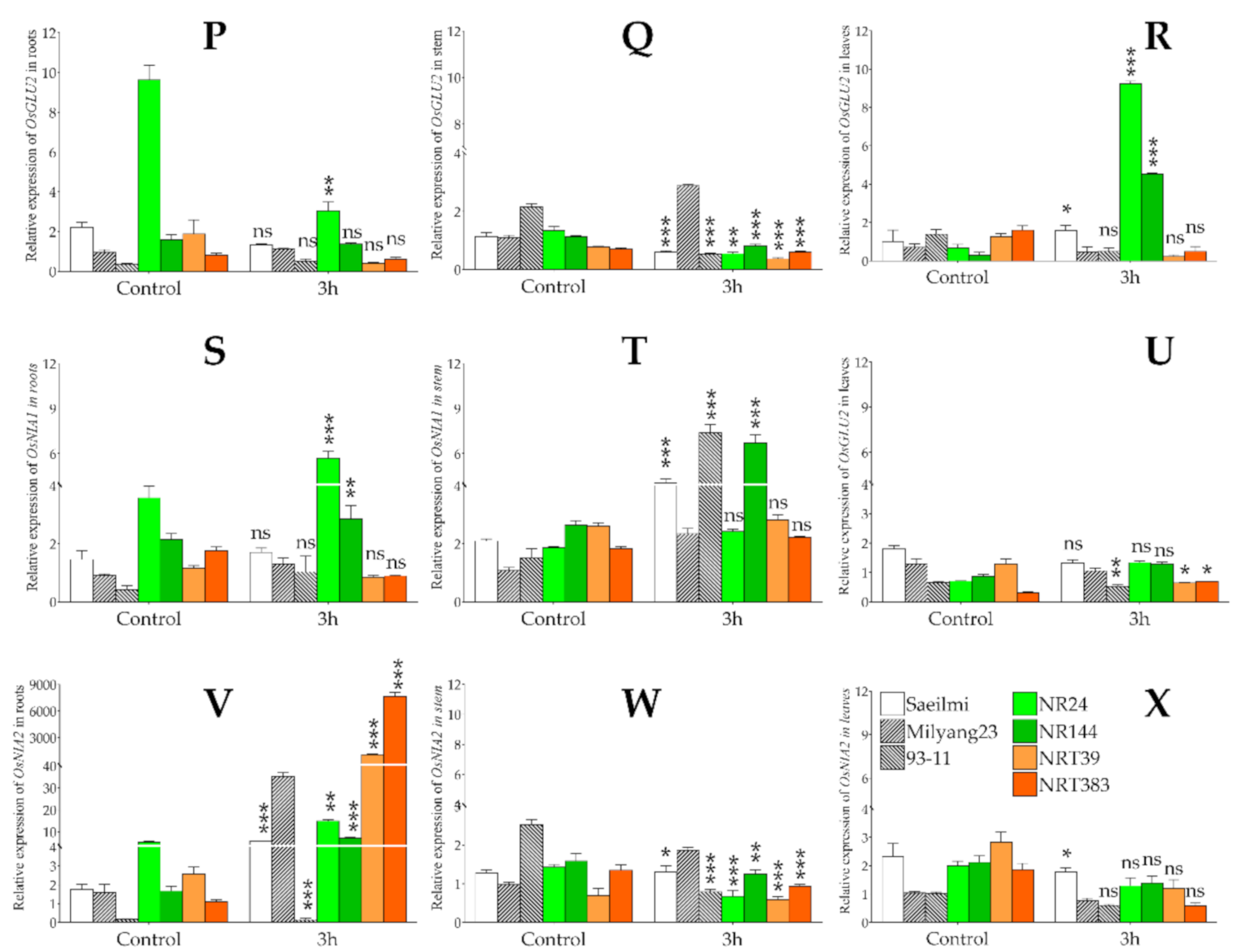
2.5. Potassium Chlorate Differentially Regulated the Expression of Genes Involved in Nitrogen Uptake, Transport, and Assimilation in Roots, Stem, and Leaf Tissues
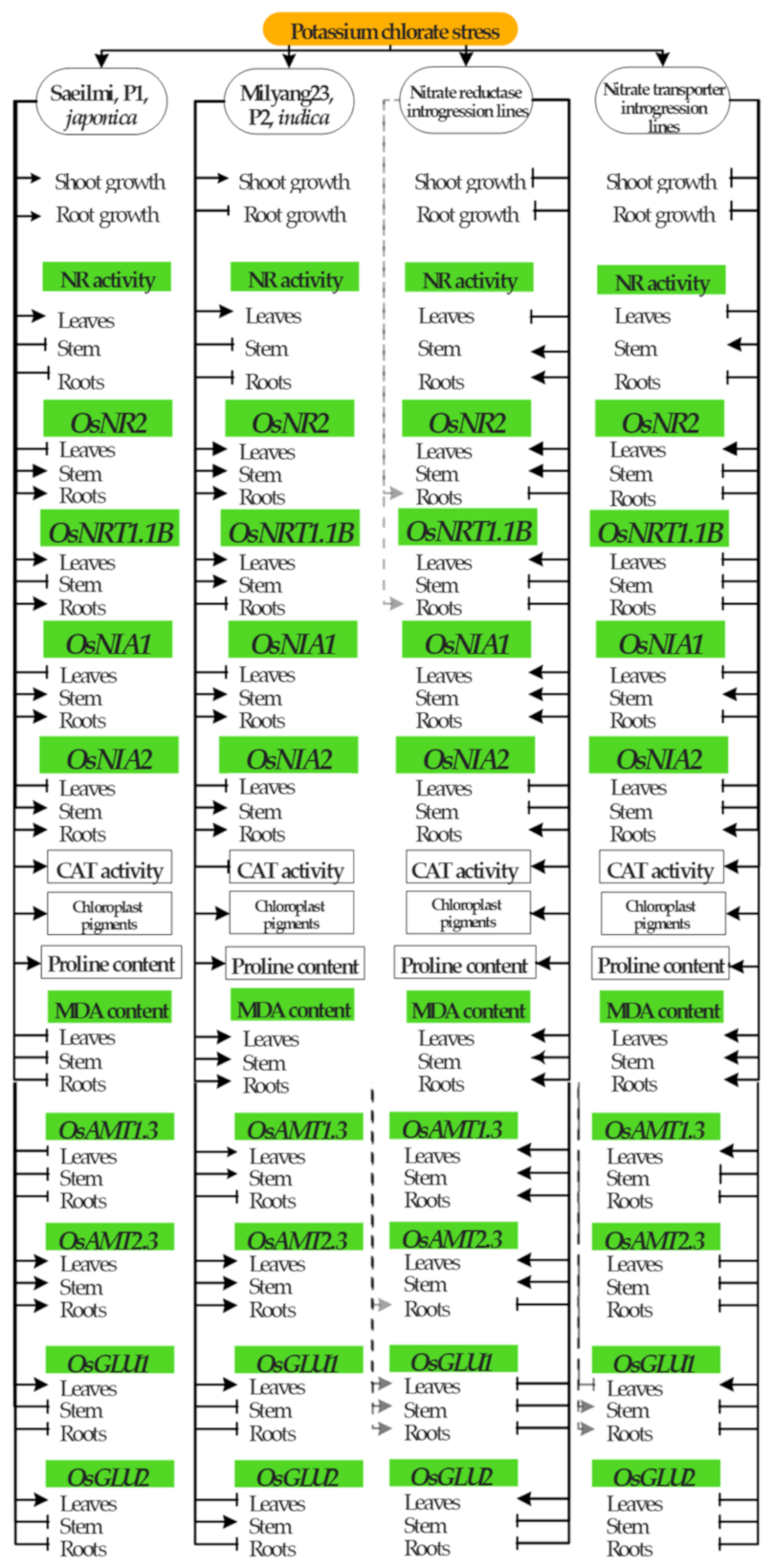
3. Discussion
3.1. Potassium Chlorate Inhibits Shoot and Root Growth in a Cultivar Dependent Manner
3.2. Potassium Chlorate Differentially Regulates Genes Involved in Nitrate Uptake, Transport, and Assimilation in a Tissue-Specific Dependent Manner
3.3. Potassium Chlorate Differentially Regulates Antioxidant Enzymes Between Parental and NR or NRT Introgression Rice Lines
3.4. Exogenous Application of Potassium Chlorate Triggers the Accumulation of Chloroplast Pigments
4. Materials and Methods
4.1. Plant Materials and Potassium Chlorate Treatment
4.2. Genomic DNA Extraction, Genotyping, and Molecular Marker Analysis
4.3. Total RNA Extraction, cDNA Synthesis, and qPCR Analysis
4.4. Proline Measurement Assay
4.5. Catalase, Polyphenol Oxidase, and Peroxidase Activity Assay
4.6. Chlorophyll, Pheophytin, and Carotenoids Content Measurements
4.7. Lipid Peroxidation Assay
4.8. Nitrate Reductase Activity Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, W.M.; Xu, W.F.; Li, S.M.; Zhao, X.Q.; Dong, G.Q. Responses of two rice cultivars differing in seedling-stage nitrogen use efficiency to growth under low-nitrogen conditions. Plant Soil 2010, 326, 291. [Google Scholar] [CrossRef]
- Mustapha, S.; Voncir, N.; Umar, S. Content and distribution of nitrogen forms in some black cotton soils in Akko LGA, Gombe State, Nigeria. Int. J. Soil Sci. 2011, 6, 275–281. [Google Scholar] [CrossRef]
- Harmsen, G.; Van Schreven, D. Mineralization of organic nitrogen in soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1955; Volume 7, pp. 299–398. [Google Scholar]
- Cameron, R.; Posner, A. Mineralisable organic nitrogen in soil fractionated according to particle size. J. Soil Sci. 1979, 30, 565–577. [Google Scholar] [CrossRef]
- Bock, E.; Wagner, M. Oxidation of inorganic nitrogen compounds as an energy source. Prokaryotes 2006, 2, 457–495. [Google Scholar]
- Weidner, S.; Pühler, A.; Küster, H. Genomics insights into symbiotic nitrogen fixation. Curr. Opin. Biotechnol. 2003, 14, 200–205. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Nie, H.; Zeng, Q.; Wu, B.; Qian, J.; Fang, Z. Rice nitrate transporter OsNPF7. 2 positively regulates tiller number and grain yield. Rice 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Fan, X.; Li, Q.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol. 2012, 160, 2052–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Fan, X.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Shen, Q.; Ma, Z.; Zhu, H.; Yin, X.; Miller, A.J. A comparison of nitrate transport in four different rice (Oryza sativa L.) cultivars. Sci. China Ser. C Life Sci. 2005, 48, 897–911. [Google Scholar]
- Fan, X.; Feng, H.; Tan, Y.; Xu, Y.; Miao, Q.; Xu, G. A putative 6-transmembrane nitrate transporter OsNRT1. 1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 2016, 58, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.-Y.; Lin, H.-X. Nitrogen-use efficiency: Transport solution in rice variations. Nat. Plants 2015, 1, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shi, W. Expression patterns of OsAMT (1.1–1.3), OsAMT3.1 and OsAMT4.1 in rice (Oryza sativa L.). Soil 2007, 39, 460–464. [Google Scholar]
- Zhao, S.; Ye, X.; Shi, W. Expression of OsAMT1 (1.1–1.3) in rice varieties differing in nitrogen accumulation. Russ. J. Plant Physiol. 2014, 61, 707–713. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, J.; Wang, Y.-Y.; Hu, R.; Xia, K.; Duan, J.; Wang, Y.; Tsay, Y.-F.; Zhang, M. Disruption of the rice nitrate transporter OsNPF2.2 hinders root-to-shoot nitrate transport and vascular development. Sci. Rep. 2015, 5, 1–10. [Google Scholar]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Beeckman, T.; Xu, G. Plant nitrogen nutrition: Sensing and signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ashoub, A.; Baeumlisberger, M.; Neupaertl, M.; Karas, M.; Brüggemann, W. Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol. Biol. 2015, 87, 459–471. [Google Scholar] [CrossRef]
- Wendelboe-Nelson, C.; Morris, P.C. Proteins linked to drought tolerance revealed by DIGE analysis of drought resistant and susceptible barley varieties. Proteomics 2012, 12, 3374–3385. [Google Scholar] [CrossRef]
- Tcherkez, G.; Hodges, M. How stable isotopes may help to elucidate primary nitrogen metabolism and its interaction with (photo) respiration in C3 leaves. J. Exp. Bot. 2007, 59, 1685–1693. [Google Scholar] [CrossRef] [Green Version]
- Lea, P.J.; Miflin, B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Kabange, N.R.; Park, S.-Y.; Shin, D.; Lee, S.-M.; Jo, S.-M.; Kwon, Y.; Cha, J.-K.; Song, Y.-C.; Ko, J.-M.; Lee, J.-H.J.A. Identification of a Novel QTL for Chlorate Resistance in Rice (Oryza sativa L.). Agriculture 2020, 10, 360. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, C. Nitrogen-use divergence between indica and japonica rice: Variation at nitrate assimilation. Mol. Plant 2020, 13, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Zhang, H. A single SNP in NRT1. 1B has a major impact on nitrogen use efficiency in rice. Sci. China Life Sci. 2015, 58, 827. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Yamashita, N. Chlorate reducing activity of spinach nitrate reductase. Agric. Biol. Chem. 1986, 50, 1893–1894. [Google Scholar]
- Solomonson, L.; Vennesland, B. Nitrate reductase and chlorate toxicity in Chlorella vulgaris Beijerinck. Plant Physiol. 1972, 50, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Roldan, M.; Reyes, F.; Moreno-Vivian, C.; Castillo, F. Chlorate and nitrate reduction in the phototrophic bacteriaRhodobacter capsulatus andRhodobacter sphaeroides. Curr. Microbiol. 1994, 29, 241–245. [Google Scholar] [CrossRef]
- Mészáros, A.; Pauk, J. Chlorate resistance as a tool to study the effect of nitrate reductase antisense gene in wheat. Cereal Res. Commun. 2002, 30, 245–252. [Google Scholar] [CrossRef]
- Kamal, N.M.; Gorafi, Y.S.A.; Mega, R.; Tsujimoto, H.J.A. Physiological response of wheat to chemical desiccants used to simulate post-anthesis drought stress. Agronomy 2018, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Teng, S.; Tian, C.; Chen, M.; Zeng, D.; Guo, L.; Zhu, L.; Han, B.; Qian, Q.J.E. QTLs and candidate genes for chlorate resistance in rice (Oryzasativa L.). Euphytica 2006, 152, 141–148. [Google Scholar] [CrossRef]
- Inzé, D.; Van Montagu, M. Oxidative stress in plants. Curr. Opin. Biotechnol. 1995, 6, 153–158. [Google Scholar] [CrossRef]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Corpas, F.J.; Chaki, M.; Fernandez-Ocana, A.; Valderrama, R.; Palma, J.M.; Carreras, A.; Begara-Morales, J.C.; Airaki, M.; del Río, L.A.; Barroso, J.B.J.P.; et al. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008, 49, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transducation in plants. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Pavlovic, D.; Nikolic, B.; Djurovic, S.; Waisi, H.; Andjelkovic, A.; Marisavljevic, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Fitomed. 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Rebeiz, C.A. Chlorophyll Biosynthesis and Technological Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. R. Soc. 2012, 367, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Suter, H.; He, J.-Z.; Hu, H.-W.; Chen, D. Dissimilatory nitrate reduction to ammonium dominates nitrate reduction in long-term low nitrogen fertilized rice paddies. Soil Biol. Biochem. 2019, 131, 149–156. [Google Scholar] [CrossRef]
- Kuiper, P.J.; Kuiper, D.; Schuit, J. Root functioning under stress conditions: An introduction. Plant Soil 1988, 249–253. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef]
- Khan, M.; Gemenet, D.C.; Villordon, A. Root system architecture and abiotic stress tolerance: Current knowledge in root and tuber crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.; Miguel, E.C.; Dias, J.M.; da Cunha, M.; Bressan-Smith, R.E.; de Oliveira, J.G.; de Souza Filho, G.A. Ultrastructural, physiological and biochemical analyses of chlorate toxicity on rice seedlings. Plant Sci. 2004, 166, 1057–1062. [Google Scholar] [CrossRef]
- LaBrie, S.T.; Wilkinson, J.Q.; Crawford, N.M. Effect of chlorate treatment on nitrate reductase and nitrite reductase gene expression in Arabidopsis thaliana. Plant Physiol. 1991, 97, 873–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.-m.; Hasegawa, H.; Ichii, M. A chlorate resistant mutant of rice (Oryza sativa L.) with normal nitrate uptake and nitrate reductase activity. Breed. Sci. 2000, 50, 9–15. [Google Scholar] [CrossRef]
- Rolly, N.K.; Lee, S.-U.; Imran, Q.M.; Hussain, A.; Mun, B.-G.; Kim, K.-M.; Yun, B.-W. Nitrosative stress-mediated inhibition of OsDHODH1 gene expression suggests roots growth reduction in rice (Oryza sativa L.). 3 Biotech 2019, 9, 273. [Google Scholar] [CrossRef]
- Wilkinson, J.Q.; Crawford, N.M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef]
- Islam, M.S. Sensing and Uptake of Nitrogen in Rice Plant: A Molecular View. Rice Sci. 2019, 26, 343–355. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Li, A.; Chu, C. NRT1.1s in plants: Functions beyond nitrate transport. J. Exp. Bot. 2020, 71, 4373–4379. [Google Scholar] [CrossRef]
- Zhang, G.-B.; Meng, S.; Gong, J.-M. The expected and unexpected roles of nitrate transporters in plant abiotic stress resistance and their regulation. Int. J. Mol. Sci. 2018, 19, 3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Wirén, N.; Gojon, A.; Chaillou, S.; Raper, D. Mechanisms and regulation of ammonium uptake in higher plants. In Plant Nitrogen; Springer: Berlin/Heidelberg, Germany, 2001; pp. 61–77. [Google Scholar]
- Bao, A.; Liang, Z.; Zhao, Z.; Cai, H. Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int. J. Mol. Sci. 2015, 16, 9037–9063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.M.; de Souza, V.M.; Tavares, O.C.H.; Zonta, E.; Santa-Catarina, C.; de Souza, S.R.; Fernandes, M.S.; Santos, L.A. OsAMT1.3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol. Rep. 2015, 9, 221–229. [Google Scholar] [CrossRef]
- Gaur, V.S.; Singh, U.; Gupta, A.K.; Kumar, A. Influence of different nitrogen inputs on the members of ammonium transporter and glutamine synthetase genes in two rice genotypes having differential responsiveness to nitrogen. Mol. Biol. Rep. 2012, 39, 8035–8044. [Google Scholar] [CrossRef]
- Kamachi, K.; Yamaya, T.; Mae, T.; Ojima, K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991, 96, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J. What is the role of hydrogen peroxide in plant peroxisomes? Plant Biol. 2015, 17, 1099–1103. [Google Scholar] [CrossRef]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.-U.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Fisher, H.; Orth, H. Die Chemie des pyrrols. Acad. Verl. Leipz. Ger. 1937, 623, 673. [Google Scholar]
- Rolly, N.K.; Imran, Q.M.; Lee, I.-J.; Yun, B.-W. Salinity Stress-Mediated Suppression of Expression of Salt Overly Sensitive Signaling Pathway Genes Suggests Negative Regulation by AtbZIP62 Transcription Factor in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armengaud, P.; Thiery, L.; Buhot, N.; Grenier-de March, G.; Savouré, A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol. Plant. 2004, 120, 442–450. [Google Scholar] [CrossRef]
- Newton, R.; Sen, S.; Puryear, J. Free proline changes in Pinus taeda L. callus in response to drought stress. Tree Physiol. 1986, 1, 325–332. [Google Scholar] [CrossRef]
- Mun, B.-G.; Lee, S.-U.; Hussain, A.; Kim, H.-H.; Rolly, N.K.; Jung, K.-H.; Yun, B.-W. S-nitrosocysteine-responsive genes modulate diverse regulatory pathways in Oryza sativa: A transcriptome profiling study. Funct. Plant Biol. 2018, 45, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 317–331. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 273–280. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Khan, A.R.; Ullah, I.; Waqas, M.; Park, G.-S.; Khan, A.L.; Hong, S.-J.; Ullah, R.; Jung, B.K.; Park, C.E.; Ur-Rehman, S. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol. Environ. Saf. 2017, 136, 180–188. [Google Scholar] [CrossRef]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase isozymes from horseradish roots I. Isolation and physical properties. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. [Google Scholar]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. [Google Scholar]
- Márton, L.; Sidorov, V.; Biasini, G.; Maliga, P. Complementation in somatic hybrids indicates four types of nitrate reductase deficient lines in Nicotiana plumbaginifolia. Mol. Gen. Genet. MGG 1982, 187, 1–3. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabange, N.R.; Park, S.-Y.; Lee, J.-Y.; Shin, D.; Lee, S.-M.; Kwon, Y.; Cha, J.-K.; Cho, J.-H.; Duyen, D.V.; Ko, J.-M.; et al. New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 22, 2192. https://doi.org/10.3390/ijms22042192
Kabange NR, Park S-Y, Lee J-Y, Shin D, Lee S-M, Kwon Y, Cha J-K, Cho J-H, Duyen DV, Ko J-M, et al. New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2021; 22(4):2192. https://doi.org/10.3390/ijms22042192
Chicago/Turabian StyleKabange, Nkulu Rolly, So-Yeon Park, Ji-Yun Lee, Dongjin Shin, So-Myeong Lee, Youngho Kwon, Jin-Kyung Cha, Jun-Hyeon Cho, Dang Van Duyen, Jong-Min Ko, and et al. 2021. "New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 22, no. 4: 2192. https://doi.org/10.3390/ijms22042192
APA StyleKabange, N. R., Park, S.-Y., Lee, J.-Y., Shin, D., Lee, S.-M., Kwon, Y., Cha, J.-K., Cho, J.-H., Duyen, D. V., Ko, J.-M., & Lee, J.-H. (2021). New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.). International Journal of Molecular Sciences, 22(4), 2192. https://doi.org/10.3390/ijms22042192







