Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines
Abstract
1. Introduction
2. Sedentary Behavior, Adiposity, and Low-Grade Chronic Inflammation: Risk Factors for Tumor Development and Metastasis
3. The Influence of Physical Activity on Adipokines in the Development and Course of Cancer
3.1. Adiponectin
3.2. Leptin
3.3. Apelin
3.4. Visfatin
3.5. Resistin
3.6. Ghrelin
3.7. Chemerin
3.8. Lipocalin 2
3.9. Osteopontin
3.10. IL-6 and TNF-α
3.11. Adipokines and Bone Metastases
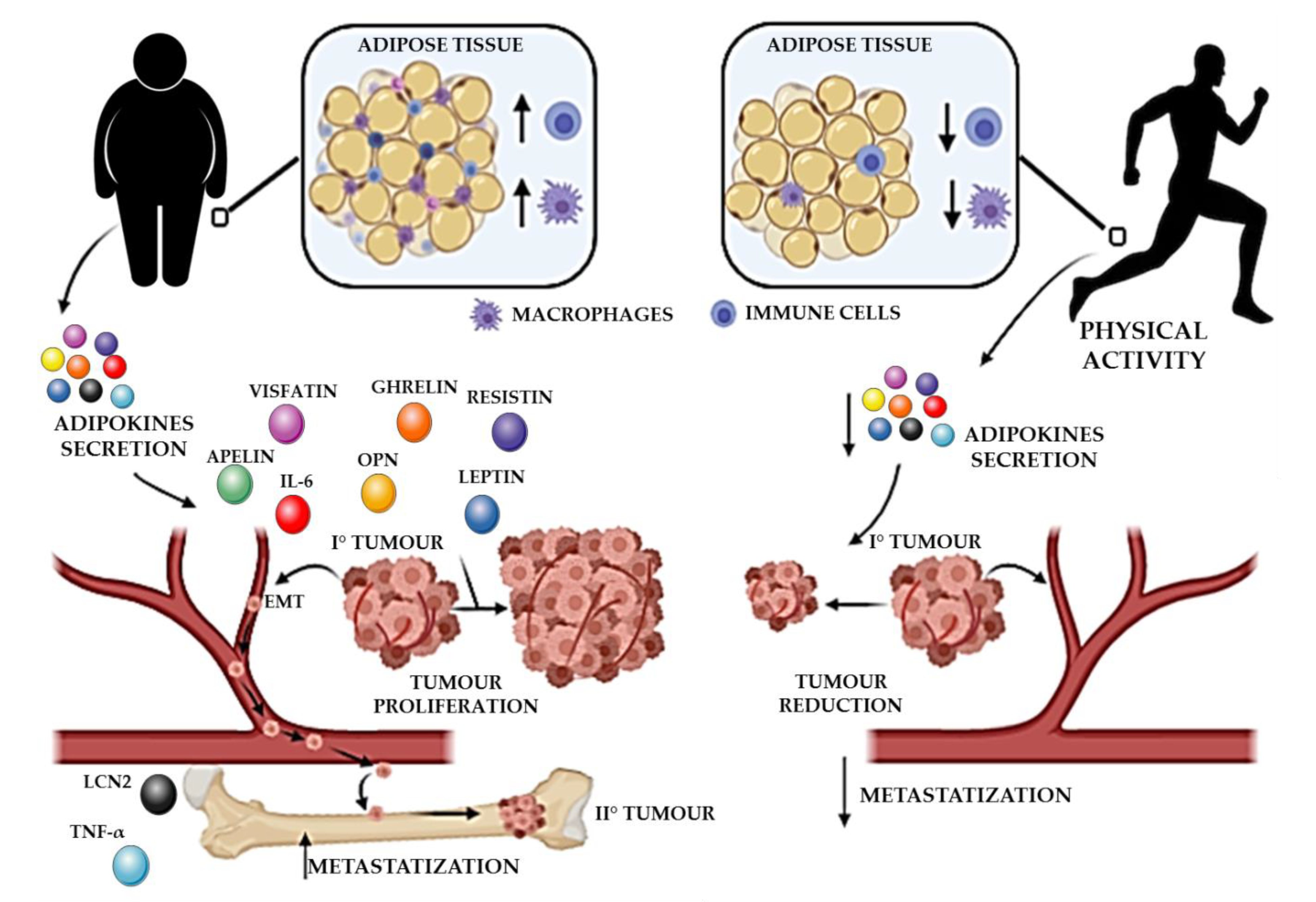
4. Effects of Physical Activity on Adipose Tissue, Low-Grade Inflammation, and Tumor Progression
5. How the PA-Dependent Effects on Adipokine and Low-Grade Inflammation Affect the Risk of Developing Metastases
6. Practical Recommendations and Conclusive Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACSM | American College of Sports Medicine |
| ADAM | A Disintegrin And Metalloproteinase Domain-Containing Protein |
| AdipoR1/2 | Adiponectin Receptor 1/2 |
| AIB1 | Amplified in Breast Cancer 1 |
| AMPK | Adenosine Monophosphate-Dependent Kinase |
| APPL1 | Adaptor Protein, Phosphotyrosine Interacting with PH Domain, and Leucine Zipper 1 |
| ASC | Adipose-Tissue-Derived Stromal Cell |
| AT | Adipose Tissue |
| BAT | Brown Adipose Tissue |
| BC | Breast Cancer |
| BMA | Bone Marrow Adipocyte |
| BMI | Body Mass Index |
| CAP1 | Adenylyl Cyclase-Associated Protein 1 |
| CCLR2 | C-C Motif Chemokine-Like Receptor 2 |
| CD | Cluster of Differentiation |
| CMKLR1 | Chemokine-Like Receptor 1 |
| CNS | Central Nervous System |
| COX2 | Cyclooxygenase 2 |
| CRC | Colorectal Cancer |
| CRP | C-reactive Protein |
| CSC | Cancer Stem Cell |
| CV | Cardiovascular Disease |
| DC | Dendritic Cell |
| ECM | Extracellular Matrix |
| EE | Endurance Exercise |
| Egr-1 | Early Growth Response Factor-1 |
| EMT | Epithelial–Mesenchymal Transition |
| ER | Oestrogen Receptor |
| ERK | Extracellular Signalling-Regulated Kinase |
| ESCC | Esophageal Squamous Cell Carcinoma |
| fFA | Free Fatty Acid |
| GC | Gastric Cancer |
| GH | Growth Hormone |
| GHSR | Growth Hormone Secretagogue Receptor |
| gp130 | Glycoprotein 130 |
| GPR-1 | G Protein-Coupled Receptor 1 |
| HCC | Hepatocellular Carcinoma |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HFD | High-Fat Diet |
| HIF-1α | Hypoxia Inducible Factor-1α |
| HIIT | High-Intensity Interval Training |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFNγ | Interferon γ |
| IGF1R | Insulin-Like Growth Factor 1 Receptor |
| IL | Interleukin |
| IRS | Insulin Receptor Substrate |
| LCN2 | Lipocalin 2 |
| LDL | Low-Density Lipoprotein |
| LEPR | Leptin Receptor |
| LGI | Low-Grade Inflammation |
| MAPK | Mitogen-Activated Protein Kinase |
| mAT | Marrow Adipose Tissue |
| MET | Metabolic Equivalent Task |
| Metrnl | Meteorin-Like |
| MM | Multiple Myeloma |
| MMP | Matrix Metalloproteinase |
| MSC | Mesenchymal Stem Cell |
| mtDNA | Mitochondrial DNA |
| NAMPT | Nicotinamide Phosphoribosyltransferase |
| NF-κB | Nuclear Factor κB |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NK | Natural Killer |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| NSCLC | Non-Small-Cell Lung Carcinoma |
| OPG | Osteoprotegerin |
| OPN | Osteopontin |
| PA | Physical Activity |
| PBEF | Pre-B-Cell Colony-Enhancing Factor |
| PBMC | Peripheral Blood Mononuclear Cell |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol-3-Kinase |
| PTEN | Phosphatase and Tensin Homolog |
| PTHrP | Parathyroid Hormone-Related Peptide |
| RANKL | Receptor Activator of NF-κB Ligand |
| RARRES2 | Retinoic Acid Receptor Responder 2 |
| RE | Resistance Exercise |
| ROR1 | Receptor Tyrosine Kinase-Like Orphan Receptor 1 |
| ROS | Reactive Oxygen Species |
| SDF-1 | Stromal-Cell-Derived Factor-1 |
| sIL-6R | Soluble Interleukin 6 Receptor |
| SKM | Skeletal Muscle |
| T2DM | Type 2 Diabetes Mellitus |
| TACE | Tumor Necrosis Factor-α-Converting Enzyme |
| TC | Thyroid Cancer |
| TLR4 | Toll-Like Receptor 4 |
| TNFα | Tumor Necrosis Factor α |
| TNFR | Tumor Necrosis Factor Receptor |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TRAIL-R2 | TNF-Related Apoptosis-Inducing Ligand Receptor 2 |
| UCP1 | Uncoupling Protein 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VCAM | Vascular Cell Adhesion Molecule |
| VSMC | Vascular Smooth Muscle Cells |
| WAT | White Adipose Tissue |
References
- Begg, S.J.; Vos, T.; Barker, B.; Stanley, L.; Lopez, A.D. Burden of disease and injury in Australia in the new millennium: Measuring health loss from diseases, injuries and risk factors. Med. J. Aust. 2008, 188, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef]
- Barker, K.; Eickmeyer, S. Therapeutic Exercise. Med. Clin. N. Am. 2020, 104, 189–198. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 1 September 2020).
- Eickmeyer, S.M.; Gamble, G.L.; Shahpar, S.; Do, K.D. The role and efficacy of exercise in persons with cancer. PM&R 2012, 4, 874–881. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Participants, S.T.C.P. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Jochem, C.; Wallmann-Sperlich, B.; Leitzmann, M.F. The Influence of Sedentary Behavior on Cancer Risk: Epidemiologic Evidence and Potential Molecular Mechanisms. Curr. Nutr. Rep. 2019, 8, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Yates, T.; Edwardson, C.L.; Khunti, K.; Talbot, D.; Gray, L.J.; Leigh, T.M.; Carter, P.; Davies, M.J. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE 2013, 8, e78350. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Anderson, A.S.; Key, T.J.; Norat, T.; Scoccianti, C.; Cecchini, M.; Berrino, F.; Boutron-Ruault, M.C.; Espina, C.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Obesity, body fatness and cancer. Cancer Epidemiol. 2015, 39 (Suppl. 1), S34–S45. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Hursting, S.D.; Digiovanni, J.; Dannenberg, A.J.; Azrad, M.; Leroith, D.; Demark-Wahnefried, W.; Kakarala, M.; Brodie, A.; Berger, N.A. Obesity, energy balance, and cancer: New opportunities for prevention. Cancer Prev. Res. 2012, 5, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.M.; Neilson, H.K.; Friedenreich, C.M. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011, 186, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E. Physical activity and gynecologic cancer prevention. Recent Results Cancer Res. 2011, 186, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, P.; Lombardi, G.; Colombini, A.; Banfi, G. Vitamin D in exercise: Physiologic and analytical concerns. Clin. Chim. Acta 2013, 16, 45–53. [Google Scholar] [CrossRef]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 030501. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Abiri, B.; Iravani, M.; Razavi, S.M.; Vafa, M. The Effects of UVB and Vitamin D on Decreasing Risk of Colorectal Cancer Incidence and Mortality: A Review of the Epidemiology, Clinical Trials, and Mechanisms. Nutr. Cancer 2019, 71, 709–717. [Google Scholar] [CrossRef]
- Tsiloulis, T.; Watt, M.J. Exercise and the Regulation of Adipose Tissue Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 175–201. [Google Scholar] [CrossRef]
- Correa, L.H.; Correa, R.; Farinasso, C.M.; de Sant’Ana Dourado, L.P.; Magalhaes, K.G. Adipocytes and Macrophages Interplay in the Orchestration of Tumor Microenvironment: New Implications in Cancer Progression. Front. Immunol. 2017, 8, 1129. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and meta-inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Santander, A.M.; Lopez-Ocejo, O.; Casas, O.; Agostini, T.; Sanchez, L.; Lamas-Basulto, E.; Carrio, R.; Cleary, M.P.; Gonzalez-Perez, R.R.; Torroella-Kouri, M. Paracrine Interactions between Adipocytes and Tumor Cells Recruit and Modify Macrophages to the Mammary Tumor Microenvironment: The Role of Obesity and Inflammation in Breast Adipose Tissue. Cancers 2015, 7, 143–178. [Google Scholar] [CrossRef]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tseng, C.; Zhang, Y.; Sirin, O.; Corn, P.G.; Li-Ning-Tapia, E.M.; Troncoso, P.; Davis, J.; Pettaway, C.; Ward, J.; et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016, 7, 11674. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Ahn, S.; Saha, A.; DiGiovanni, J.; Kolonin, M.G. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 2019, 38, 1979–1988. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Barb, D.; Williams, C.J.; Neuwirth, A.K.; Mantzoros, C.S. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am. J. Clin. Nutr. 2007, 86, s858–s866. [Google Scholar] [CrossRef]
- Dieudonne, M.N.; Bussiere, M.; Dos Santos, E.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2006, 345, 271–279. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, Y.Y.; Yu, B.Y.; Yang, B.S.; Cho, K.H.; Yoon, D.K.; Roh, Y.K. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch. Pharm. Res. 2005, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Miyoshi, Y.; Ishihara, H.; Noguchi, S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008, 112, 405–410. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell Adh. Mig. 2010, 4, 358–362. [Google Scholar] [CrossRef]
- Alessi, D.R.; Sakamoto, K.; Bayascas, J.R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006, 75, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L.; et al. Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef]
- Moon, H.S.; Chamberland, J.P.; Aronis, K.; Tseleni-Balafouta, S.; Mantzoros, C.S. Direct role of adiponectin and adiponectin receptors in endometrial cancer: In vitro and ex vivo studies in humans. Mol. Cancer Therap. 2011, 10, 2234–2243. [Google Scholar] [CrossRef]
- Erdogan, S.; Sezer, S.; Baser, E.; Gun-Eryilmaz, O.; Gungor, T.; Uysal, S.; Yilmaz, F.M. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr. Relat. Cancer 2013, 20, 669–675. [Google Scholar] [CrossRef]
- Gonullu, G.; Kahraman, H.; Bedir, A.; Bektas, A.; Yucel, I. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int. J. Colorectal Dis. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Cui, E.; Guo, H.; Shen, M.; Yu, H.; Gu, D.; Mao, W.; Wang, X. Adiponectin inhibits migration and invasion by reversing epithelialmesenchymal transition in nonsmall cell lung carcinoma. Oncol. Rep. 2018, 40, 1330–1338. [Google Scholar] [CrossRef]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. 2002, 26, 1407–1433. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.Y.; Hamnvik, O.P.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endcorinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef]
- Yang, R.; Barouch, L.A. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 2007, 101, 545–559. [Google Scholar] [CrossRef]
- Tessitore, L.; Vizio, B.; Jenkins, O.; De Stefano, I.; Ritossa, C.; Argiles, J.M.; Benedetto, C.; Mussa, A. Leptin expression in colorectal and breast cancer patients. Int. J. Mol. Med. 2000, 5, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Robles, M.J.; Segura-Ortega, J.E.; Fafutis-Morris, M. The biology of leptin and its implications in breast cancer: A general view. J. Interferon Cytokine Res. 2013, 33, 717–727. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Wei, X.H.; Li, S.J.; Liu, Y.; Hu, H.L.; Li, Z.Z.; Kuang, X.H.; Wang, L.; Shi, X.; Yuan, S.T.; et al. Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun. Signal. 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Jarde, T.; Perrier, S.; Vasson, M.P.; Caldefie-Chezet, F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer 2011, 47, 33–43. [Google Scholar] [CrossRef]
- Catalano, S.; Mauro, L.; Marsico, S.; Giordano, C.; Rizza, P.; Rago, V.; Montanaro, D.; Maggiolini, M.; Panno, M.L.; Ando, S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J. Biol. Chem. 2004, 279, 19908–19915. [Google Scholar] [CrossRef]
- Cirillo, D.; Rachiglio, A.M.; la Montagna, R.; Giordano, A.; Normanno, N. Leptin signaling in breast cancer: An overview. J. Cell Biochem. 2008, 105, 956–964. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Wang, R.; Wang, K.; Xu, Y.; Song, G.; Wu, C.; Yin, Y. Leptin and HER-2 are associated with gastric cancer progression and prognosis of patients. Biomed. Pharm. 2012, 66, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Fu, S.; Xu, X.; Yang, Y.; Du, L.; Li, W.; Kan, S.; Li, Z.; Zhang, X.; Wang, L.; et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br. J. Cancer 2014, 110, 1801–1810. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, X.; Du, L.; Yang, Y.; Cheng, H.; Zhang, X.; Li, Z.; Wang, L.; Li, J.; Liu, H.; et al. Leptin-mediated regulation of MT1-MMP localization is KIF1B dependent and enhances gastric cancer cell invasion. Carcinogenesis 2013, 34, 974–983. [Google Scholar] [CrossRef]
- Ratke, J.; Entschladen, F.; Niggemann, B.; Zanker, K.S.; Lang, K. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocr. Relat. Cancer 2010, 17, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gan, Y.; Shen, Y.; Cai, X.; Song, Y.; Zhao, F.; Yao, M.; Gu, J.; Tu, H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget 2015, 6, 16120–16134. [Google Scholar] [CrossRef] [PubMed]
- Harbuzariu, A.; Rampoldi, A.; Daley-Brown, D.S.; Candelaria, P.; Harmon, T.L.; Lipsey, C.C.; Beech, D.J.; Quarshie, A.; Ilies, G.O.; Gonzalez-Perez, R.R. Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget 2017, 8, 7740–7752. [Google Scholar] [CrossRef] [PubMed]
- Kleinz, M.J.; Davenport, A.P. Emerging roles of apelin in biology and medicine. Pharmacol. Ther. 2005, 107, 198–211. [Google Scholar] [CrossRef]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef]
- Wu, L.; Chen, L.; Li, L. Apelin/APJ system: A novel promising therapy target for pathological angiogenesis. Clin. Chim. Acta 2017, 466, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yuan, L.Q.; Luo, X.H.; Huang, J.; Cui, R.R.; Guo, L.J.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Apelin suppresses apoptosis of human osteoblasts. Apoptosis 2007, 12, 247–254. [Google Scholar] [CrossRef]
- Masoumi, J.; Jafarzadeh, A.; Khorramdelazad, H.; Abbasloui, M.; Abdolalizadeh, J.; Jamali, N. Role of Apelin/APJ axis in cancer development and progression. Adv. Med. Sci. 2020, 65, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, F.; Wang, P.; Jia, S.; Sun, L.; Huo, H. Apelin-13 induces MCF-7 cell proliferation and invasion via phosphorylation of ERK1/2. Int. J. Mol. Med. 2015, 36, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Su, T.; Lv, D.; Xie, F.; Liu, W.; Cao, J.; Sheikh, I.A.; Qin, X.; Li, L.; Chen, L. ERK1/2 mediates lung adenocarcinoma cell proliferation and autophagy induced by apelin-13. Acta Biochim. Biophys. Sin. 2014, 46, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Berta, J.; Kenessey, I.; Dobos, J.; Tovari, J.; Klepetko, W.; Jan Ankersmit, H.; Hegedus, B.; Renyi-Vamos, F.; Varga, J.; Lorincz, Z.; et al. Apelin expression in human non-small cell lung cancer: Role in angiogenesis and prognosis. J. Thorac. Oncol. 2010, 5, 1120–1129. [Google Scholar] [CrossRef]
- Li, L.L.L.; He, L.; Zhang, Z.; Xie, F.; Guo, Y.; Xiao, J.; Chen, L.; Li, J. Effects of Apelin-13 on Rat Bone Marrow-Derived Mesenchymal Stem Cell Proliferation Through the AKT/GSK3β/Cyclin D1 Pathway. Int. J. Pept. Res. Therap. 2014, 20, 421–425. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Zhang, Z.; Jiang, Z. Hypoxia promotes bone marrow-derived mesenchymal stem cell proliferation through apelin/APJ/autophagy pathway. Acta Biochim. Biophys. Sin. 2015, 47, 362–367. [Google Scholar] [CrossRef]
- Zhang, N.K.; Cao, Y.; Zhu, Z.M.; Zheng, N.; Wang, L.; Xu, X.H.; Gao, L.R. Activation of Endogenous Cardiac Stem Cells by Apelin-13 in Infarcted Rat Heart. Cell Transpl. 2016, 25, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Hung, A.C.; Lo, S.; Hou, M.F.; Lee, Y.C.; Tsai, C.H.; Chen, Y.Y.; Liu, W.; Su, Y.H.; Lo, Y.H.; Wang, C.H.; et al. Extracellular Visfatin-Promoted Malignant Behavior in Breast Cancer Is Mediated Through c-Abl and STAT3 Activation. Clin. Cancer Res. 2016, 22, 4478–4490. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Holm, S.; Aukrust, P.; Halvorsen, B. Visfatin/NAMPT: A multifaceted molecule with diverse roles in physiology and pathophysiology. Annu. Rev. Nutr. 2012, 32, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The Role of Secretory Activity Molecules of Visceral Adipocytes in Abdominal Obesity in the Development of Cardiovascular Disease: A Review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef]
- Lin, T.C. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer Manag. Res. 2019, 11, 3481–3491. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Waligorska-Stachura, J.; Andrusiewicz, M.; Biczysko, M.; Sowinski, J.; Skrobisz, J.; Ruchala, M. Nicotinamide phosphorybosiltransferase overexpression in thyroid malignancies and its correlation with tumor stage and with survivin/survivin DEx3 expression. Tumor Biol. 2015, 36, 7859–7863. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tian, W.; Liu, Y.; Ju, Y.; Shen, Y.; Zhao, S.; Zhang, B.; Li, Y. Visfatin Triggers the Cell Motility of Non-Small Cell Lung Cancer via UpRegulation of Matrix Metalloproteinases. Basic Clin. Pharmacol. Toxicol. 2016, 119, 548–554. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, C.; Sun, X.; Zhang, L.; Liu, L.; Ouyang, J.; Li, B. Visfatin promotes osteosarcoma cell migration and invasion via induction of epithelial-mesenchymal transition. Oncol. Rep. 2015, 34, 987–994. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.C.; Kwon, Y.W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.Y.; Lee, J.; Yang, H.M.; et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Diabetes. The missing link with obesity? Nature 2001, 409, 292–293. [Google Scholar] [CrossRef]
- Zhao, C.W.; Gao, Y.H.; Song, W.X.; Liu, B.; Ding, L.; Dong, N.; Qi, X. An Update on the Emerging Role of Resistin on the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2019, 2019, 1532164. [Google Scholar] [CrossRef] [PubMed]
- Codoner-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta 2015, 438, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.S.; Won, E.H.; Chang, I.H.; Kim, T.H.; Park, E.S.; Kim, M.K.; Kim, W.; Myung, S.C. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011, 108, E77–E83. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci. Rep. 2016, 6, 18923. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Wang, G.; Englander, E.W.; Kojima, M.; Greeley, G.H., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: Enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002, 143, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Inui, A.; Asakawa, A.; Bowers, C.Y.; Mantovani, G.; Laviano, A.; Meguid, M.M.; Fujimiya, M. Ghrelin, appetite, and gastric motility: The emerging role of the stomach as an endocrine organ. FASEB J. 2004, 18, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C. Structure and physiological actions of ghrelin. Scientifica 2013, 2013, 518909. [Google Scholar] [CrossRef]
- Ibrahim Abdalla, M.M. Ghrelin—Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef]
- Lien, G.S.; Lin, C.H.; Yang, Y.L.; Wu, M.S.; Chen, B.C. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur. J. Pharmacol. 2016, 776, 124–131. [Google Scholar] [CrossRef]
- Kraus, D.; Reckenbeil, J.; Wenghoefer, M.; Stark, H.; Frentzen, M.; Allam, J.P.; Novak, N.; Frede, S.; Gotz, W.; Probstmeier, R.; et al. Ghrelin promotes oral tumor cell proliferation by modifying GLUT1 expression. Cell Mol. Life Sci. 2016, 73, 1287–1299. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, L.; Hu, D.; Ji, J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-kappaB signaling pathway. Mol. Cell Biochem. 2013, 382, 163–172. [Google Scholar] [CrossRef]
- Duxbury, M.S.; Waseem, T.; Ito, H.; Robinson, M.K.; Zinner, M.J.; Ashley, S.W.; Whang, E.E. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 2003, 309, 464–468. [Google Scholar] [CrossRef]
- Li, A.; Cheng, G.; Zhu, G.H.; Tarnawski, A.S. Ghrelin stimulates angiogenesis in human microvascular endothelial cells: Implications beyond GH release. Biochem. Biophys. Res. Commun. 2007, 353, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cassoni, P.; Papotti, M.; Ghe, C.; Catapano, F.; Sapino, A.; Graziani, A.; Deghenghi, R.; Reissmann, T.; Ghigo, E.; Muccioli, G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 2001, 86, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Cordoba-Chacon, J.; Hergueta-Redondo, M.; Martinez-Fuentes, A.J.; Kineman, R.D.; Moreno-Bueno, G.; Luque, R.M.; Castano, J.P. A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: Potential pathophysiological relevance. PLoS ONE 2011, 6, e23302. [Google Scholar] [CrossRef]
- Xu, X.; Jhun, B.S.; Ha, C.H.; Jin, Z.G. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology 2008, 149, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. CMKLR1 and GPR1 mediate chemerin signaling through the RhoA/ROCK pathway. Mol. Cell Endocrinol. 2015, 417, 36–51. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Wanninger, J.; Schmidhofer, S.; Weigert, J.; Neumeier, M.; Dorn, C.; Hellerbrand, C.; Zimara, N.; Schaffler, A.; Aslanidis, C.; et al. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology 2011, 152, 26–35. [Google Scholar] [CrossRef]
- Treeck, O.; Buechler, C.; Ortmann, O. Chemerin and Cancer. Int. J. Mol. Sci. 2019, 20, 3750. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Pachynski, R.K. Chemerin modulation of tumor growth: Potential clinical applications in cancer. Discov. Med. 2018, 26, 31–37. [Google Scholar]
- Shin, W.J.; Zabel, B.A.; Pachynski, R.K. Mechanisms and Functions of Chemerin in Cancer: Potential Roles in Therapeutic Intervention. Front. Immunol. 2018, 9, 2772. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Wang, P.; Salazar, N.; Zheng, Y.; Nease, L.; Rosalez, J.; Leong, W.I.; Virdi, G.; Rennier, K.; Shin, W.J.; et al. Chemerin Suppresses Breast Cancer Growth by Recruiting Immune Effector Cells Into the Tumor Microenvironment. Front. Immunol. 2019, 10, 983. [Google Scholar] [CrossRef]
- El-Sagheer, G.; Gayyed, M.; Ahmad, A.; Abd El-Fattah, A.; Mohamed, M. Expression of chemerin correlates with a poor prognosis in female breast cancer patients. Breast Cancer 2018, 10, 169–176. [Google Scholar] [CrossRef]
- Sarmadi, P.; Tunali, G.; Esendagli-Yilmaz, G.; Yilmaz, K.B.; Esendagli, G. CRAM-A indicates IFN-gamma-associated inflammatory response in breast cancer. Mol. Immunol. 2015, 68, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yin, H.K.; Guan, D.X.; Zhao, J.S.; Feng, Y.X.; Deng, Y.Z.; Wang, X.; Li, N.; Wang, X.F.; Cheng, S.Q.; et al. Chemerin suppresses hepatocellular carcinoma metastasis through CMKLR1-PTEN-Akt axis. Br. J. Cancer 2018, 118, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Liu-Chittenden, Y.; Jain, M.; Gaskins, K.; Wang, S.; Merino, M.J.; Kotian, S.; Kumar Gara, S.; Davis, S.; Zhang, L.; Kebebew, E. RARRES2 functions as a tumor suppressor by promoting beta-catenin phosphorylation/degradation and inhibiting p38 phosphorylation in adrenocortical carcinoma. Oncogene 2017, 36, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, W.K.; Liu, X.; To, K.F.; Chen, G.G.; Yu, J.; Ng, E.K. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: A clinical and experimental study. Peptides 2014, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.D.; Kandola, S.; Tiszlavicz, L.; Reisz, Z.; Dockray, G.J.; Varro, A. The role of chemerin and ChemR23 in stimulating the invasion of squamous oesophageal cancer cells. Br. J. Cancer 2016, 114, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Tummler, C.; Snapkov, I.; Wickstrom, M.; Moens, U.; Ljungblad, L.; Maria Elfman, L.H.; Winberg, J.O.; Kogner, P.; Johnsen, J.I.; Sveinbjornsson, B. Inhibition of chemerin/CMKLR1 axis in neuroblastoma cells reduces clonogenicity and cell viability in vitro and impairs tumor growth in vivo. Oncotarget 2017, 8, 95135–95151. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef] [PubMed]
- Somiari, S.B.; Shriver, C.D.; Heckman, C.; Olsen, C.; Hu, H.; Jordan, R.; Arciero, C.; Russell, S.; Garguilo, G.; Hooke, J.; et al. Plasma concentration and activity of matrix metalloproteinase 2 and 9 in patients with breast disease, breast cancer and at risk of developing breast cancer. Cancer Lett. 2006, 233, 98–107. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ott, K.M. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury--where do we stand today? Nephrol. Dial. Transpl. 2011, 26, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Borkham-Kamphorst, E.; van de Leur, E.; Zimmermann, H.W.; Karlmark, K.R.; Tihaa, L.; Haas, U.; Tacke, F.; Berger, T.; Mak, T.W.; Weiskirchen, R. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim. Biophys. Acta 2013, 1832, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 (LCN2) Expression in Hepatic Malfunction and Therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Bauer, R.; Rehwald, C.; Brune, B. Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol. Res. 2017, 120, 146–156. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.H. Lipocalin2 expressions correlate significantly with tumor differentiation in epithelial ovarian cancer. J. Histochem. Cytochem. 2009, 57, 513–521. [Google Scholar] [CrossRef]
- Tong, Z.; Wu, X.; Ovcharenko, D.; Zhu, J.; Chen, C.S.; Kehrer, J.P. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem. J. 2005, 391, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtroder, K.; Christensen, L.L.; Olesen, S.H.; Frederiksen, C.M.; Laiho, P.; Aaltonen, L.A.; Laurberg, S.; Sorensen, F.B.; Hagemann, R.; ØRntoft, T.F. Gene expression in colorectal cancer. Cancer Res. 2002, 62, 4352–4363. [Google Scholar] [PubMed]
- Tong, Z.; Kunnumakkara, A.B.; Wang, H.; Matsuo, Y.; Diagaradjane, P.; Harikumar, K.B.; Ramachandran, V.; Sung, B.; Chakraborty, A.; Bresalier, R.S.; et al. Neutrophil gelatinase-associated lipocalin: A novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008, 68, 6100–6108. [Google Scholar] [CrossRef]
- Hu, C.; Yang, K.; Li, M.; Huang, W.; Zhang, F.; Wang, H. Lipocalin 2: A potential therapeutic target for breast cancer metastasis. OncoTargets Ther. 2018, 11, 8099–8106. [Google Scholar] [CrossRef] [PubMed]
- Oren, B.; Urosevic, J.; Mertens, C.; Mora, J.; Guiu, M.; Gomis, R.R.; Weigert, A.; Schmid, T.; Grein, S.; Brune, B.; et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J. Pathol. 2016, 239, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McNeish, B.; Butterfield, C.; Moses, M.A. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013, 27, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.P.; Wu, B.L.; Xie, Y.M.; Zhang, Y.L.; Liao, L.D.; Zhou, F.; Xie, J.J.; Zeng, F.M.; Xu, X.E.; Fang, W.K.; et al. Lipocalin 2 promotes the migration and invasion of esophageal squamous cell carcinoma cells through a novel positive feedback loop. Biochim. Biophys. Acta 2015, 1853, 2240–2250. [Google Scholar] [CrossRef]
- Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Montalto, G.; Cervello, M.; Polesel, J.; et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim. Biophys. Acta 2016, 1863, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Min, I.S.; Park, Y.R.; Lee, S.T.; Kim, S.W. Lipocalin 2 inversely regulates TRAIL sensitivity through p38 MAPK-mediated DR5 regulation in colorectal cancer. Int. J. Oncol. 2018, 53, 2789–2799. [Google Scholar] [CrossRef]
- Haylock, D.N.; Nilsson, S.K. Osteopontin: A bridge between bone and blood. Br. J. Hematol. 2006, 134, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Nat. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K.; Rittling, S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol. Sci. 2008, 103, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ambrosi, J.; Catalan, V.; Ramirez, B.; Rodriguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of osteopontin with IL-18 in obese individuals: Implications for insulin resistance. PLoS ONE 2013, 8, e63944. [Google Scholar] [CrossRef]
- Cabia, B.; Andrade, S.; Carreira, M.C.; Casanueva, F.F.; Crujeiras, A.B. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes. Rev. 2016, 17, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol. Lett. 2019, 17, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, P.; Kumar, D.; Chakraborty, G.; Gorain, M.; Kundu, G.C. Functional characterization of stromal osteopontin in melanoma progression and metastasis. PLoS ONE 2013, 8, e69116. [Google Scholar] [CrossRef]
- Kothari, A.N.; Arffa, M.L.; Chang, V.; Blackwell, R.H.; Syn, W.K.; Zhang, J.; Mi, Z.; Kuo, P.C. Osteopontin-A Master Regulator of Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1alpha-mediated VEGF-dependent angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef]
- Wai, P.Y.; Kuo, P.C. The role of Osteopontin in tumor metastasis. J. Surg. Res. 2004, 121, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Xia, Y.H.; Xue, T.C.; Zhang, H.; Ye, S.L. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol. Rep. 2011, 25, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Guo, L.; Lin, G.; Chen, Z.; Chen, T.; Lin, J.; Zhang, B.; Gu, X. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015, 39, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26. [Google Scholar] [CrossRef]
- Desai, B.; Rogers, M.J.; Chellaiah, M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol. Cancer 2007, 6, 18. [Google Scholar] [CrossRef]
- Ito, T.; Hashimoto, Y.; Tanaka, E.; Kan, T.; Tsunoda, S.; Sato, F.; Higashiyama, M.; Okumura, T.; Shimada, Y. An inducible short-hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin. Cancer Res. 2006, 12, 1308–1316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lazar, M.; Sullivan, J.; Chipitsyna, G.; Gong, Q.; Ng, C.Y.; Salem, A.F.; Aziz, T.; Witkiewicz, A.; Denhardt, D.T.; Yeo, C.J.; et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J. Gastrointest. Surg. 2010, 14, 1566–1577. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Direct. Autoimmun. 2010, 11, 145–156. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metast. Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, I.; Hutton, B.; Moretto, P.; Ng, T.; Addison, C.L.; Clemons, M. Incidence, consequences and treatment of bone metastases in breast cancer patients-Experience from a single cancer centre. J. Bone Oncol. 2013, 2, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, F.; Vargas, G.; Clezardin, P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016, 5, 93–95. [Google Scholar] [CrossRef]
- Sen, S.; Dasgupta, P.; Kamath, G.; Srikanth, H.S. Paratharmone related protein (peptide): A novel prognostic, diagnostic and therapeutic marker in Head & Neck cancer. J. Stomatol. Maxillofac. Surg. 2018, 119, 33–36. [Google Scholar] [CrossRef]
- Caers, J.; Deleu, S.; Belaid, Z.; De Raeve, H.; Van Valckenborgh, E.; De Bruyne, E.; Defresne, M.P.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 2007, 21, 1580–1584. [Google Scholar] [CrossRef]
- Rosen, C.J.; Ackert-Bicknell, C.; Rodriguez, J.P.; Pino, A.M. Marrow fat and the bone microenvironment: Developmental, functional, and pathological implications. Crit. Rev. 2009, 19, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.V.; Edwards, C.M. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front. Endocrinol. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Laharrague, P.; Fontanilles, A.M.; Tkaczuk, J.; Corberand, J.X.; Penicaud, L.; Casteilla, L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur. Cytokine Netw. 2000, 11, 634–639. [Google Scholar] [PubMed]
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia 2015, 17, 849–861. [Google Scholar] [CrossRef]
- Maroni, P. Leptin, Adiponectin, and Sam68 in Bone Metastasis from Breast Cancer. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Adipokines as therapeutic targets in breast cancer treatment. Exp. Opin. Therap. Targets 2018, 22, 941–953. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef]
- Zhang, J.; Choi, Y.; Mavromatis, B.; Lichtenstein, A.; Li, W. Preferential killing of PTEN-null myelomas by PI3K inhibitors through Akt pathway. Oncogene 2003, 22, 6289–6295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, G.; He, Y.; Yu, X. Bone Marrow Adipocyte: An Intimate Partner with Tumor Cells in Bone Metastasis. Front. Endocrinol. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; He, J.; Liu, H.; Lin, P.; Wan, X.; Navone, N.M.; Tong, Q.; Kwak, L.W.; Orlowski, R.Z.; et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget 2015, 6, 34329–34341. [Google Scholar] [CrossRef] [PubMed]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin. Exp. Metast. 2015, 32, 353–368. [Google Scholar] [CrossRef]
- Kim, A.Y.; Lee, Y.S.; Kim, K.H.; Lee, J.H.; Lee, H.K.; Jang, S.H.; Kim, S.E.; Lee, G.Y.; Lee, J.W.; Jung, S.A.; et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 2010, 24, 1441–1452. [Google Scholar] [CrossRef]
- Wang, C.; Wen, J.; Zhou, Y.; Li, L.; Cui, X.; Wang, J.; Pan, L.; Ye, Z.; Liu, P.; Wu, L. Apelin induces vascular smooth muscle cells migration via a PI3K/Akt/FoxO3a/MMP-2 pathway. Int. J. Biochem. Cell Biol. 2015, 69, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Rogers, R.J.; Davis, K.K.; Collins, K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018, 64, 99–107. [Google Scholar] [CrossRef]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Sutherland, L.N.; Bomhof, M.R.; Capozzi, L.C.; Basaraba, S.A.; Wright, D.C. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J. Phsyiol. 2009, 587, 1607–1617. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef]
- Macpherson, R.E.; Huber, J.S.; Frendo-Cumbo, S.; Simpson, J.A.; Wright, D.C. Adipose Tissue Insulin Action and IL-6 Signaling after Exercise in Obese Mice. Med. Sci. Sport Exerc. 2015, 47, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Chavanelle, V.; Boisseau, N.; Otero, Y.F.; Combaret, L.; Dardevet, D.; Montaurier, C.; Delcros, G.; Peltier, S.L.; Sirvent, P. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci. Rep. 2017, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Styner, M.; Thompson, W.R.; Galior, K.; Uzer, G.; Wu, X.; Kadari, S.; Case, N.; Xie, Z.; Sen, B.; Romaine, A.; et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 2014, 64, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Styner, M.; Pagnotti, G.M.; McGrath, C.; Wu, X.; Sen, B.; Uzer, G.; Xie, Z.; Zong, X.; Styner, M.A.; Rubin, C.T.; et al. Exercise Decreases Marrow Adipose Tissue Through ss-Oxidation in Obese Running Mice. J. Bone Miner. Res. 2017, 32, 1692–1702. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sport Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Ahmadi-Kani Golzar, F.; Fathi, R.; Mahjoub, S. High-fat diet leads to adiposity and adipose tissue inflammation: The effect of whey protein supplementation and aerobic exercise training. Appl. Physiol. Nutr. Metab. 2019, 44, 255–262. [Google Scholar] [CrossRef]
- Stinkens, R.; Brouwers, B.; Jocken, J.W.; Blaak, E.E.; Teunissen-Beekman, K.F.; Hesselink, M.K.; van Baak, M.A.; Schrauwen, P.; Goossens, G.H. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiol. 2018, 125, 1585–1593. [Google Scholar] [CrossRef]
- Larsen, S.; Danielsen, J.H.; Sondergard, S.D.; Sogaard, D.; Vigelsoe, A.; Dybboe, R.; Skaaby, S.; Dela, F.; Helge, J.W. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand. J. Med. Sci. Sports 2015, 25, e59–e69. [Google Scholar] [CrossRef]
- Ronn, T.; Volkov, P.; Tornberg, A.; Elgzyri, T.; Hansson, O.; Eriksson, K.F.; Groop, L.; Ling, C. Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6-month exercise intervention. Acta Physiol. 2014, 211, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Giolo De Carvalho, F.; Sparks, L.M. Targeting White Adipose Tissue with Exercise or Bariatric Surgery as Therapeutic Strategies in Obesity. Biology 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cervantes, C.; Liu, F. Common and distinct regulation of human and mouse brown and beige adipose tissues: A promising therapeutic target for obesity. Protein Cell 2017, 8, 446–454. [Google Scholar] [CrossRef]
- Hughes, D.A.; Jastroch, M.; Stoneking, M.; Klingenspor, M. Molecular evolution of UCP1 and the evolutionary history of mammalian non-shivering thermogenesis. BMC Evol. Biol. 2009, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.F.; Parsons, S.A.; Smith, S.R.; Sparks, L.M. Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity 2016, 24, 2467–2470. [Google Scholar] [CrossRef]
- Otero-Diaz, B.; Rodriguez-Flores, M.; Sanchez-Munoz, V.; Monraz-Preciado, F.; Ordonez-Ortega, S.; Becerril-Elias, V.; Baay-Guzman, G.; Obando-Monge, R.; Garcia-Garcia, E.; Palacios-Gonzalez, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9, 1781. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Sanchis-Gomar, F.; Perego, S.; Sansoni, V.; Banfi, G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine 2016, 54, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Balci, M.K.; Balci, N.; Karayalcin, U. Acute effect of exercise on plasma leptin level and insulin resistance in obese women with stable caloric intake. Endocr. Res. 2007, 32, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Klimcakova, E.; Moro, C.; Viguerie, N.; Berlan, M.; Hejnova, J.; Richterova, B.; Kraus, I.; Langin, D.; Stich, V. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metab. Clin. Exp. 2006, 55, 1375–1381. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Fenicchia, L.M.; Miller, C.S.; Ploutz-Synder, L.L.; Weinstock, R.S.; Carhart, R.; Azevedo, J.L., Jr. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int. J. Obes. 2001, 25, 1474–1480. [Google Scholar] [CrossRef]
- Gorgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and Regulation of Adipokine and Myokine Production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G.; et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. Open 2015, 1, 19. [Google Scholar] [CrossRef]
- Koh, Y.; Park, K.S. Responses of inflammatory cytokines following moderate intensity walking exercise in overweight or obese individuals. J. Exerc. Rehabil. 2017, 13, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.A.; Dachs, G.U. Effects of Exercise on the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 31–51. [Google Scholar] [CrossRef]
- Vellers, H.L.; Kleeberger, S.R.; Lightfoot, J.T. Inter-individual variation in adaptations to endurance and resistance exercise training: Genetic approaches towards understanding a complex phenotype. Mammal. Genome 2018, 29, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sport Exerc. 2001, 33, S446–S451; discussion S452–S453. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Fu, A.; Luo, K.Q. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci. Rep. 2017, 7, 39975. [Google Scholar] [CrossRef]
- Mei, X.; Middleton, K.; Shim, D.; Wan, Q.; Xu, L.; Ma, Y.V.; Devadas, D.; Walji, N.; Wang, L.; Young, E.W.K.; et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. 2019, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef]
- Wang, J.S.; Chang, C.Y.; Chow, S.E.; Chen, Y.W.; Yang, C.M. Exercise modulates platelet-nasopharyngeal carcinoma cell aggregation and subsequent tissue factor and matrix metalloproteinase activities. J. Appl. Physiol. 2007, 103, 763–770. [Google Scholar] [CrossRef]
- Alvarado, A.; Gil da Costa, R.M.; Faustino-Rocha, A.I.; Ferreira, R.; Lopes, C.; Oliveira, P.A.; Colaco, B. Effects of exercise training on breast cancer metastasis in a rat model. Int. J. Exp. Pathol. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.A.; Dachs, G.U. Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE(-/-) mice. J. Appl. Physiol. 2018, 124, 938–949. [Google Scholar] [CrossRef]
- Yan, L.; Demars, L.C. Effects of non-motorized voluntary running on experimental and spontaneous metastasis in mice. Anticancer Res. 2011, 31, 3337–3344. [Google Scholar]
- Rincon-Castanedo, C.; Morales, J.S.; Martin-Ruiz, A.; Valenzuela, P.L.; Ramirez, M.; Santos-Lozano, A.; Lucia, A.; Fiuza-Luces, C. Physical exercise effects on metastasis: A systematic review and meta-analysis in animal cancer models. Cancer Metast. Rev. 2020, 39, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Rief, H.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Debus, J.; Forster, R. Resistance training concomitant to radiotherapy of spinal bone metastases—Survival and prognostic factors of a randomized trial. Radiat. Oncol. 2016, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Fels, D.R.; West, M.; Allen, J.D.; Broadwater, G.; Barry, W.T.; Wilke, L.G.; Masko, E.; Douglas, P.S.; Dash, R.C.; et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev. Res. 2013, 6, 925–937. [Google Scholar] [CrossRef]
- Brown, J.C.; Rhim, A.D.; Manning, S.L.; Brennan, L.; Mansour, A.I.; Rustgi, A.K.; Damjanov, N.; Troxel, A.B.; Rickels, M.R.; Ky, B.; et al. Effects of exercise on circulating tumor cells among patients with resected stage I-III colon cancer. PLoS ONE 2018, 13, e0204875. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Y.; Wang, B.; Wang, X.; Cheng, A.; Kolb, S.; Stanford, J.L.; Wright, J.L. Vigorous Physical Activity Is Associated with Lower Risk of Metastatic-Lethal Progression in Prostate Cancer and Hypomethylation in the CRACR2A Gene. Cancer Epidemiol. Biomark. Prev. 2019, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Jung, P.; Kulkoyluoglu-Cotul, E.; Liguori, C.; Lumibao, J.; Mazewski, C.; Ranard, K.; Rowles, J.L., 3rd; Wang, Y.; Xue, L.; et al. Impact of Diet and Nutrition on Cancer Hallmarks. J. Cancer Prev. Curr. Res. 2017, 7. [Google Scholar] [CrossRef]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Perry, I.J.; Phillips, C.M. Dietary Quality Determined by the Healthy Eating Index-2015 and Biomarkers of Chronic Low-Grade Inflammation: A Cross-Sectional Analysis in Middle-to-Older Aged Adults. Nutrients 2021, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Forbes, C.C.; Swan, F.; Greenley, S.L.; Lind, M.; Johnson, M.J. Physical activity and nutrition interventions for older adults with cancer: A systematic review. J. Cancer Surviv. 2020, 14, 689–711. [Google Scholar] [CrossRef]
- Baillargeon, J.; Rose, D.P. Obesity, adipokines, and prostate cancer (review). Int. J. Oncol. 2006, 28, 737–745. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Newton, C.C.; Silverman, D.T.; Pollak, M.; Nogueira, L.M.; Weinstein, S.J.; Albanes, D.; Mannisto, S.; Jacobs, E.J. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am. J. Epidemiol. 2015, 182, 187–197. [Google Scholar] [CrossRef]
- Ma, Y.V.; Lam, C.; Dalmia, S.; Gao, P.; Young, J.; Middleton, K.; Liu, C.; Xu, H.; You, L. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J. Cell Biochem. 2018, 119, 5665–5675. [Google Scholar] [CrossRef]
- Assi, M.; Kenawi, M.; Ropars, M.; Rebillard, A. Interleukin-6, C/EBP-beta and PPAR-gamma expression correlates with intramuscular liposarcoma growth in mice: The impact of voluntary physical activity levels. Biochem. Biophys. Res. Commun. 2017, 490, 1026–1032. [Google Scholar] [CrossRef]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.; Nagendran, J.; Flores, C.T.; et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhang, B.H.; Zhang, K.Z.; Meng, X.T.; Jia, Q.A.; Zhang, Q.B.; Bu, Y.; Zhu, X.D.; Ma, D.N.; Ye, B.G.; et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: With reference to nervous system. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef]
- Shim, Y.J.; Kim, H.J.; Oh, S.C.; Lee, S.I.; Choi, S.W. Exercise during adjuvant treatment for colorectal cancer: Treatment completion, treatment-related toxicities, body composition, and serum level of adipokines. Cancer Manag. Res. 2019, 11, 5403–5412. [Google Scholar] [CrossRef] [PubMed]
- Travier, N.; Buckland, G.; Vendrell, J.J.; Fernandez-Veledo, S.; Peiro, I.; Del Barco, S.; Pernas, S.; Zamora, E.; Bellet, M.; Margeli, M.; et al. Changes in metabolic risk, insulin resistance, leptin and adiponectin following a lifestyle intervention in overweight and obese breast cancer survivors. Eur. J. Cancer Care 2018, 27, e12861. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Parmentier, J.H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose tissue inflammation in breast cancer survivors: Effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Przyborowski, K.; Proniewski, B.; Zakrzewska, A.; Kaczor, D.; Stojak, M.; Buczek, E.; Nieckarz, Z.; Zoladz, J.A.; Wietrzyk, J.; et al. Breast cancer pulmonary metastasis is increased in mice undertaking spontaneous physical training in the running wheel; a call for revising beneficial effects of exercise on cancer progression. Am. J. Cancer Res. 2017, 7, 1926–1936. [Google Scholar]
- Pareja-Galeano, H.; Garatachea, N.; Lucia, A. Exercise as a Polypill for Chronic Diseases. Prog. Mol. Biol. Transl. Sci. 2015, 135, 497–526. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sport Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.C.; Kalda, A.L. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study. J. Clin. Oncol. 2003, 21, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Albright, A.L.; Blissmer, B.J.; Braun, B.; Chasan-Taber, L.; Fernhall, B.; Regensteiner, J.G.; Rubin, R.R.; Sigal, R.J.; American College of Sports Medicine; et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2010, 42, 2282–2303. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K.; American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Physical Activity and the Cancer Patient. Available online: http://www.cancer.org/treatment/survivorshipduringandaftertreatment/stayingactive/physicalactivity-and-the-cancer-patient (accessed on 1 November 2020).
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- De Backer, I.C.; Schep, G.; Hoogeveen, A.; Vreugdenhil, G.; Kester, A.D.; van Breda, E. Exercise testing and training in a cancer rehabilitation program: The advantage of the steep ramp test. Arch. Phys. Med. Rehab. 2007, 88, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Krause, M.P.; Milne, K.J.; Hawke, T.J. Adiponectin-Consideration for its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528. [Google Scholar] [CrossRef]
- Xu, L.; Rogers, C.R.; Halliday, T.M.; Wu, Q.; Wilmouth, L. Correlates of Physical Activity, Psychosocial Factors, and Home Environment Exposure among U.S. Adolescents: Insights for Cancer Risk Reduction from the FLASHE Study. Int. J. Environ. J. Pub. Health 2020, 17, 5753. [Google Scholar] [CrossRef] [PubMed]
| Tumor Progression and Metastases | Ref. | |
|---|---|---|
| Adiponectin | • Inhibits proliferation, adhesion, invasion, and migration of BC cells • Inhibits proliferation of CRC cells by blocking the cell cycle • In NSCLC positively modulate the EMT markers • In endometrial carcinoma reduction of AdipoR1 and AdipoR2 increase malignancy | [42,44] [182] [50] [47] |
| Leptin | • Transform breast epithelial cells to mediate further proliferation of tumor cells • Induce angiogenesis by stimulating the production of VEGF • Acts via the JAK/STAT3 and PI3K pathways • High levels, in GC, correlates with invasion, metastasis and VEGF expression • Significantly improved the migratory activity of human CRC cells • Over-expression of LEPR induces proliferation, migration and angiogenesis in pancreatic cancer cells | [60] [60] [56] [61] [64] [65] |
| Visfatin | • In NSCLC enhanced cell migration and invasion via activation of MMP2 and MMP9 • In osteosarcoma cell lines induces the EMT transition via NF-κB pathway | [84] [86] |
| Resistin | • In prostate cancer cells induce cell proliferation through PI3K/AKT pathway • Promote the invasiveness of BC cells • Activating the signalling of PI3K and MAPK improves tumor growth and metastasis | [91] [92] [33] |
| Apelin | • In BC and lung adenocarcinoma cancer cells promote the invasion of malignant cells via upregulation of MMP1 expression • Induce VSMCs migration through the PI3K/Akt/FoxO3a signalling and by upregulating MMP2 • In NSCLC its higher expression correlates with tumor growth and progression | [72,73] [183] [74] |
| Ghrelin | • Increase angiogenesis via ERK2 signalling pathway • In CRC cell induces proliferation through the GHS-R/Ras/PI3K/Akt/mTOR axis • Promote oral cancer proliferation via modulation of GLUT1 expression • Promote migration and invasion in GC and pancreatic adenocarcinoma | [102] [98] [99] [100,101] |
| Chemerin | • In BC exerts a tumor suppressor role by binding to its CMKLR1 and GPR1 receptors • Suppresses HCC migration, invasion and metastasis through upregulation of tumor suppressor PTEN • In GC cells increase invasiveness and upregulation of VEGF and MMP7 | [112] [116] [118] |
| Lipocalin2 | • In BC cells induce EMT, and increase motility and invasion • Induce production of HIF-1α and VEGF to stimulate angiogenesis • In ESCC its overexpression promote migration, invasion and lung metastasis and increased MMP9 activity | [133] [135] [136] |
| Osteopontin | • In BC cells increase in EMT-related transcription factors and in HIF-1α and promote angiogenesis, skeletal metastasis and enhancing tumor progression • In HCC facilitates cancer growth and metastases by activation of (PI3K)/Akt, MAPK, NF-kB and MMP2 pathways. • In NSCLC induces tumor cell migration acting on integrins and CD4437 | [151,152] [153] [155] |
| IL-6 | • Through activation of Jak2/STAT-3 signalling induce EMT, upregulate MMP7 and MMP9, and IL-6 • Activates the PI3K/Akt pathway and promote the survival of cancer cells | [177] [178] |
| TNFα | • Through NF-κB activation promotes cell proliferation, apoptosis, angiogenesis and metastasis | [163] |
| In Vitro Studies | Model of Exercise | Effects on Tumor Progression | Ref. | |
|---|---|---|---|---|
| Human MDA-MB-231 and UACC-893 BC cells, A549 lung cancer cells, 2008 ovarian cancer cells | Microfluidic circulatory system: low shear stress of 15 dynes/cm2 (resting state) vs. high shear stress of 60 dynes/cm2 (intensive exercise) | - 4 h of high shear stress produce necrosis in 90% of circulating cancer cells derived from multiple types of tumor cells - 16–24 h of high shear stress cause apoptosis via anoikis in 92% of circulating cancer cells (vs. 11% of low shear stress) - 8 h of high shear stress destroyed 74% of metastatic BC cells | [219] | |
| Osteocytes-like cells, MDA-MB-231 BC cells, RAW264.7 osteoclasts, and HUVECs | Osteocyte-like cells subjected to 2 h of oscillatory fluid flow with peak shear stress of 1 Pa (mild exercise) | - CM from flow-stimulated osteocyte-like cells increase by 45% migration of BC cells - CM from osteoclast cultured in CM from flow-stimulated osteocytes reduced BC cell migration by 47% - CM from HUVEC cultured in CM from flow-stimulated osteocytes increased BC cell apoptosis by 29% → anti-metastatic potential of flow-stimulated osteocytes mediated by osteoclasts and endothelial cells | [240] | |
| Osteocyte-like cells, MDA-MB-231 BC cells, and HUVECs | 3D microfluidic tissue model with osteocytes mechanically stimulated by a physiological oscillatory fluid flow with a peak shear stress of 1 Pa | ↓ trans-endothelial BC cell extravasation (32.4% with mechanically stimulated osteocytes vs. 53.5% static osteocytes) | [220] | |
| In Vivo Studies | Groups | Exercise Intervention | Effects on Tumor Progression | Ref. |
| Rats with BC induced by carcinogen MNU | -sedentary MNU (n = 15) -sedentary CTRL (n = 10) -EX MNU (n = 15) -EX CTRL (n = 10) | 35 week-moderate exercise training on a treadmill 60 min/day, 5 days/week | EX MNU developed less tumors per animal than sedentary MNU (2.30 vs. 2.55). sedentary MNU showed pulmonary nodules No metastasis in EX MNU | [223] |
| Mice inoculated with liposarcoma (LIPO) | -EX CTRL (n = 9) -EX LIPO (n = 9) -CTRL (n = 9) -LIPO (n = 9) | -6 weeks spontaneous wheel PA before tumor injection (n = 36) -8 weeks voluntary wheel running post-tumor injection (n = 18) | ↑ IL-6 circulating levels in EX LIPO vs. LIPO and EX CTRL vs. CTRL ↑ tumor growth in EX LIPO vs. LIPO ↓ body weight loss in EX LIPO vs. all remaining groups ↑ risk of developing lung metastasis in EX LIPO vs. LIPO | [241] |
| C57BL/6 male mice inoculated with mouse prostate adenocarcinoma cells | -EX group (n = 28) -CTRL group (n = 31) | 8 weeks voluntary access to a wheel 24 h/day | ↓ pro-metastatic genes in EX group vs. CTRL ↓ IL-6 and CXCL-1 in EX group vs. CTRL ↑ HIF-1α and VEGF (tumor vascularization) in EX group vs. CTRL | [242] |
| C57BL/6 mice transplanted with murine liver cancer cells | -CTRL (n = 12) -8min/d (n = 12) -16min/d (n = 12) -32min/d (n = 12) | -9 weeks of regular moderate swimming, 8 min/day -9 weeks of overload swimming, 16 and 32 min/day | ↓ tumor volume and lung metastasis in 8 min/d vs. CTRL ↓ TGF-β1-induced EMT in 8 min/d vs. CTRL ↑ tumor growth and lung metastasis in both 16 min/d 32 min/d vs. CTRL | [243] |
| Hyperlipidaemia ApoE-/- mice with orthotopic murine BC | -CTRL (n = 10)-HEx high-exercise (n = 10)-LEx low-exercise (n = 10) | -HEx group: 10 km/day of continuous wheel access -LEx group: 8 km/day of wheel access every 2nd day | No differences between HEx, LEx, and CTRL in tumor growth ↓ internal metastases and tumor hypoxia in both Hex and LEx vs. CTRL ↑ beneficial changes in tumor microenvironment | [224] |
| Mice injected with metastasis of Lewis lung carcinoma (LLC) or with metastasis of B16BL/6 melanoma | -subcutaneous injection of spontaneous LLC cells (n = 30)-intravenous injection of experimental B16BL/6 cells (n = 30)-CTRL (n = 30) | -9 weeks of voluntary running before cancer cells injection -2 weeks of voluntary running after metastases injection (B16BL/6) or surgical removal of the primary tumor (LLC) | no differences in the numbers or sizes of lung metastases between B16BL/6 or LLC groups ↓ plasma insulin and leptin levels in LLC vs. CTRL ↑ adiponectin levels and PDGF-BB in LLC vs. CTRL no effect on VEGF and MCP-1 levels | [225] |
| Balb/C mice injected with mouse BC cells | -CTRL (n = 20) -EX group (n = 16) | 4 weeks of voluntary wheel running after cancer injection | ↑ secondary metastases nodules in lungs in EX group vs. CTRL ↓ endothelial function in EX group vs. CTRL | [247] |
| Clinical Studies | Groups | Exercise Intervention | Effects on Tumor Progression | Ref. |
| BC women | -CTRL: healthy women (n = 7) -BC: breast cancer patients receiving adjuvant chemotherapy (n = 20) | -CTRL: acute bout of ergometer cycling at 55% of VO2peak for 2 h -BC: 1 h resistance whole-body training and 30 min of high-intensity spinning (pulse > 80% HR max) on stationary ergometer bicycles | ↓ cell viability by both CTRL and BC group exercise-conditioned sera: 19% and 11%, respectively, in MCF-7; and 9% and 13%, respectively, in MDA-MB-231 BC cells -45% of mice inoculated with MCF-7 cells pre-incubated with exercise-conditioned sera formed tumors | [221] |
| Subjects with stable spinal bone metastases undergoing radiotherapy | -Group A: resistance training (n = 30) -Group B: passive physical therapy (n = 30) | -Group A performed 30 min of 3 different exercises: in ‘‘all-fours’’ position, in the ‘‘gluteus arch’’ position, and in the ‘‘supine position’’ -Group B received 15 min of passive PA in form of breathing exercises | No differences in either overall or bone survival between groups A and B ↑ in local bone progression in 16.7% group B vs. A | [229] |
| Stage IIB–IIIC BC women | -AC group: combination with doxorubicin– cyclophosphamide (n = 10) -AC+AET group: aerobic exercise training (n = 10) | -AC received 4 cycles of doxorubicin (60 mg/m2) in combination with cyclophosphamide (600 mg/ m2) -AC+AET had also performed 12 weeks of 3 cycle ergometer sessions/week at 60–100% of VO2 peak, 30 to 45 min/session. | ↓ IL-1β in both groups ↓ IL-2 in AC+AET group vs. AC ↑ IL-8 in AC+AET group vs. AC ↑ of 38% in tumor vascularization in AC+AET group vs. AC | [230] |
| Stage I–III CRC subjects | -CTRL: usual-care control (n = 13) -Low-dose EX: 150 min/week aerobic exercise (n = 14) -High-dose EX: 300 min/week of aerobic exercise (n = 12) | EX groups provided with an in-home treadmill and a HR monitor, performed 6 months aerobic training at 50–70% of the HR max | ↓ circulating tumor cells in both low-dose and high-dose EX vs. CTRL ↓ BMI, insulin, and soluble ICAM-1 in all 3 groups | [231] |
| Stage II or III CRC subjects | -CTRL: CRC subject (n = 15) -EX group: CRC subject (n = 27) | EX group performed home-based 50 min circular workout 3 times/week, composed of a series of aerobic and anaerobic exercises | ↑ Serum leptin in CTRL group ↓ Serum adiponectin in CTRL group ↑ Serum adiponectin in EX group | [244] |
| BC overweight or obese women | -EX group: (n = 37) | 24 bi-weekly sessions of 25 min aerobic exercise using static bicycles at 70% of max workload and 25 min muscle strengthening exercises | ↓ metabolic risk biomarkers and insulin resistance before vs. after training ↓ leptin, adiponectin, and BMI before vs. after training | [245] |
| Stage I–III BC obese women | -CTRL: BC obese women (n = 10) -EX group: BC obese women (n = 11) | 16 weeks of 150 min/week of aerobic exercise, including treadmill walking/running, rowing machine, or stationary bicycle at 65–80% of HR max combined with 2–3 days of resistance exercise | ↓ CRP, leptin, IL-6, IL-8 in EX group vs. CTRL ↑ adiponectin in EX group vs. CTRL ↓ macrophages M1 and ↑ M2 in EX group vs. CTRL ↓ AT secretion of IL-6 and TNFα ↑ IL-12 in EX group vs. CTRL | [246] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perego, S.; Sansoni, V.; Ziemann, E.; Lombardi, G. Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines. Int. J. Mol. Sci. 2021, 22, 2005. https://doi.org/10.3390/ijms22042005
Perego S, Sansoni V, Ziemann E, Lombardi G. Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines. International Journal of Molecular Sciences. 2021; 22(4):2005. https://doi.org/10.3390/ijms22042005
Chicago/Turabian StylePerego, Silvia, Veronica Sansoni, Ewa Ziemann, and Giovanni Lombardi. 2021. "Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines" International Journal of Molecular Sciences 22, no. 4: 2005. https://doi.org/10.3390/ijms22042005
APA StylePerego, S., Sansoni, V., Ziemann, E., & Lombardi, G. (2021). Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines. International Journal of Molecular Sciences, 22(4), 2005. https://doi.org/10.3390/ijms22042005







