Adipocyte-Derived Extracellular Vesicles: State of the Art
Abstract
1. Introduction
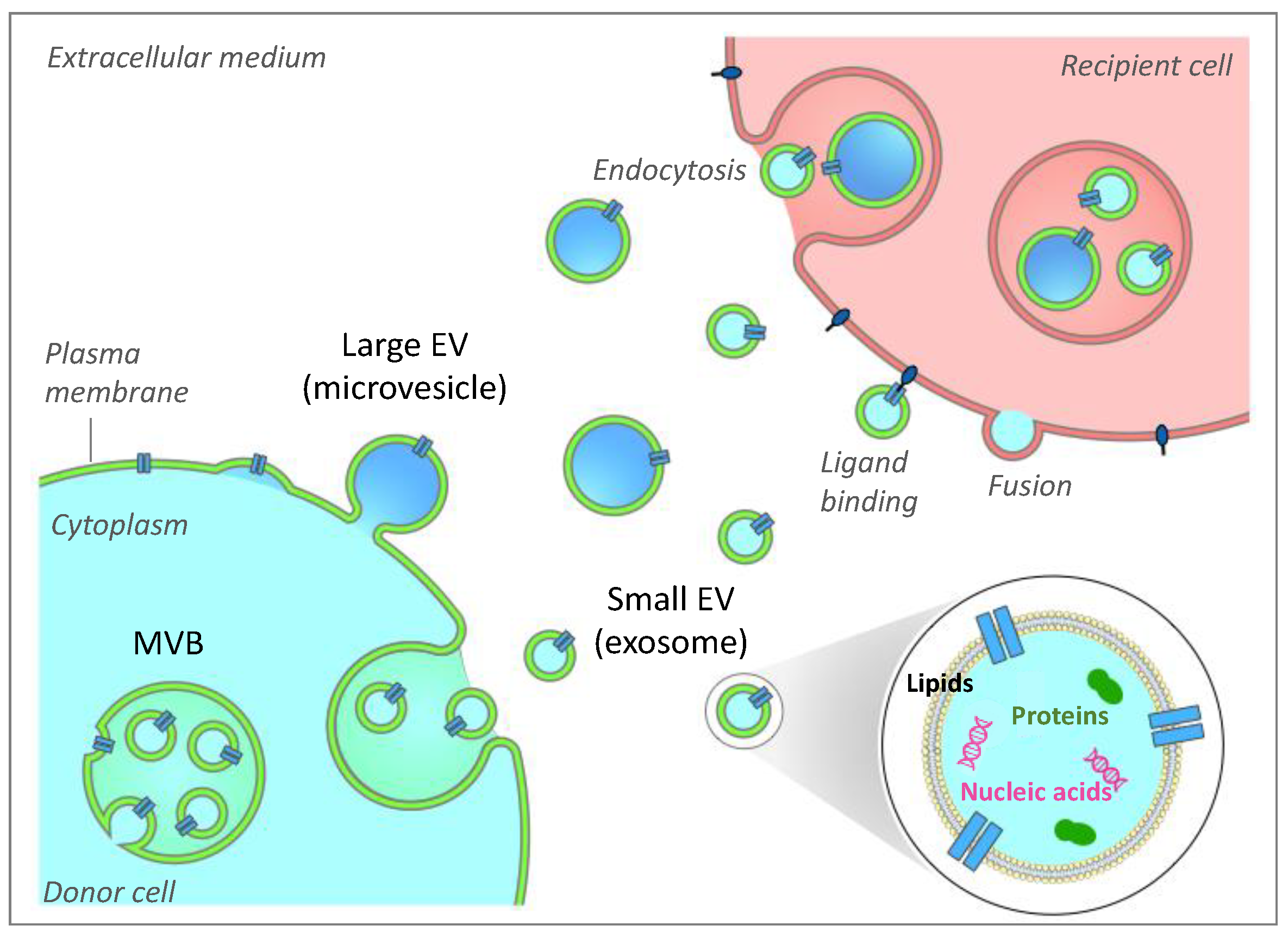
2. Generalities on Extracellular Vesicles
3. Adipocytes Are Important EV Providers
3.1. Adipose-Derived EV: A Complex Network of Metabolic Signals Inside WAT
3.2. Contribution of Adipocyte-Derived EV to the Circulating Pool of EV in Biofluids
3.3. Adipose-Derived EV Regulate Glucose Homeostasis and Inflammation
4. Adipose-Derived EV Content Explains Their Biological Functions
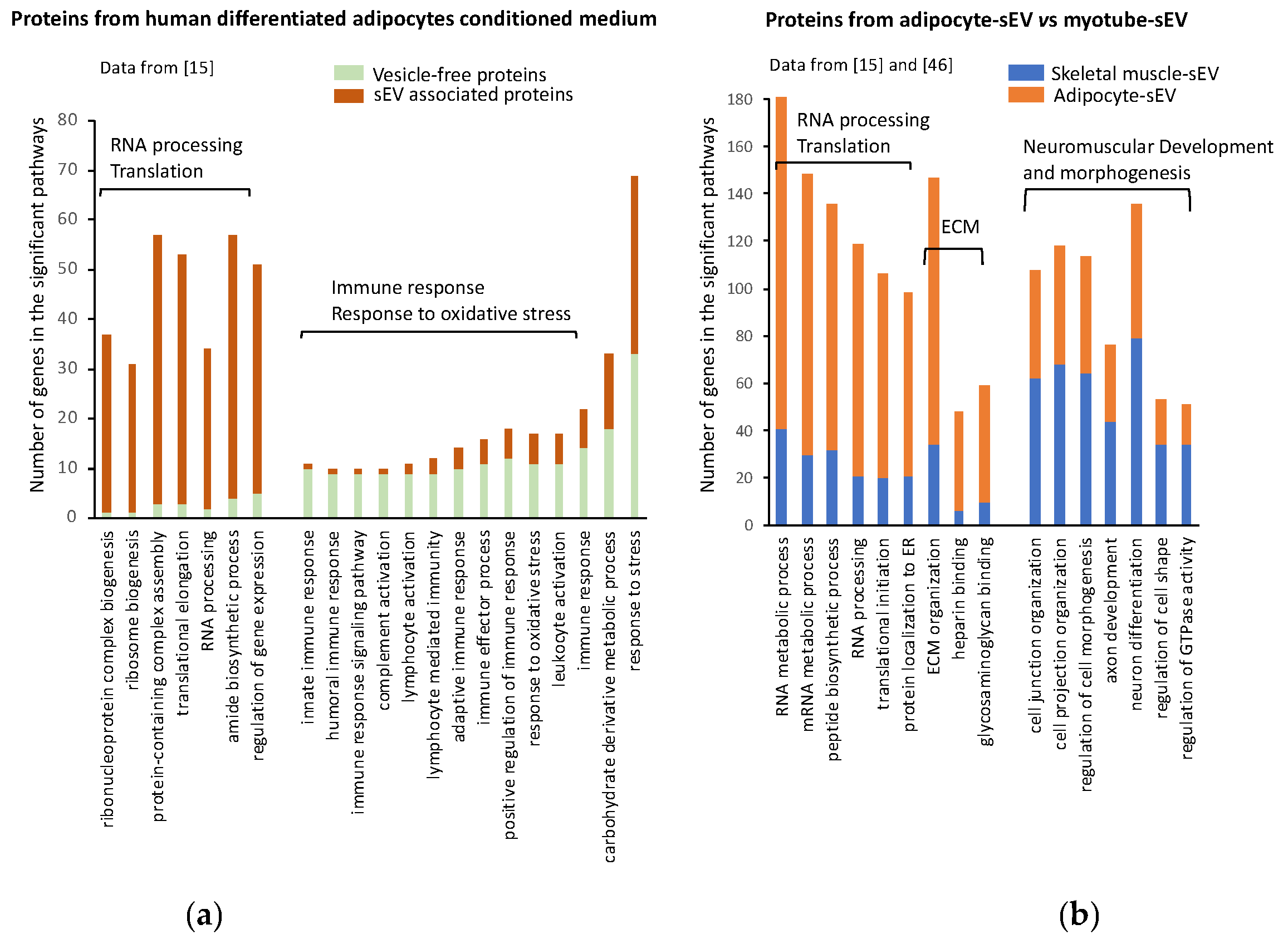
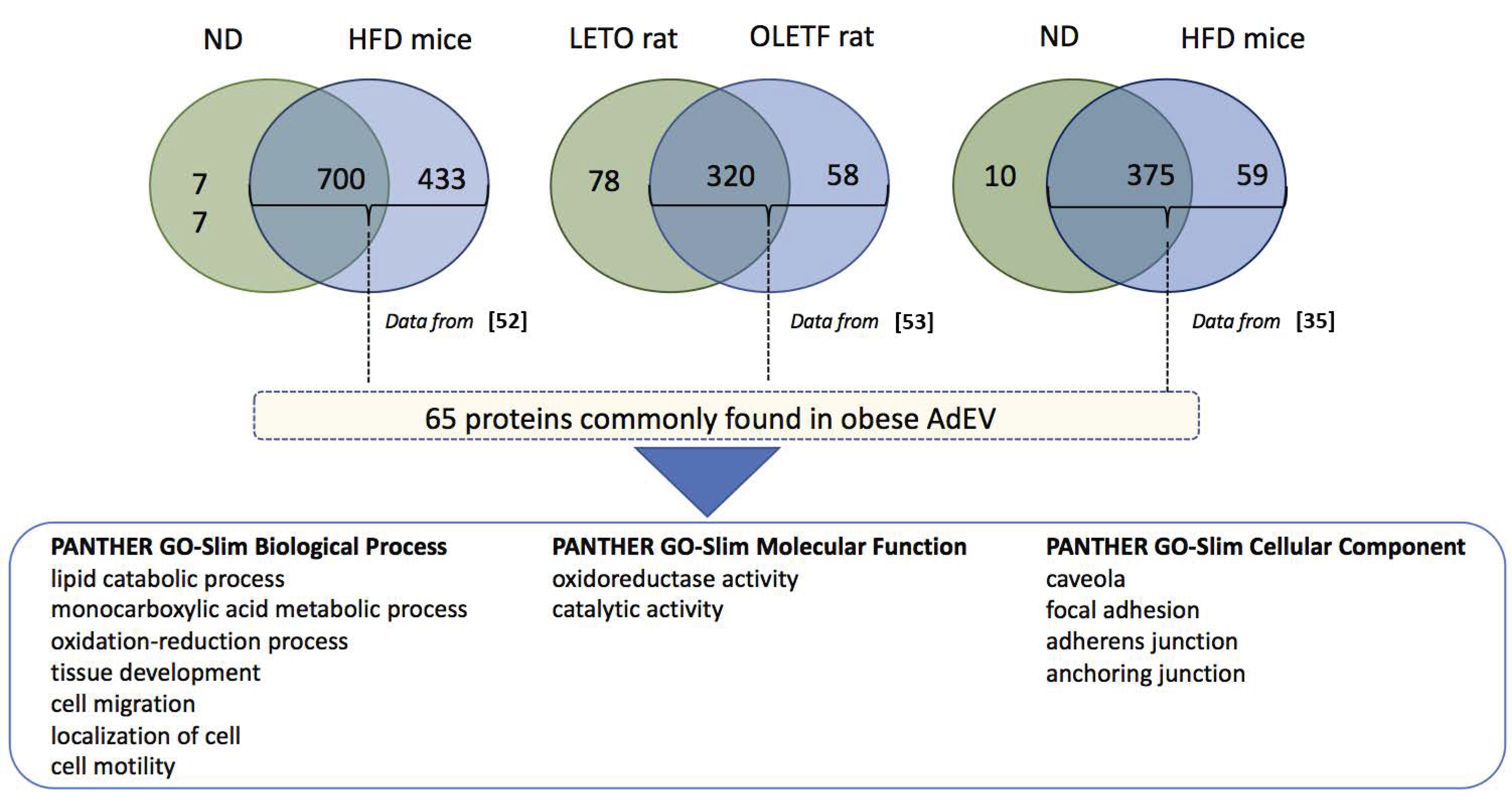
4.1. Protein Content of Adipocyte-Derived Extracellular Vesicles
4.2. RNA in Adipocyte-Derived Extracellular Vesicles
4.3. Lipids in AdEV
5. Therapeutic Strategies to Decrease Ad EV Deleterious Effects
5.1. Targeting AdEV Extracellular Vesicle Biogenesis and Release
5.2. Modulation of AdEV Lipid Composition by the Diet
5.3. Use of Extracellular Vesicles from Healthy Subjects
5.4. Use of Antibodies against AdEV
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Despreés, J.-P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; LaRose, E.; Rodeés-Cabau, J.; Bertrand, O.F.; Poirier, P. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arter. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Le Lay, S.; Dugail, I. Connecting lipid droplet biology and the metabolic syndrome. Prog. Lipid Res. 2009, 48, 191–195. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage ac-cumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Vidal, M. Exosomes: Revisiting their role as “garbage bags”. Traffic 2019, 20, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Żekanowska, E.; Kornek, M. Large extracellular vesicles: Have we found the holy grail of inflammation? Front. Immunol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Freyssinet, J.-M.; Toti-Orfanoudakis, F. Formation of procoagulant microparticles and properties. Thromb. Res. 2010, 125, S46–S48. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 140172. [Google Scholar] [CrossRef]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trötzmüller, M.; Köfeler, H.; Mabilleau, G.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef]
- Connolly, K.D.; Wadey, R.M.; Mathew, D.; Johnson, E.; Rees, D.A.; James, P.E. Evidence for adipocyte-derived extracellular vesicles in the human circulation. Endocrinology 2018, 159, 3259–3267. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; Legonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef]
- Flaherty, S.E., III; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Müller, G.A.; Schneider, M.; Biemer-Daub, G.; Wied, S. Upregulation of lipid synthesis in small rat adipocytes by microvesicle-associated CD73 from large adipocytes. Obesity 2011, 19, 1531–1544. [Google Scholar] [CrossRef]
- Müller, G.; Jung, C.; Straub, J.; Wied, S.; Kramer, W. Induced release of membrane vesicles from rat adipocytes containing glycosylphosphatidylinositol-anchored microdomain and lipid droplet signalling proteins. Cell. Signal. 2009, 21, 324–338. [Google Scholar] [CrossRef]
- Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Martis-Sueiro, A.; Couto, I.; Santos, F.; Baltar, J.; Casanueva, F.; Pardo, M. Vesicles shed by pathological murine adipocytes spread pathology: Characterization and functional role of insulin resistant/hypertrophied adiposomes. Int. J. Mol. Sci. 2020, 21, 2252. [Google Scholar] [CrossRef]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 2018, 175, 695–708.e13. [Google Scholar] [CrossRef]
- Mleczko, J.; Ortega, F.J.; Falcón-Pérez, J.M.; Wabitsch, M.; Fernández-Real, J.M.; Mora, S. Extracellular vesicles from hypoxic adipocytes and obese subjects reduce insulin-stimulated glucose uptake. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Togliatto, G.; Dentelli, P.; Gili, M.; Gallo, S.A.; Deregibus, C.; Biglieri, E.G.; Iavello, A.; Santini, E.; Rossi, C.; Solini, A.; et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: Impact on clinical applications. Int. J. Obes. 2016, 40, 102–111. [Google Scholar] [CrossRef]
- Eblázquez, R.; Sanchez-Margallo, F.M.; La Rosa, O.E.; Edalemans, W.; Ãlvarez, V.; Etarazona, R.; Macías-García, B.; Eblázquez, R.; Sanchez-Margallo, F.M.; La Rosa, O.E.; et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Lining, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.E.; Visseren, F.L.J.; Van Balkom, B.W.; Hoen, E.N.N.; Van Herwaarden, J.A.; De Jager, W.; Schipper, H.S.; Brenkman, A.B.; Verhaar, M.; Wauben, M.H.; et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity 2014, 22, 1296–1308. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.; Samblas, M.; Martínez, J.A.; Milagro, F.I. Effects of exosomes from LPS-activated macrophages on adipocyte gene expression, differentiation, and insulin-dependent glucose uptake. J. Physiol. Biochem. 2018, 74, 559–568. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Amosse, J.; Durcin, M.; Malloci, M.; Vergori, L.; Fleury, A.; Gagnadoux, F.; Dubois, S.; Simard, G.; Boursier, J.; Hue, O.; et al. Phenotyping of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of Macrophage Migration Inhibitory Factor. Mol. Metab. 2018, 18, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.R.; Douagi, I.; Andersson, D.P.; Bäckdahl, J.; Rydén, M.; Arner, P.; Laurencikiene, J. Increased fat cell size: A major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 2016, 59, 560–570. [Google Scholar] [CrossRef]
- Deng, Z.-B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Mulya, A.; Lazic, M.; Radhakrishnan, D.; Berk, M.P.; Povero, D.; Gornicka, A.; Feldstein, A.E. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PLoS ONE 2015, 10, e0123110. [Google Scholar] [CrossRef] [PubMed]
- Wadey, R.M.; Connolly, K.D.; Mathew, D.; Walters, G.; Rees, D.A.; James, P.E. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis 2019, 283, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.-F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.-W.; Shen, D.; Zhang, J.-Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget 2017, 8, 81880–81891. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Ni, K.; Jin, Y. Post-translational modification regulates formation and cargo-loading of extracellular vesicles. Front. Immunol. 2020, 11, 948. [Google Scholar] [CrossRef]
- Pálfy, M.; Reményi, A.; Korcsmáros, T. Endosomal crosstalk: Meeting points for signaling pathways. Trends Cell Biol. 2012, 22, 447–456. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Sikkeland, J.; Fenaroli, F.; Griffiths, G.; Daniels, M.P.; Cao, H.; Saatcioglu, F.; Hotamisligil, G.S. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J. Lipid Res. 2015, 56, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, M.-C.; Bigot, A.; Jensen, S.S.; Dennis, J.L.; Rogowska-Wrzesinska, A.; Lainé, J.; Gache, V.; Furling, D.; Jensen, O.N.; Voit, T.; et al. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J. Proteom. 2012, 77, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017, 45, D183–D189. [Google Scholar] [CrossRef]
- Phoonsawat, W.; Aoki-Yoshida, A.; Tsuruta, T.; Sonoyama, K. Adiponectin is partially associated with exosomes in mouse serum. Biochem. Biophys. Res. Commun. 2014, 448, 261–266. [Google Scholar] [CrossRef]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J. Extracell. Vesicles 2015, 4, 29159. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Mittelbrunn, M. Role of exosomes in the protection of cellular homeostasis. Cell Adhes. Migr. 2017, 11, 127–134. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Attané, C.; Carrié, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Lee, J.-E.; Moon, P.-G.; Lee, I.-K.; Baek, M.-C. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Protein J. 2015, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Tamara, C.; Nerea, L.-B.; Belén, B.S.; Alberto, M.-V.; Aurelio, S.; Iván, C.; Javier, B.; Felipe, C.F.; María, P. Human obese white adipose tissue sheds depot-specific extracellular vesicles and reveals candidate biomarkers for monitoring obesity and its comorbidities. Transl. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Janas, M.M.; Sapoń, K.; Janas, T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015, 589, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.; Lee, H. Sorting mechanisms for MicroRNAs into extracellular vesicles and their associated diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Koppers-Lalic, D.; Hackenberg, M.; Bijnsdorp, I.V.; Van Eijndhoven, M.A.; Sadek, P.; Sie, D.; Zini, N.; Middeldorp, J.M.; Ylstra, B.; De Menezes, R.X.; et al. Nontemplated nucleotide additions distinguish the small rna composition in cells from exosomes. Cell Rep. 2014, 8, 1649–1658. [Google Scholar] [CrossRef]
- Batagov, A.O.; Kurochkin, I.V. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol. Direct 2013, 8, 12. [Google Scholar] [CrossRef]
- Müller, G.; Schneider, M.; Biemer-Daub, G.; Wied, S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell. Signal. 2011, 23, 1207–1223. [Google Scholar] [CrossRef]
- Yue, B.; Yang, H.; Wu, J.; Wang, J.; Ru, W.; Cheng, J.; Huang, Y.; Lei, C.; Lan, X.; Chen, H. Characterization and transcriptome analysis of exosomal and nonexosomal RNAs in bovine adipocytes. Int. J. Mol. Sci. 2020, 21, 9313. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Ge, S.; Liu, Y.; Bai, M.; Zhu, K.; Fan, Q.; Li, J.; Ning, T.; Tian, F.; et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 2019, 38, 2844–2859. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic adipocyte–derived exosomal miR-802-5p contributes to insulin resistance in cardiac myocytes through targeting HSP60. Obesity 2020, 28, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-derived exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J. Physiol. Biochem. 2019, 75, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Le Lay, S.; Krief, S.; Farnier, C.; Lefrère, I.; Le Liepvre, X.; Bazin, R.; Ferré, P.; Dugail, I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J. Biol. Chem. 2001, 276, 16904–16910. [Google Scholar] [CrossRef]
- Zeghari, N.; Vidal, H.; Younsi, M.; Ziegler, O.; Drouin, P.; Donner, M. Adipocyte membrane phospholipids and PPAR-γ expression in obese women: Relationship to hyperinsulinemia. Am. J. Physiol. Metab. 2000, 279, E736–E743. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.B.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef]
- Muniappan, L.; Javidan, A.; Jiang, W.; Mohammadmoradi, S.; Moorleghen, J.J.; Katz, W.S.; Balakrishnan, A.; Howatt, D.A.; Subramanian, V. Calpain inhibition attenuates adipose tissue inflammation and fibrosis in diet-induced obese mice. Sci. Rep. 2017, 7, 14398. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-B.; Lee, W.H.; Shao, N.-Y.; Ismail, N.I.; Katwadi, K.; Lim, M.-M.; Kwek, X.-Y.; Michel, N.A.; Li, J.; Newson, J.; et al. Calpain inhibition restores autophagy and prevents mitochondrial fragmentation in a human iPSC model of diabetic endotheliopathy. Stem Cell Rep. 2019, 12, 597–610. [Google Scholar] [CrossRef]
- Xu, J.; Camfield, R.; Gorski, S.M. The interplay between exosomes and autophagy—Partners in crime. J. Cell Sci. 2018, 131, jcs215210. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, S.; Du, X.; Zhang, W.; Jing, R.; Wang, X.; Pan, L. RhoA inhibitor suppresses the production of microvesicles and rescues high ventilation induced lung injury. Int. Immunopharmacol. 2019, 72, 74–81. [Google Scholar] [CrossRef]
- Wang, G.H.; Ma, K.L.; Zhang, Y.; Hu, Z.B.; Liu, L.; Lu, J.; Chen, P.P.; Lu, C.C.; Ruan, X.Z.; Liu, B.C. Caspase 3/ROCK1 pathway mediates high glucose-induced platelet microparticles shedding. Biochem. Biophys. Res. Commun. 2019, 509, 596–602. [Google Scholar] [CrossRef]
- Soliman, H.; Varela, J.N.; Nyamandi, V.; Garcia-Patino, M.; Lin, G.; Bankar, G.R.; Jia, Z.; MacLeod, K.M. Attenuation of obesity-induced insulin resistance in mice with heterozygous deletion of ROCK2. Int. J. Obes. 2016, 40, 1435–1443. [Google Scholar] [CrossRef]
- Lee, S.; Huang, H.; Choi, K.; Lee, D.H.; Shi, J.; Liu, T.; Chun, K.H.; Seo, J.-A.; Lima, I.S.; Zabolotny, J.M.; et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am. J. Physiol. Metab. 2014, 306, E332–E343. [Google Scholar] [CrossRef]
- Soliman, H.; Nyamandi, V.; Garcia-Patino, M.; Varela, J.N.; Bankar, G.; Lin, G.; Jia, Z.; MacLeod, K.M. Partial deletion of ROCK2 protects mice from high-fat diet-induced cardiac insulin resistance and contractile dysfunction. Am. J. Physiol. Circ. Physiol. 2015, 309, H70–H81. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, F.; Ahmad, Z.; Thomas, R.; Melhem, M.; Snider, A.J.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; Ahmad, R. Neutral sphingomyelinase 2 regulates inflammatory responses in monocytes/macrophages induced by TNF-α. Sci. Rep. 2020, 10, 16802. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ru, Z.; Xiao, W.; Xiong, Z.; Wang, C.; Yuan, C.; Zhang, X.; Yang, H. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem. Biophys. Res. Commun. 2018, 506, 122–129. [Google Scholar] [CrossRef]
- Mauer, A.S.; Hirsova, P.; Maiers, J.L.; Shah, V.H.; Malhi, H. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am. J. Physiol. Liver Physiol. 2017, 312, G300–G313. [Google Scholar] [CrossRef] [PubMed]
- Rivas, D.A.; Rice, N.P.; Ezzyat, Y.; McDonald, D.J.; Cooper, B.E.; Fielding, R.A. Sphingosine-1-phosphate analog FTY720 reverses obesity but not age-induced anabolic resistance to muscle contraction. Am. J. Physiol. Physiol. 2019, 317, C502–C512. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sasase, T.; Ishii, Y.; Katsuda, Y.; Miyajima, K.; Yamada, T.; Ohta, T. The sphingosine-1-phosphate receptor modulator, FTY720, prevents the incidence of diabetes in Spontaneously Diabetic Torii rats. Clin. Exp. Pharmacol. Physiol. 2020. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Eitan, E.; Tosti, V.; Suire, C.N.; Cava, E.; Berkowitz, S.T.; Bertozzi, B.; Raefsky, S.M.; Veronese, N.; Spangler, R.; Spelta, F.; et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 2017, 16, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Cuffe, H.; Marshall, S.M.; McDaniel, A.L.; Ha, J.-H.; Kavanagh, K.; Hong, C.; Tontonoz, P.; Temel, R.E.; Parks, J.S. Dietary cholesterol promotes adipocyte hypertrophy and adipose tissue inflammation in visceral, but not in subcutaneous, fat in monkeys. Arter. Thromb. Vasc. Biol. 2014, 34, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Möbius, W.; Ohno-Iwashita, Y.; Van Donselaar, E.G.; Oorschot, V.M.J.; Shimada, Y.; Fujimoto, T.; Heijnen, H.F.G.; Geuze, H.J.; Slot, J.W. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 2002, 50, 43–55. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Vitale, N. Thematic review series: Exosomes and microvesicles: Lipids as key components of their biogenesis and functions, cholesterol and the journey of extracellular vesicles. J. Lipid Res. 2018, 59, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.; et al. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kim, J.-H.; Choi, E.-S.; Cho, J.H.; Kim, E. Effect of young exosomes injected in aged mice. Int. J. Nanomed. 2018, 13, 5335–5345. [Google Scholar] [CrossRef]
- Fleury, A.; Martinez, M.C.; Le Lay, S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Aoki, N.; Tominaga, N.; Takeshita, F.; Sonoda, H.; Yoshioka, Y.; Ochiya, T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol. Ther. 2017, 25, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Rappa, G.; Karbanová, J.; Vanier, C.; Morimoto, C.; Corbeil, D.; Lorico, A. Anti-human CD 9 antibody Fab fragment impairs the internalization of extracellular vesicles and the nuclear transfer of their cargo proteins. J. Cell. Mol. Med. 2019, 23, 4408–4421. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbols | Protein Accession Numbers | Gene Names |
|---|---|---|
| Acadl | P51174 | acyl-Coenzyme A dehydrogenase, long-chain |
| Acads | Q07417 | acyl-Coenzyme A dehydrogenase, short chain |
| Aco2 | Q99KI0 | aconitase 2, mitochondrial |
| Acsl1 | P41216 | acyl-CoA synthetase long-chain family member 1 |
| Adipoq | Q60994 | adiponectin, C1Q and collagen domain containing |
| Agpat2 | Q8K3K7 | 1-acylglycerol-3-phosphate O-acyltransferase 2 (lysophosphatidic acid acyltransferase, beta) |
| Aifm2 | Q8BUE4 | apoptosis-inducing factor, mitochondrion-associated 2 |
| Aldh2 | P47738 | aldehyde dehydrogenase 2, mitochondrial |
| Aldh3a2 | P47740 | aldehyde dehydrogenase family 3, subfamily A2 |
| Anxa1 | P10107 | annexin A1 |
| Anxa6 | P14824 | annexin A6 |
| Aoc3 | O70423 | amine oxidase, copper containing 3 |
| Atp2a2 | O55143 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| Atp5a1 | Q03265 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 |
| Atp5b | P56480 | ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit |
| Cat | P24270 | catalase |
| Cav1 | P49817 | caveolin, caveolae protein 1 |
| Cav2 | Q9WVC3 | caveolin 2 |
| Cct3 | P80318 | chaperonin subunit 3 (gamma) |
| Cd36 | Q08857 | CD36 antigen |
| Cd47 | Q61735 | CD47 antigen (Rh-related antigen, integrin-associated signal transducer) |
| Cd9 | P40240 | CD9 antigen |
| Cltc | Q68FD5 | clathrin, heavy polypeptide (Hc) |
| Col6a1 | Q04857 | collagen, type VI, alpha 1 |
| Decr1 | Q9CQ62 | 2,4-dienoyl CoA reductase 1, mitochondrial |
| Dlat | Q8BMF4 | dihydrolipoamide S-acetyltransferase (E2 component of pyruvate dehydrogenase complex) |
| Eef1a1 | P10126 | eukaryotic translation elongation factor 1 alpha 1 |
| Ehd2 | Q8BH64 | EH-domain containing 2 |
| Etfa | Q99LC5 | electron transferring flavoprotein, alpha polypeptide |
| Fasn | P19096 | fatty acid synthase |
| Gnaq | P21279 | guanine nucleotide binding protein, alpha q polypeptide |
| Gpd1 | P13707 | glycerol-3-phosphate dehydrogenase 1 (soluble) |
| Gpi | P06745 | glucose phosphate isomerase 1 |
| Hadh | Q61425 | hydroxyacyl-Coenzyme A dehydrogenase |
| Hadhb | Q99JY0 | hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl- Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), beta subunit |
| Hsd17b12 | O70503 | hydroxysteroid (17-beta) dehydrogenase 12 |
| Hsd17b4 | P51660 | hydroxysteroid (17-beta) dehydrogenase 4 |
| Itgb1 | P09055 | integrin beta 1 (fibronectin receptor beta) |
| Kpnb1 | P70168 | karyopherin (importin) beta 1 |
| Lamb2 | Q61292 | laminin, beta 2 |
| Lamc1 | P02468 | laminin, gamma 1 |
| Ldha | P06151 | lactate dehydrogenase A |
| Lipe | P54310 | lipase, hormone sensitive |
| Lpcat3 | Q91V01 | membrane bound O-acyltransferase domain containing 5 |
| Lpl | P11152 | lipoprotein lipase; similar to Lipoprotein lipase precursor (LPL) |
| Lrp1 | Q91ZX7 | low density lipoprotein receptor-related protein 1 |
| Mcam | Q8R2Y2 | melanoma cell adhesion molecule |
| Mdh2 | P08249 | malate dehydrogenase 2, NAD (mitochondrial) |
| Ogdh | Q60597 | oxoglutarate dehydrogenase (lipoamide) |
| Pc | Q05920 | pyruvate carboxylase |
| Pdhb | Q9D051 | pyruvate dehydrogenase (lipoamide) beta |
| Pdia3 | P27773 | protein disulfide isomerase associated 3 |
| Phb | P67778 | prohibitin |
| Prkar2b | P31324 | protein kinase, cAMP dependent regulatory, type II beta |
| Rab18 | P35293 | RAB18, member RAS oncogene family |
| Rab8b | P61028 | RAB8B, member RAS oncogene family |
| Rras | P10833 | Harvey rat sarcoma oncogene, subgroup R |
| Sdha | Q8K2B3 | succinate dehydrogenase complex, subunit A, flavoprotein (Fp) |
| Sfxn1 | Q99JR1 | sideroflexin 1 |
| Sts | P50427 | steroid sulfatase |
| Tmed10 | Q9D1D4 | transmembrane emp24-like trafficking protein 10 (yeast) |
| Tubb3 | Q9ERD7 | tubulin, beta 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rome, S.; Blandin, A.; Le Lay, S. Adipocyte-Derived Extracellular Vesicles: State of the Art. Int. J. Mol. Sci. 2021, 22, 1788. https://doi.org/10.3390/ijms22041788
Rome S, Blandin A, Le Lay S. Adipocyte-Derived Extracellular Vesicles: State of the Art. International Journal of Molecular Sciences. 2021; 22(4):1788. https://doi.org/10.3390/ijms22041788
Chicago/Turabian StyleRome, Sophie, Alexia Blandin, and Soazig Le Lay. 2021. "Adipocyte-Derived Extracellular Vesicles: State of the Art" International Journal of Molecular Sciences 22, no. 4: 1788. https://doi.org/10.3390/ijms22041788
APA StyleRome, S., Blandin, A., & Le Lay, S. (2021). Adipocyte-Derived Extracellular Vesicles: State of the Art. International Journal of Molecular Sciences, 22(4), 1788. https://doi.org/10.3390/ijms22041788






