Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection
Abstract
:1. Introduction
2. Results
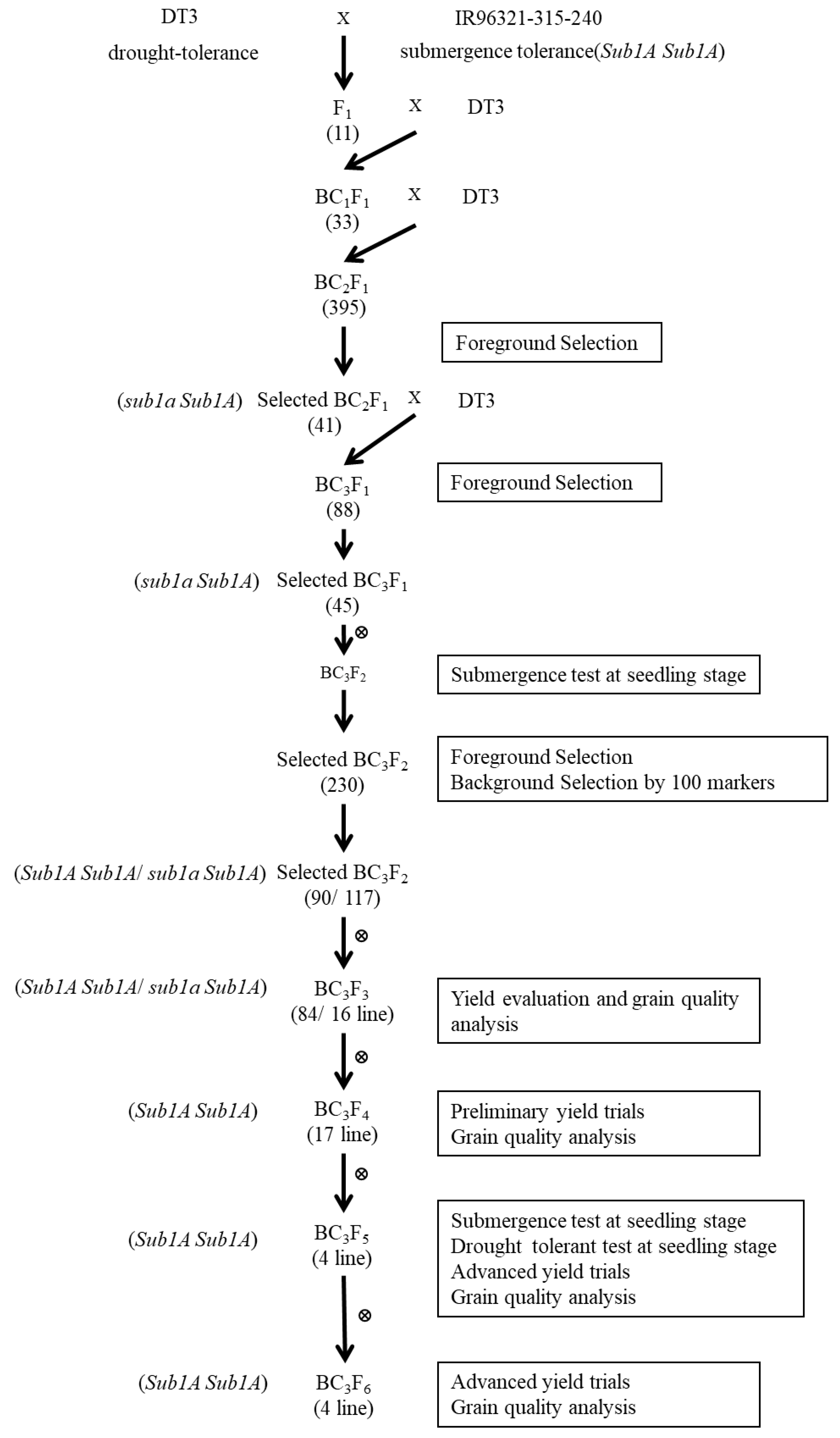
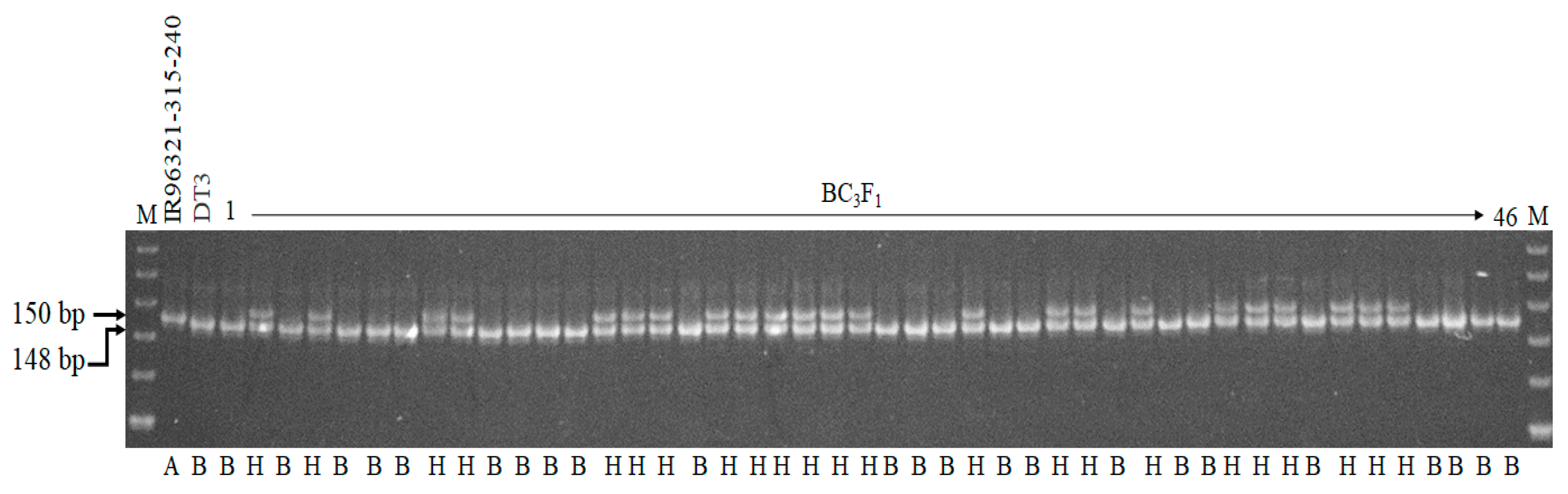
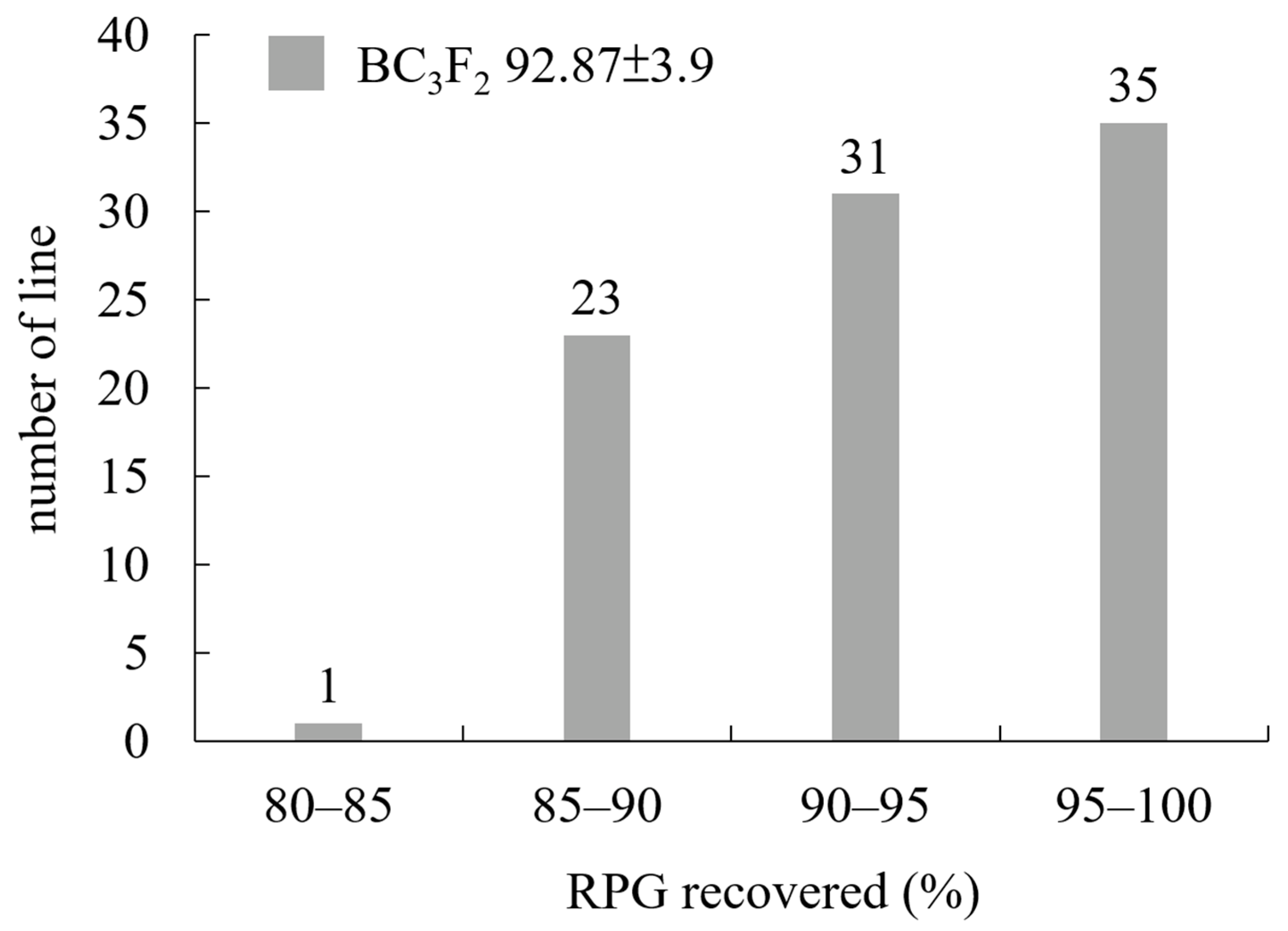
2.1. Development of BC3F6 Submergence-Tolerant Lines Using Marker-Assisted Breeding
2.2. Phenotyping and Evaluation of the Agronomic Performance of Newly Developed Sub1A Lines
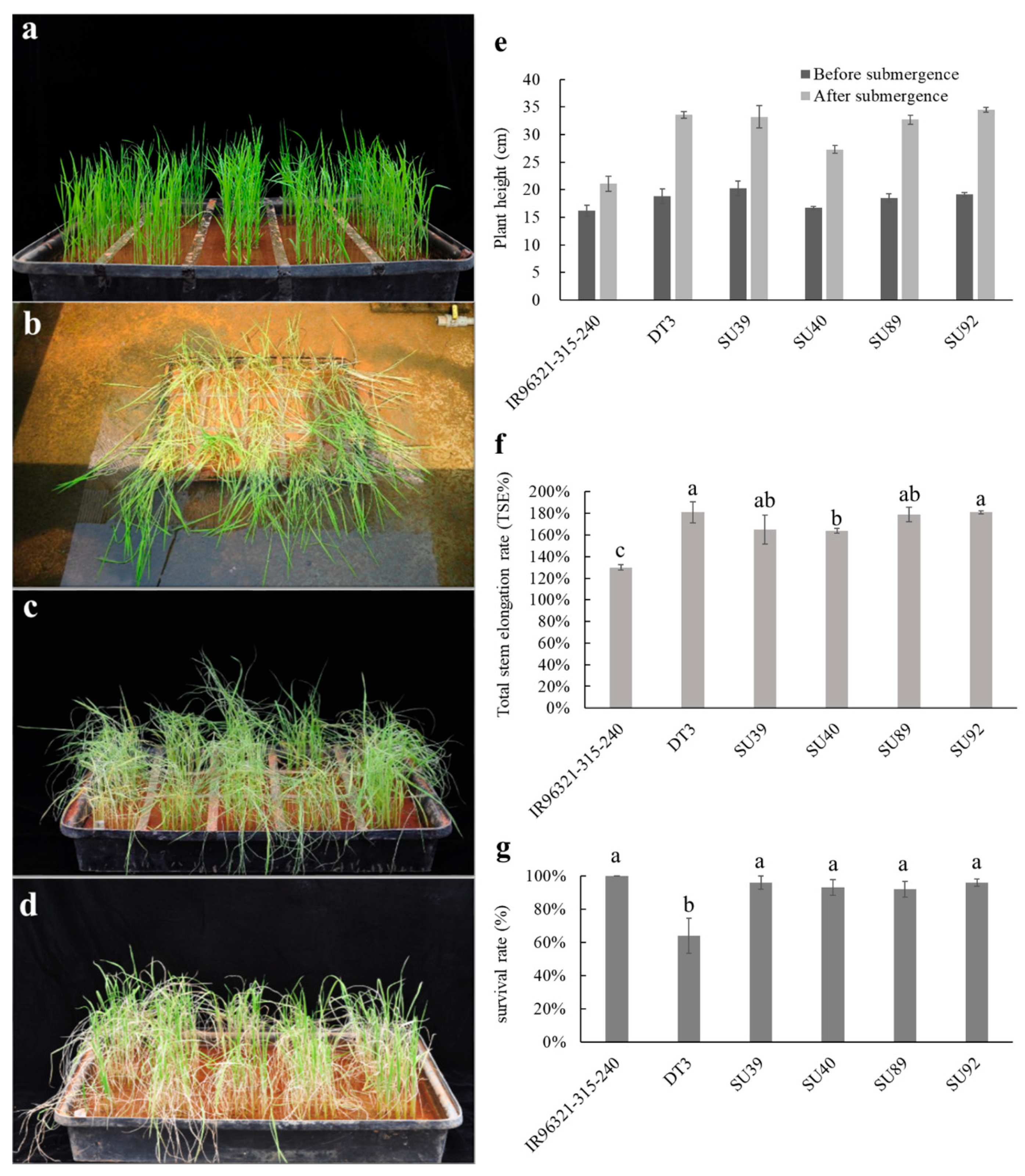
2.3. Performance of the Sub1A Lines (BC3F5) under Submergence and Drought Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Evaluation of Sub1A Lines for Submergence Tolerance
4.3. Evaluation of Sub1A Lines for Osmotic Stress Tolerance
4.4. Evaluation of Sub1A Lines for Blast Resistance
4.5. Evaluation of Agronomic Traits
4.6. DNA Isolation and PCR Amplification
4.7. Marker Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chantal, L.M.; Marie, D.G.; Olivier, M. Land Use and Food Security in 2050: A Narrow Road. Éditions Quae; Editions Quae: Paris, France, 2018. [Google Scholar]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Schneider, P.; Asch, F. Rice production and food security in Asian Mega deltas—A review on characteristics, vulnerabilities and agricultural adaptation options to cope with climate change. J. Agron. Crop Sci. 2020, 206, 491–503. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Billa, L.; Singh, A. Effect of climate change on seasonal monsoon in Asia and its impact on the variability of monsoon rainfall in Southeast Asia. Geosci. Front. 2015, 6, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: SUB1′s journey from landrace to modern cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Muhammad, I.; Kareem, I.; Salisu, M.A.; Arolu, I.W. Submergence tolerance in rice: Review of, mechanism, breeding and, future rrospects. Sustainability 2020, 12, 1632. [Google Scholar] [CrossRef] [Green Version]
- Neeraja, C.N.; Maghirang-Rodriguez, R.; Pamplona, A.; Heuer, S.; Collard, B.C.; Septiningsih, E.M.; Vergara, G.; Sanchez, D.; Xu, K.; Ismail, A.M.; et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Mackill, D.J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Ronald, P.C.; Mackill, D.J. A high-resolution linkage map of the vicinity of the rice submergence tolerance locus Sub1. Mol. Gen. Genet. 2000, 263, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor–like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Iftekharuddaula, K.M.; Newaz, M.A.; Salam, M.A.; Ahmed, H.U.; Mahbub, M.A.A.; Septiningsih, E.M.; Collard, B.C.Y.; Sanchez, D.L.; Pamplona, A.M.; Mackill, D.J. Rapid and high-precision marker assisted backcrossing to introgress the SUB1 QTL into BR11, the rainfed lowland rice mega variety of Bangladesh. Euphytica 2011, 178, 83–97. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Hidayatun, N.; Sanchez, D.L.; Nugraha, Y.; Carandang, J.; Pamplona, A.M.; Collard, B.C.Y.; Ismail, A.M.; Mackill, D.J. Accelerating the development of new submergence tolerant rice varieties: The case of Ciherang-Sub1 and PSB Rc18-Sub1. Euphytica 2015, 202, 259–268. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Pamplona, A.M.; Sanchez, D.L.; Neeraja, C.N.; Vergara, G.V.; Heuer, S.; Ismail, A.M.; Mackill, D.J. Development of submergence-tolerant rice cultivars: The Sub1 locus and beyond. Ann. Bot. 2009, 103, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Singh, A.; Sandhu, N.; Bhandari, A.; Vikram, P.; Kumar, A. Combining drought and submergence tolerance in rice: Marker-assisted breeding and QTL combination effects. Mol. Breed. 2017, 37, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iftekharuddaula, K.M.; Ahmed, H.U.; Ghosal, S.; Moni, Z.R.; Amin, A.; Ali, M.S. Development of new submergence tolerant rice variety for Bangladesh using marker-assisted backcrossing. Rice Sci. 2015, 22, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-P.; Yang, Y.-T.; Kuo, H.-C. Large increasing trend of tropical cyclone rainfall in Taiwan and the roles of terrain. J. Clim. 2013, 26, 4138–4147. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.H.; Kuo, C.W.; Hsu, Y.C.; Lin, Y.R.; Wu, Y.P. Increasing drought tolerance of rice cultivar ‘TK9′ using marker- assisted selection. Crop Environ. Bioinform. 2014, 11, 143–164. [Google Scholar]
- Das, K.; Sarkar, R.; Ismail, A. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005, 168, 131–136. [Google Scholar] [CrossRef]
- Singh, S.; Mackill, D.J.; Ismail, A.M. Responses of SUB1 rice introgression lines to submergence in the field: Yield and grain quality. Field Crops Res. 2009, 113, 12–23. [Google Scholar] [CrossRef]
- Mackill, D.J.; Amante, M.M.; Vergara, B.S.; Sarkarung, S. Improved semidwarf rice lines with tolerance to submergence of seedlings. Crop Sci. 1993, 33, 749–753. [Google Scholar] [CrossRef]
- Sandhu, N.; Dixit, S.; Swamy, B.P.M.; Raman, A.; Kumar, S.; Singh, S.P.; Yadaw, R.B.; Singh, O.N.; Reddy, J.N.; Anandan, A.; et al. Marker assisted breeding to developmultiple stress tolerant varieties for flood and drought prone areas. Rice 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthu, V.; Abbai, R.; Nallathambi, J.; Rahman, H.; Ramasamy, S.; Kambale, R.; Thulasinathan, T.; Ayyenar, B.; Muthurajan, R. Pyramiding QTLs controlling tolerance against drought, salinity, and submergence in rice through marker assisted breeding. PLoS ONE 2020, 15, e0227421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, G.; Rao, G.J.N. Molecular marker assisted gene stacking for biotic and abiotic stress resistance genes in an elite rice cultivar. Front. Plant Sci. 2015, 6, 698. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 16814–16819. [Google Scholar] [CrossRef] [Green Version]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 2011, 23, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Dang, T.T.; Vergara, G.V.; Pandey, D.M.; Sanchez, D.; Neeraja, C.N.; Septiningsih, E.M.; Mendioro, M.; Tecson-Mendoza, E.M.; Ismail, A.M.; et al. Molecular marker survey and expression analyses of the rice submergence-tolerance gene SUB1A. Theor. Appl. Genet. 2010, 121, 1441–1453. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, T.; Zhang, A.; Ong, K.H.; Luo, Z.; Li, Z.; Yang, J.; Yin, Z. Marker-assisted breeding of Chinese elite rice cultivar 9311 for disease resistance to rice blast and bacterial blight and tolerance to submergence. Mol. Breed. 2017, 37, 106. [Google Scholar] [CrossRef]
- Jantaboon, J.; Siangliw, M.; Im-mark, S.; Jamboonsri, W.; Vanavichit, A.; Toojinda, T. Ideotype breeding for submergence tolerance and cooking quality by marker-assisted selection in rice. Field Crops Res. 2011, 123, 206–213. [Google Scholar] [CrossRef]
- Mohapatra, S.; Panda, A.K.; Bastia, A.K.; Mukherjee, A.K.; Sanghamitra, P.; Meher, J.; Mohanty, S.P.; Pradhan, S.K. Development of submergence-tolerant, bacterial blight-resistant, and high-yielding near isogenic lines of popular variety, ‘Swarna’ through marker-assisted breeding approach. Front. Plant Sci. 2021, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Pandit, E.; Pawar, S.; Barik, S.R.; Mohanty, S.P.; Meher, J.; Pradhan, S.K. Marker-assisted backcross breeding for improvement of submergence tolerance and hrain yield in the popular rice variety ‘Maudamani’. Agronomy 2021, 11, 1263. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magneschi, L.; Perata, P. Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 2009, 103, 181–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toojinda, T.; Siangliw, M.; Tragoonrung, S.; Vanavichit, A. Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Ann. Bot. 2003, 91, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.Y.; Chen, Z.F.; Xing, Z.P.; Zhou, L.; Liu, Q.Y.; Zhang, Z.Z.; Jiang, Y.; Hu, Y.J.; Zhu, J.Y.; Cui, P.Y.; et al. Effects of slow or controlled release fertilizer types and fertilization modes on yield and quality of rice. J. Integr. Agric. 2018, 17, 2222–2234. [Google Scholar] [CrossRef]
- Patindol, J.A.; Siebenmorgen, T.J.; Wang, Y.-J. Impact of environmental factors on rice starch structure: A review. Starch-Stärke 2015, 67, 42–54. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ram, P.C. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Wu, Y.S.; Chen, C.T.; Lai, M.H.; Yen, H.M.; Yang, C.Y. Physiological and molecular responses of seedlings of an upland rice (‘Tung Lu 3’) to total submergence compared to those of a submergence-tolerant lowland rice (‘FR13A’). Rice 2017, 10, 42. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cook, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Los Banos, Philippines, 1976; pp. 61–65. [Google Scholar]
- IRRI. Standard Evaluation System for Rice; International Rice Research Institute: Los Banos, Philippines, 2013. [Google Scholar]
- Hsu, Y.C.; Tseng, M.C.; Wu, Y.P.; Lin, M.Y.; Wei, F.J.; Hwu, K.K.; Hsing, Y.I.; Lin, Y.R. Genetic factors responsible for eating and cooking qualities of rice grains in a recombinant inbred population of an inter-subspecific cross. Mol. Breed. 2014, 34, 655–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Berloo, R. GGT: Software for the display of graphical genotypes. J. Hered. 1999, 90, 328–330. [Google Scholar] [CrossRef] [Green Version]
| Line or Variety | Grain Yield Evaluation | Yield Components | ||||||
|---|---|---|---|---|---|---|---|---|
| Grain Yield (kg ha−1) | Yield Index z (%) | Panicle Number (No.) | Panicle Length (cm) | Panicle Weight (g) | Spikelets per Panicle (No.) | Seed Set (%) | 1000-Grain Weight (g) | |
| IR96321-315-240 | 4617 b | 73.7 | 19.8 | 19.0 | 2.08 c | 117.0 | 84.7 | 19.0 c |
| DT3 | 6309 ab | 100.0 | 12.8 | 19.8 | 2.72 b | 138.3 | 79.6 | 22.3 b |
| SU39 | 5853 ab | 95.9 | 14.2 | 18.9 | 3.39 a | 150.4 | 80.4 | 25.9 a |
| SU40 | 6168 ab | 101.8 | 14.4 | 20.4 | 2.88 ab | 154.5 | 84.5 | 20.9 b |
| SU89 | 6101 ab | 97.8 | 14.1 | 19.5 | 3.14 ab | 143.2 | 84.2 | 24.7 a |
| SU92 | 7345 a | 114.6 | 15.1 | 19.7 | 3.01 ab | 133.6 | 85.0 | 25.3 a |
| Line or Variety | Seed Appearance | Palatability | ||||
|---|---|---|---|---|---|---|
| Chalky Rice (%) | Seed Length (mm) | Seed Width (mm) | Seed Length-Width Ratio | Seed Thickness (mm) | ||
| IR96321-315-240 | 53.1 bc | 4.69 a | 2.11 d | 2.23 a | 1.81 d | 35.5 c |
| DT3 | 80.2 a | 4.28 d | 2.69 b | 1.59 d | 2.04 a | 58.6 b |
| SU39 | 86.5 a | 4.38 c | 2.81 a | 1.56 e | 2.03 a | 61.4 ab |
| SU40 | 59.2 b | 4.35 c | 2.36 c | 1.85 b | 1.90 c | 61.9 ab |
| SU89 | 33.3 d | 4.51 b | 2.70 b | 1.67 c | 2.03 a | 62.1 a |
| SU92 | 43.3 c | 4.51 b | 2.69 b | 1.67 c | 2.00 b | 60.5 ab |
| Line or Variety | Peak Viscosity (PKV) | Hot Paste Viscosity (HPV) | Breakdown Viscosity (BDV) | Cool Paste Viscosity (CPV) | Setback Viscosity (CSV) |
|---|---|---|---|---|---|
| IR96321-315-240 | 2350.33 c | 1463.33 b | 887.00 c | 3470.33 a | 2007.00 a |
| DT3 | 3159.50 b | 1435.75 b | 1723.75 b | 2302.25 b | 866.50 b |
| SU39 | 3197.75 b | 1506.75 a | 1691.00 b | 2414.25 b | 907.50 b |
| SU40 | 3229.25 b | 1393.75 b | 1835.50 b | 2228.50 b | 834.75 b |
| SU89 | 3404.25 ab | 1521.50 a | 1882.75 b | 2424.00 b | 902.50 b |
| SU92 | 3551.25 a | 1330.25 b | 2221.00 a | 2200.50 b | 870.25 b |
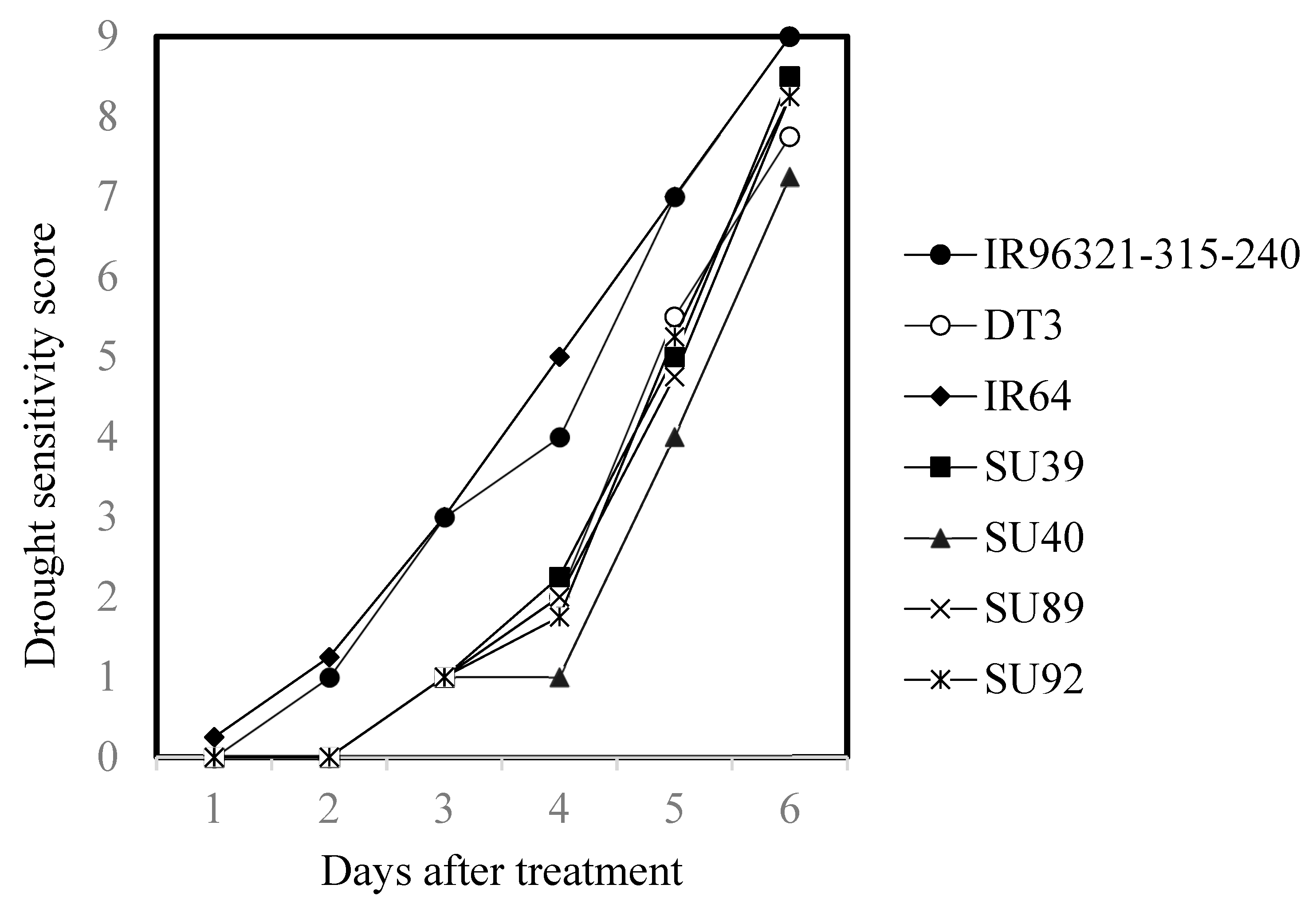
| Line or Variety | Days after Treatment | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| IR96321-315-240 | 0.00 b | 1.00 a | 3 a | 4.00 a | 7.00 a | 9.00 a |
| DT3 | 0.00 b | 0.00 b | 1 b | 2.00 b | 5.50 b | 7.75 ab |
| IR64 z | 0.25 a | 1.25 a | 3 a | 5.00 a | 7.00 a | 9.00 a |
| SU39 | 0.00 b | 0.00 b | 1 b | 2.25 b | 5.00 b | 8.50 ab |
| SU40 | 0.00 b | 0.00 b | 1 b | 1.00 b | 4.00 c | 7.25 b |
| SU89 | 0.00 b | 0.00 b | 1 b | 2.00 b | 4.75 bc | 8.25 ab |
| SU92 | 0.00 b | 0.00 b | 1 b | 1.75 b | 5.25 b | 8.25 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-P.; Wang, S.-M.; Chang, Y.-C.; Ho, C.; Hsu, Y.-C. Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection. Int. J. Mol. Sci. 2021, 22, 13365. https://doi.org/10.3390/ijms222413365
Wu Y-P, Wang S-M, Chang Y-C, Ho C, Hsu Y-C. Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection. International Journal of Molecular Sciences. 2021; 22(24):13365. https://doi.org/10.3390/ijms222413365
Chicago/Turabian StyleWu, Yong-Pei, Shu-Mei Wang, Yu-Chi Chang, Chi Ho, and Yu-Chia Hsu. 2021. "Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection" International Journal of Molecular Sciences 22, no. 24: 13365. https://doi.org/10.3390/ijms222413365
APA StyleWu, Y.-P., Wang, S.-M., Chang, Y.-C., Ho, C., & Hsu, Y.-C. (2021). Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection. International Journal of Molecular Sciences, 22(24), 13365. https://doi.org/10.3390/ijms222413365






