Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria
Abstract
1. Introduction
2. Results
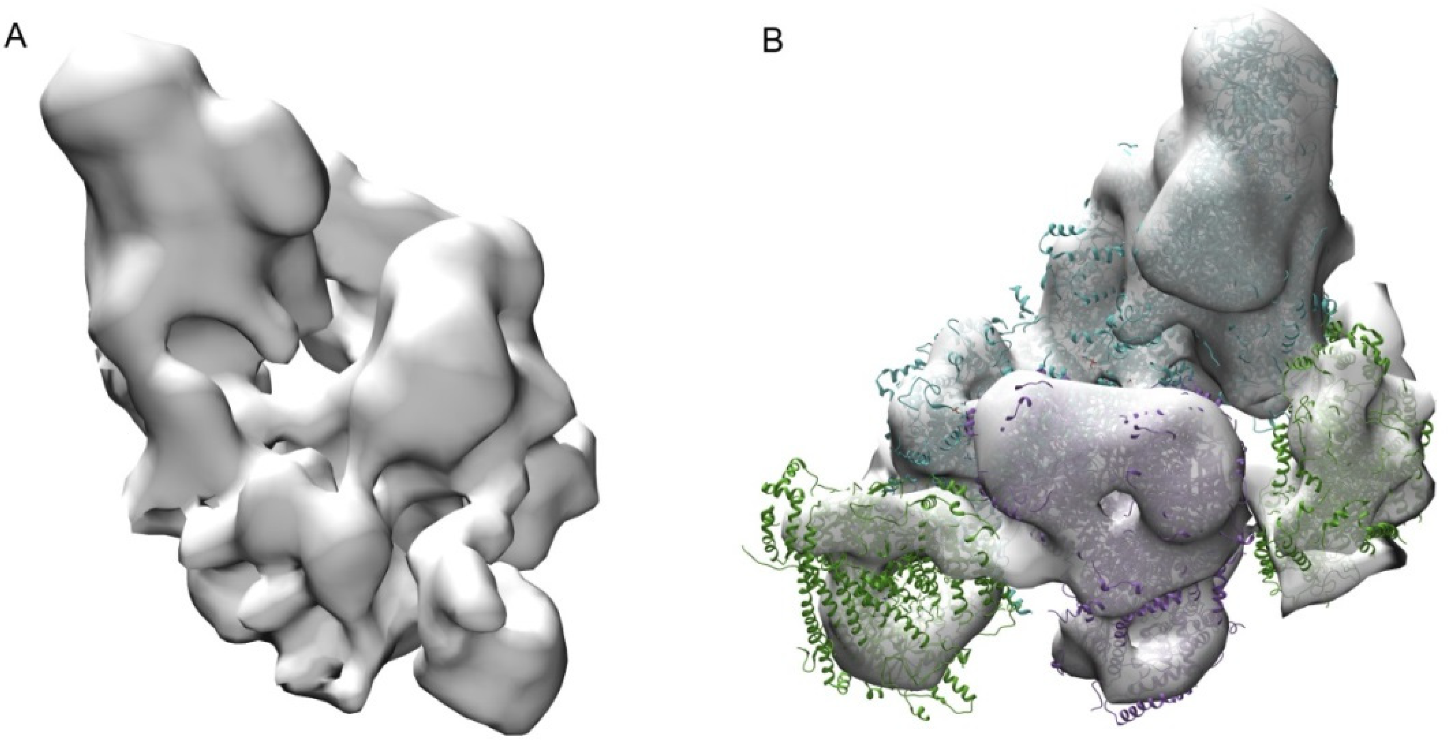
2.1. Respirasome Structure
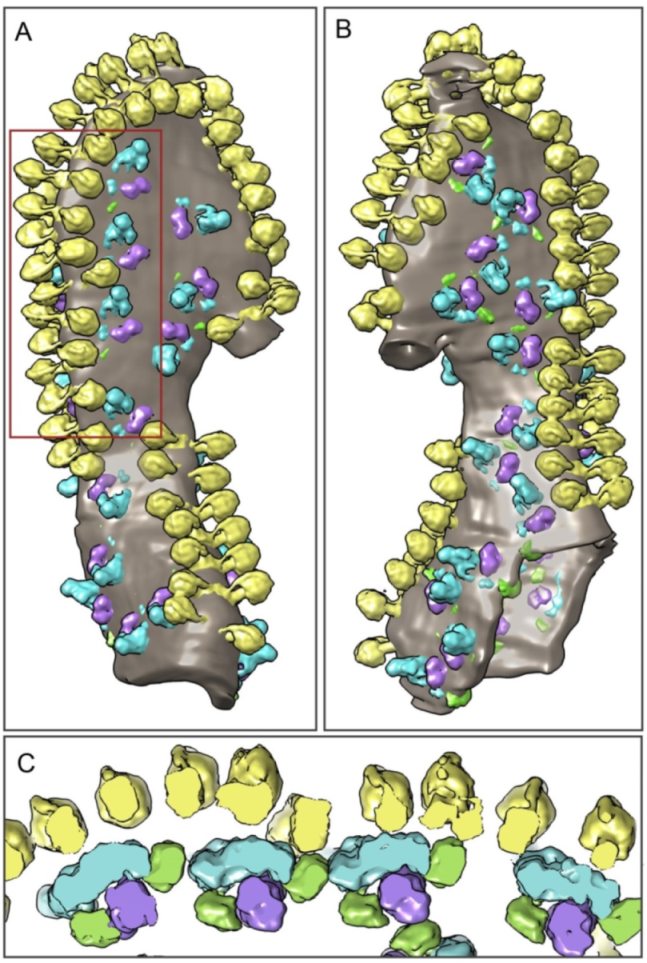
2.2. The Structure of Oligomeric Oxidative Phosphorylation System (OXPHOS) Clusters
3. Discussion
4. Methods
4.1. Mitochondria Isolation
4.2. Blue Native Gel Electrophoresis
4.3. Sample Preparation for Cryo-Electron Microscopy
4.4. Cryo-Electron Tomography
4.5. Tomogram Reconstruction
4.6. Segmentation and Sub-Tomogram Averaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krasinskaya, I.P.; Marshansky, V.N.; Dragunova, S.F.; Yaguzhinsky, L.S. Relationships of respiratory chain and ATP-synthetase in energized mitochondria. FEBS Lett. 1984, 167, 176–180. [Google Scholar] [CrossRef]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Drachev, L.A.; Kaulen, A.D.; Skulachev, V.P. Correlation of photochemical cycle, H+ release and uptake, and electric events in bacteriorhodopsin. FEBS Lett. 1984, 178, 331–335. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Kovbasnjuk, O.N.; Yaguzhinsky, L.S. Evidence in favor of the existence of a kinetic barrier for proton transfer from a surface of bilayer phospholipid membrane to bulk water. Biochim. Biophys. Acta BBA Biomembr. 1993, 1150, 45–50. [Google Scholar] [CrossRef]
- Evtodienko, V.Y.; Antonenko, Y.N.; Yaguzhinsky, L.S. Increase of local hydrogen ion gradient near bilayer lipid membrane under the conditions of catalysis of proton transfer across the interface. FEBS Lett. 1998, 425, 222–224. [Google Scholar] [CrossRef]
- Antonenko, Y.; Pohl, P. Microinjection in combination with microfluorimetry to study proton diffusion along phospholipid membranes. Eur. Biophys. J. EBJ 2008, 37, 865–870. [Google Scholar] [CrossRef]
- Heberle, J.; Riesle, J.; Thiedemann, G.; Oesterhelt, D.; Dencher, N.A. Proton migration along the membrane surface and retarded surface to bulk transfer. Nature 1994, 370, 379–382. [Google Scholar] [CrossRef]
- Medvedev, E.; Stuchebrukhov, A. Mechanism of long-range proton translocation along biological membranes. FEBS Lett. 2012, 587, 345–349. [Google Scholar] [CrossRef]
- Serowy, S.; Saparov, S.; Antonenko, Y.; Kozlovsky, W.; Hagen, V.; Pohl, P. Structural Proton Diffusion along Lipid Bilayers. Biophys. J. 2003, 84, 1031–1037. [Google Scholar] [CrossRef]
- Yaguzhinsky, L.S.; Boguslavsky, L.I.; Volkov, A.G.; Rakhmaninova, A.B. Synthesis of ATP coupled with action of membrane protonic pumps at the octane–water interface. Nature 1976, 259, 494–496. [Google Scholar] [CrossRef]
- Eroshenko, L.V.; Marakhovskaya, A.S.; Vangeli, I.M.; Semenyuk, P.I.; Orlov, V.N.; Yaguzhinsky, L.S. Bronsted Acids Bounded to the Mitochondrial Membranes as a Substrate for ATP Synthase. Dokl. Biochem. Biophys. 2012, 444, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, V.S.; Motovilov, K.A.; Lobysheva, N.V.; Orlov, V.N.; Yaguzhinsky, L.S. The formation of metastable bond between protons and mitoplast surface. Dokl. Biochem. Biophys. 2011, 438, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, J.; Bergstrand, J.; Nilsson, T.; Šachl, R.; von Ballmoos, C.; Widengren, J.; Brzezinski, P. The lateral distance between a proton pump and ATP synthase determines the ATP-synthesis rate. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.A.; Trumpower, B.L. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J. Biol. Chem. 1985, 260, 2458–2467. [Google Scholar] [CrossRef]
- Boumans, H.; Grivell, L.A.; Berden, J.A. The Respiratory Chain in Yeast Behaves as a Single Functional Unit. J. Biol. Chem. 1998, 273, 4872–4877. [Google Scholar] [CrossRef]
- Eubel, H.; Jänsch, L.; Braun, H.-P. New Insights into the Respiratory Chain of Plant Mitochondria. Supercomplexes and a Unique Composition of Complex II. Plant Physiol. 2003, 133, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef]
- Kovářová, N.; Mráček, T.; Nůsková, H.; Holzerová, E.; Vrbacký, M.; Pecina, P.; Hejzlarová, K.; Kľučková, K.; Rohlena, J.; Neuzil, J.; et al. High molecular weight forms of mammalian respiratory chain complex II. PLoS ONE 2013, 8, e71869. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Mohsen, A.-W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for Physical Association of Mitochondrial Fatty Acid Oxidation and Oxidative Phosphorylation Complexes. J. Biol. Chem. 2010, 285, 29834–29841. [Google Scholar] [CrossRef]
- Porpaczy, Z.; Sumegi, B.; Alkonyi, I. Interaction between NAD-dependent isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase complex, and NADH:ubiquinone oxidoreductase. J. Biol. Chem. 1987, 262, 9509–9514. [Google Scholar] [CrossRef]
- Sumegi, B.; Srere, P.A. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J. Biol. Chem. 1984, 259, 15040–15045. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Kouřil, R.; Peters, K.; Braun, H.-P.; Boekema, E.J. Structure and function of mitochondrial supercomplexes. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.-G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Blum, T.B.; Kühlbrandt, W. Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc. Natl. Acad. Sci. USA 2018, 115, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, M.; Guo, R.; Yan, K.; Lei, J.; Gao, N.; Yang, M. The architecture of the mammalian respirasome. Nature 2016, 537, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257.e12. [Google Scholar] [CrossRef]
- Letts, J.A.; Fiedorczuk, K.; Sazanov, L.A. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ko, Y.; Delannoy, M.; Ludtke, S.J.; Chiu, W.; Pedersen, P.L. Mitochondrial ATP synthasome: Three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J. Biol. Chem. 2004, 279, 31761–31768. [Google Scholar] [CrossRef]
- Ko, Y.; Delannoy, M.; Hullihen, J.; Chiu, W.; Pedersen, P. Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J. Biol. Chem. 2003, 278, 12305–12309. [Google Scholar] [CrossRef]
- Nůsková, H.; Mráček, T.; Mikulová, T.; Vrbacký, M.; Kovářová, N.; Kovalčíková, J.; Pecina, P.; Houštěk, J. Mitochondrial ATP synthasome: Expression and structural interaction of its components. Biochem. Biophys. Res. Commun. 2015, 464, 787–793. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Chesnokov, Y.M.; Kamyshinsky, R.A.; Yaguzhinsky, L.S.; Vasilov, R.G. Determining the structure and location of the ATP synthase in the membranes of rat’s heart mitochondria using cryoelectron tomography. Nanotechnol. Russ. 2020, 15, 83–89. [Google Scholar] [CrossRef]
- Buzhynskyy, N.; Sens, P.; Prima, V.; Sturgis, J.N.; Scheuring, S. Rows of ATP Synthase Dimers in Native Mitochondrial Inner Membranes. Biophys. J. 2007, 93, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brèthes, D.; di Rago, J.-P.; Velours, J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002, 21, 221–230. [Google Scholar] [CrossRef]
- Strauss, M.; Hofhaus, G.; Schröder, R.R.; Kühlbrandt, W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008, 27, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Bultema, J.B.; Braun, H.-P.; Boekema, E.J.; Kouril, R. Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim. Biophys. Acta 2009, 1787, 60–67. [Google Scholar] [CrossRef]
- Patten, D.A.; Wong, J.; Khacho, M.; Soubannier, V.; Mailloux, R.J.; Pilon-Larose, K.; MacLaurin, J.G.; Park, D.S.; McBride, H.M.; Trinkle-Mulcahy, L.; et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014, 33, 2676–2691. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991, 199, 223–231. [Google Scholar] [CrossRef]
- Grandier-Vazeille, X.; Guérin, M. Separation by Blue Native and Colorless Native Polyacrylamide Gel Electrophoresis of the Oxidative Phosphorylation Complexes of Yeast Mitochondria Solubilized by Different Detergents: Specific Staining of the Different Complexes. Anal. Biochem. 1996, 242, 248–254. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef]
- Radermacher, M. Weighted Back-projection Methods. In Electron Tomography; Frank, J., Ed.; Springer: New York, NY, USA, 2006; pp. 245–273. [Google Scholar] [CrossRef]
- Kimanius, D.; Forsberg, B.O.; Scheres, S.H.; Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 2016, 5, e18722. [Google Scholar] [CrossRef]
- Bharat, T.A.M.; Scheres, S.H.W. Resolving macromolecular structures from electron cryo-tomography data using subtomogram averaging in RELION. Nat. Protoc. 2016, 11, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Dai, W.; Sun, S.Y.; Jonasch, D.; He, C.Y.; Schmid, M.F.; Chiu, W.; Ludtke, S.J. Convolutional neural networks for automated annotation of cellular cryo-electron tomograms. Nat. Methods 2017, 14, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Galaz-Montoya, J.G.; Flanagan, J.; Schmid, M.F.; Ludtke, S.J. Single particle tomography in EMAN2. J. Struct. Biol. 2015, 190, 279–290. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterov, S.; Chesnokov, Y.; Kamyshinsky, R.; Panteleeva, A.; Lyamzaev, K.; Vasilov, R.; Yaguzhinsky, L. Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria. Int. J. Mol. Sci. 2021, 22, 1462. https://doi.org/10.3390/ijms22031462
Nesterov S, Chesnokov Y, Kamyshinsky R, Panteleeva A, Lyamzaev K, Vasilov R, Yaguzhinsky L. Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria. International Journal of Molecular Sciences. 2021; 22(3):1462. https://doi.org/10.3390/ijms22031462
Chicago/Turabian StyleNesterov, Semen, Yury Chesnokov, Roman Kamyshinsky, Alisa Panteleeva, Konstantin Lyamzaev, Raif Vasilov, and Lev Yaguzhinsky. 2021. "Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria" International Journal of Molecular Sciences 22, no. 3: 1462. https://doi.org/10.3390/ijms22031462
APA StyleNesterov, S., Chesnokov, Y., Kamyshinsky, R., Panteleeva, A., Lyamzaev, K., Vasilov, R., & Yaguzhinsky, L. (2021). Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria. International Journal of Molecular Sciences, 22(3), 1462. https://doi.org/10.3390/ijms22031462






