High Molecular Weight Kininogen: A Review of the Structural Literature
Abstract
:1. Introduction
2. HK Biochemical and Evolutionary Characterization
3. The Importance of Kininogen in Civilization and Infectious Diseases
4. Literature Describing the Structure of Kininogen
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.E.; Mandle, R.; Kaplan, A.P. Characterization of Human High Molecular Weight Kininogen. Procoagulant Activity Associated with the Light Chain of Kinin-Free High Molecular Weight Kininogen. J. Exp. Med. 1978, 147, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Kerbiriou, D.M.; Griffin, J.H. Human High Molecular Weight Kininogen. Studies of Structure-Function Relationships and of Proteolysis of the Molecule Occurring during Contact Activation of Plasma. J. Biol. Chem. 1979, 254, 12020–12027. [Google Scholar] [CrossRef]
- Weisel, J.W.; Nagaswami, C.; Woodhead, J.L.; Cadena, R.A.D.; Page, J.D.; Colman, R.W. The Shape of High Molecular Weight Kininogen. Organization into Structural Domains, Changes with Activation, and Interactions with Prekallikrein, as Determined by Electron Microscopy. J. Biol. Chem. 1994, 269, 10100–10106. [Google Scholar] [CrossRef]
- Winter, W.E.; Greene, D.N.; Beal, S.G.; Isom, J.A.; Manning, H.; Wilkerson, G.; Harris, N. Clotting Factors: Clinical Biochemistry and Their Roles as Plasma Enzymes. Adv. Clin. Chem. 2020, 94, 31–84. [Google Scholar] [PubMed]
- Londesborough, J.C.; Hamberg, U. The Sialic Acid Content and Isoelectric Point of Human Kininogen. Biochem. J. 1975, 145, 401–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandle, R.J.; Colman, R.W.; Kaplan, A.P. Identification of Prekallikrein and High Molecular Weight Kininogen as a Complex in Human Plasma. Proc. Natl. Acad. Sci. USA 1976, 73, 4179–4183. [Google Scholar] [CrossRef] [Green Version]
- Hock, J.; Vogel, R.; Linke, R.P.; Müller-Esterl, W. High Molecular Weight Kininogen-Binding Site of Prekallikrein Probed by Monoclonal Antibodies. J. Biol. Chem. 1990, 265, 12005–12011. [Google Scholar] [CrossRef]
- Kerbiriou, D.M.; Bouma, B.N.; Griffin, J.H. Immunochemical Studies of Human High Molecular Weight Kininogen and of Its Complexes with Plasma Prekallikrein or Kallikrein. J. Biol. Chem. 1980, 255, 3952–3958. [Google Scholar] [CrossRef]
- Kokoye, Y.; Ivanov, I.; Cheng, Q.; Matafonov, A.; Dickeson, S.K.; Mason, S.; Sexton, D.J.; Renné, T.; McCrae, K.; Feener, E.P.; et al. A Comparison of the Effects of Factor XII Deficiency and Prekallikrein Deficiency on Thrombus Formation. Thromb. Res. 2016, 140, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, B.M.; Matafonov, A.; Ivanov, I.; Sun, M.-F.; Cheng, Q.; Dickeson, S.K.; Li, C.; Sun, D.; Verhamme, I.M.; Emsley, J.; et al. An Update on Factor XI Structure and Function. Thromb. Res. 2018, 161, 94–105. [Google Scholar] [CrossRef]
- Thompson, R.E.; Mandle, R.; Kaplan, A.P. Studies of Binding of Prekallikrein and Factor XI to High Molecular Weight Kininogen and Its Light Chain. Proc. Natl. Acad. Sci. USA 1979, 76, 4862–4866. [Google Scholar] [CrossRef] [Green Version]
- Ponczek, M.B.; Gailani, D.; Doolittle, R.F. Evolution of the Contact Phase of Vertebrate Blood Coagulation. J. Thromb. Haemost. 2008, 6, 1876–1883. [Google Scholar] [CrossRef] [Green Version]
- Ponczek, M.B.; Shamanaev, A.; LaPlace, A.; Dickeson, S.K.; Srivastava, P.; Sun, M.-F.; Gruber, A.; Kastrup, C.; Emsley, J.; Gailani, D. The Evolution of Factor XI and the Kallikrein-Kinin System. Blood Adv. 2020, 4, 6135–6147. [Google Scholar] [CrossRef]
- Thompson, R.E.; Mandle, R.; Kaplan, A.P. Association of Factor XI and High Molecular Weight Kininogen in Human Plasma. J. Clin. Investig. 1977, 60, 1376–1380. [Google Scholar] [CrossRef]
- Finn, R.D.; Mistry, J.; Schuster-Böckler, B.; Griffiths-Jones, S.; Hollich, V.; Lassmann, T.; Moxon, S.; Marshall, M.; Khanna, A.; Durbin, R.; et al. Pfam: Clans, Web Tools and Services. Nucleic Acids Res. 2006, 34, D247–D251. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Bateman, A.; Birney, E.; Durbin, R.; Eddy, S.R.; Howe, K.L.; Sonnhammer, E.L.L. The Pfam Protein Families Database. Nucleic Acids Res. 2000, 28, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, S.K.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2009, 38, D211–D222. [Google Scholar] [CrossRef]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L.L.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2004, 32, D138–D141. [Google Scholar] [CrossRef]
- Punta, M.; Coggill, P.C.; Eberhardt, R.Y.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, S.K.; Ceric, G.; Clements, J.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2012, 40, D290–D301. [Google Scholar] [CrossRef]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.-R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.L.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef] [Green Version]
- Coggill, P.; Finn, R.D.; Bateman, A. Identifying Protein Domains with the Pfam Database. Curr. Protoc. Bioinform. 2008, 1, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.J.; Finn, R.D.; Bateman, A. Pfam 10 Years on: 10 000 Families and Still Growing. Brief. Bioinform. 2008, 9, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Sigrist, C.J.A.; Cerutti, L.; de Castro, E.; Langendijk-Genevaux, P.S.; Bulliard, V.; Bairoch, A.; Hulo, N. PROSITE, a Protein Domain Database for Functional Characterization and Annotation. Nucleic Acids Res. 2009, 38, D161–D166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, C.J.A.; Cerutti, L.; Hulo, N.; Gattiker, A.; Falquet, L.; Pagni, M.; Bairoch, A.; Bucher, P. PROSITE: A Documented Database Using Patterns and Profiles as Motif Descriptors. Brief. Bioinform. 2002, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; de Castro, E.; Langendijk-Genevaux, P.S.; Pagni, M.; Sigrist, C.J.A. The PROSITE Database. Nucleic Acids Res. 2006, 34, D227–D230. [Google Scholar] [CrossRef] [Green Version]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; Cuche, B.A.; de castro, E.; Lachaize, C.; Langendijk-Genevaux, P.S.; Sigrist, C.J.A. The 20 Years of PROSITE. Nucleic Acids Res. 2008, 36, D245–D249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulo, N.; Sigrist, C.J.A.; le Saux, V.; Langendijk-Genevaux, P.S.; Bordoli, L.; Gattiker, A.; de Castro, E.; Bucher, P.; Bairoch, A. Recent Improvements to the PROSITE Database. Nucleic Acids Res. 2004, 32, D134–D137. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, C.J.A.; de Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and Continuing Developments at PROSITE. Nucleic Acids Res. 2013, 41, D344–D347. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, K.; Bucher, P.; Falquet, L.; Bairoch, A. The PROSITE Database, Its Status in 1999. Nucleic Acids Res. 1999, 27, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Falquet, L.; Pagni, M.; Bucher, P.; Hulo, N.; Sigrist, C.J.A.; Hofmann, K.; Bairoch, A. The PROSITE Database, Its Status in 2002. Nucleic Acids Res. 2002, 30, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, T.; Pagni, M.; Bridge, A.; Bougueleret, L.; Xenarios, I.; Cerutti, L. PfsearchV3: A Code Acceleration and Heuristic to Search PROSITE Profiles. Bioinformatics 2013, 29, 1215–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, C.J.A.; de Castro, E.; Langendijk-Genevaux, P.S.; le Saux, V.; Bairoch, A.; Hulo, N. ProRule: A New Database Containing Functional and Structural Information on PROSITE Profiles. Bioinformatics 2005, 21, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE Signature Matches and ProRule-Associated Functional and Structural Residues in Proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent Updates to the Protein Domain Annotation Resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent Updates, New Developments and Status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef]
- Schultz, J.; Copley, R.R.; Doerks, T.; Ponting, C.P.; Bork, P. SMART: A Web-Based Tool for the Study of Genetically Mobile Domains. Nucleic Acids Res. 2000, 28, 231–234. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 Years of the SMART Protein Domain Annotation Resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolution of Proteins of the Cystatin Superfamily. J. Mol. Evol. 1990, 30, 60–71. [Google Scholar] [CrossRef]
- Zhou, L.; Li-Ling, J.; Huang, H.; Ma, F.; Li, Q. Phylogenetic Analysis of Vertebrate Kininogen Genes. Genomics 2008, 91, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordiš, D.; Turk, V. Phylogenomic Analysis of the Cystatin Superfamily in Eukaryotes and Prokaryotes. BMC Evol. Biol. 2009, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Lalmanach, G.; Naudin, C.; Lecaille, F.; Fritz, H. Kininogens: More than Cysteine Protease Inhibitors and Kinin Precursors. Biochimie 2010, 92, 1568–1579. [Google Scholar] [CrossRef]
- Herwald, H.; Mörgelin, M.; Svensson, H.G.; Sjöbring, U. Zinc-Dependent Conformational Changes in Domain D5 of High Molecular Mass Kininogen Modulate Contact Activation. Eur. J. Biochem. 2001, 268, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Oehmcke, S.; Mörgelin, M.; Herwald, H. Activation of the Human Contact System on Neutrophil Extracellular Traps. J. Innate Immun. 2009, 1, 225–230. [Google Scholar] [CrossRef]
- Alquraishi, M. AlphaFold at CASP13. Bioinformatics 2019, 35, 4862–4865. [Google Scholar] [CrossRef]
- Tong, A.B.; Burch, J.D.; McKay, D.; Bustamante, C.; Crackower, M.A.; Wu, H. Could AlphaFold Revolutionize Chemical Therapeutics? Nat. Struct. Mol. Biol. 2021, 28, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. What Does AlphaFold Mean for Drug Discovery? Nat. Rev. Drug Discov. 2021, 20, 725–727. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. UCSF Chimera—A visualization system for exploratory research and analysis. J. Biol. Chem. 2004, 25, 1605–1612. [Google Scholar]
- Earl, L.A.; Falconieri, V.; Milne, J.L.; Subramaniam, S. Cryo-EM: Beyond the Microscope. Curr. Opin. Struct. Biol. 2017, 46, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nakane, T.; Kotecha, A.; Sente, A.; McMullan, G.; Masiulis, S.; Brown, P.M.G.E.; Grigoras, I.T.; Malinauskaite, L.; Malinauskas, T.; Miehling, J.; et al. Single-Particle Cryo-EM at Atomic Resolution. Nature 2020, 587, 152–156. [Google Scholar] [CrossRef]
- Cheng, Y. Single-Particle Cryo-EM—How Did It Get Here and Where Will It Go. Science 2018, 361, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Hand, E. Cryo-EM Reveals Exquisite Molecular Structures—At High Cost. A Cheaper Microscope Could Bring the Resolution Revolution to the Masses. Science 2020, 367, 354–358. [Google Scholar] [CrossRef]
- Burrows, N.D.; Penn, R.L. Cryogenic Transmission Electron Microscopy: Aqueous Suspensions of Nanoscale Objects. Microsc. Microanal. 2013, 19, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
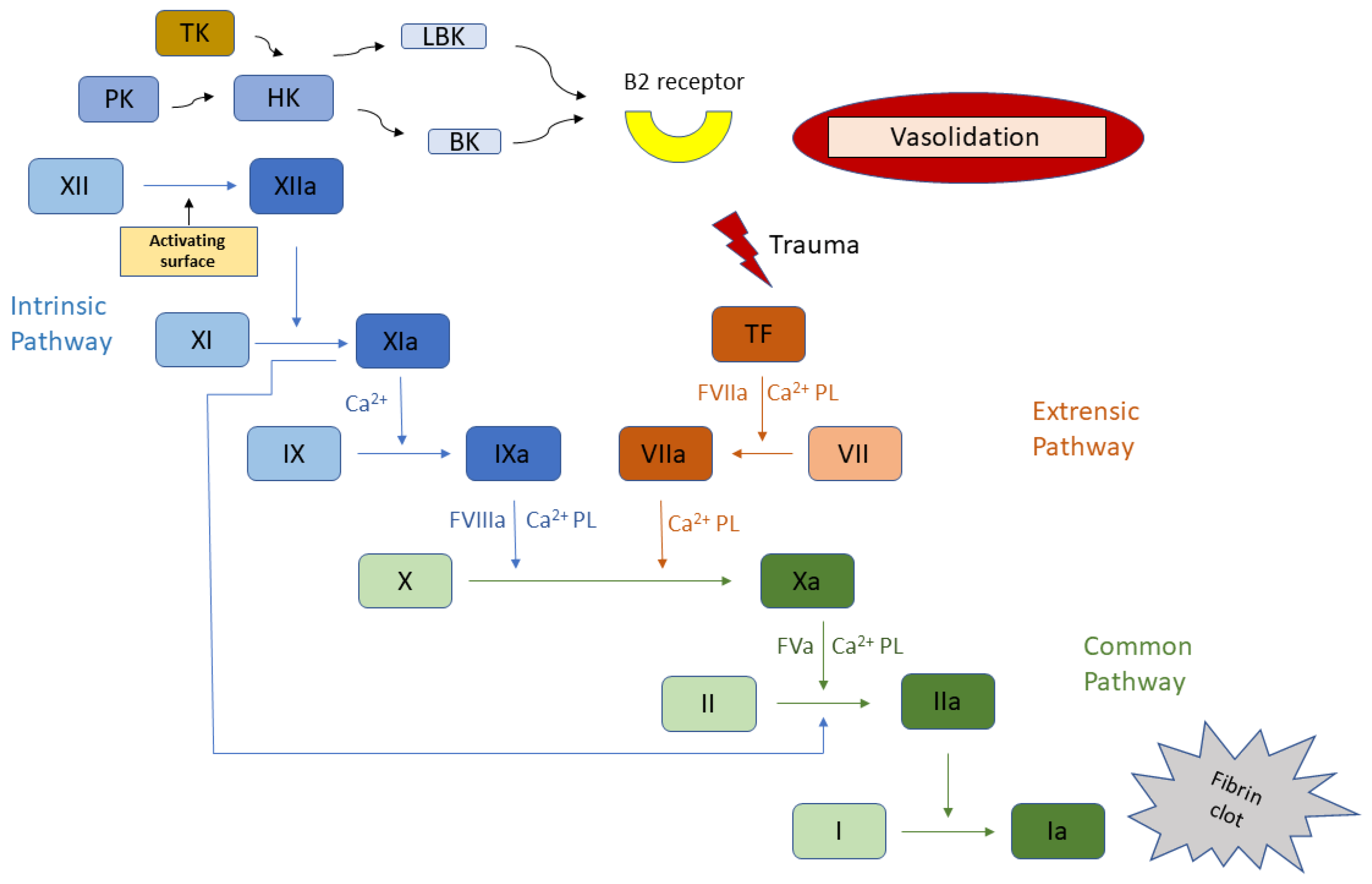
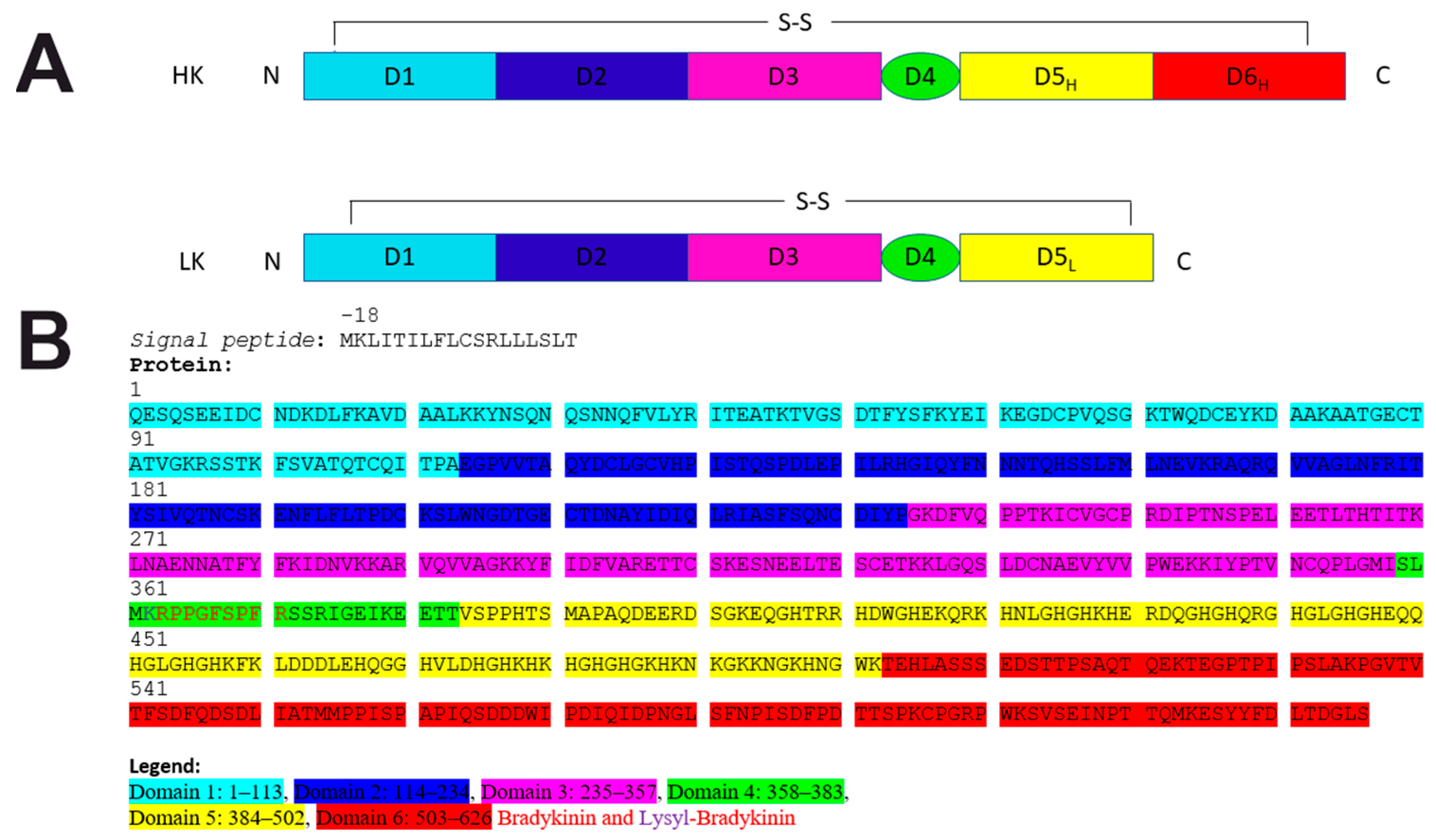
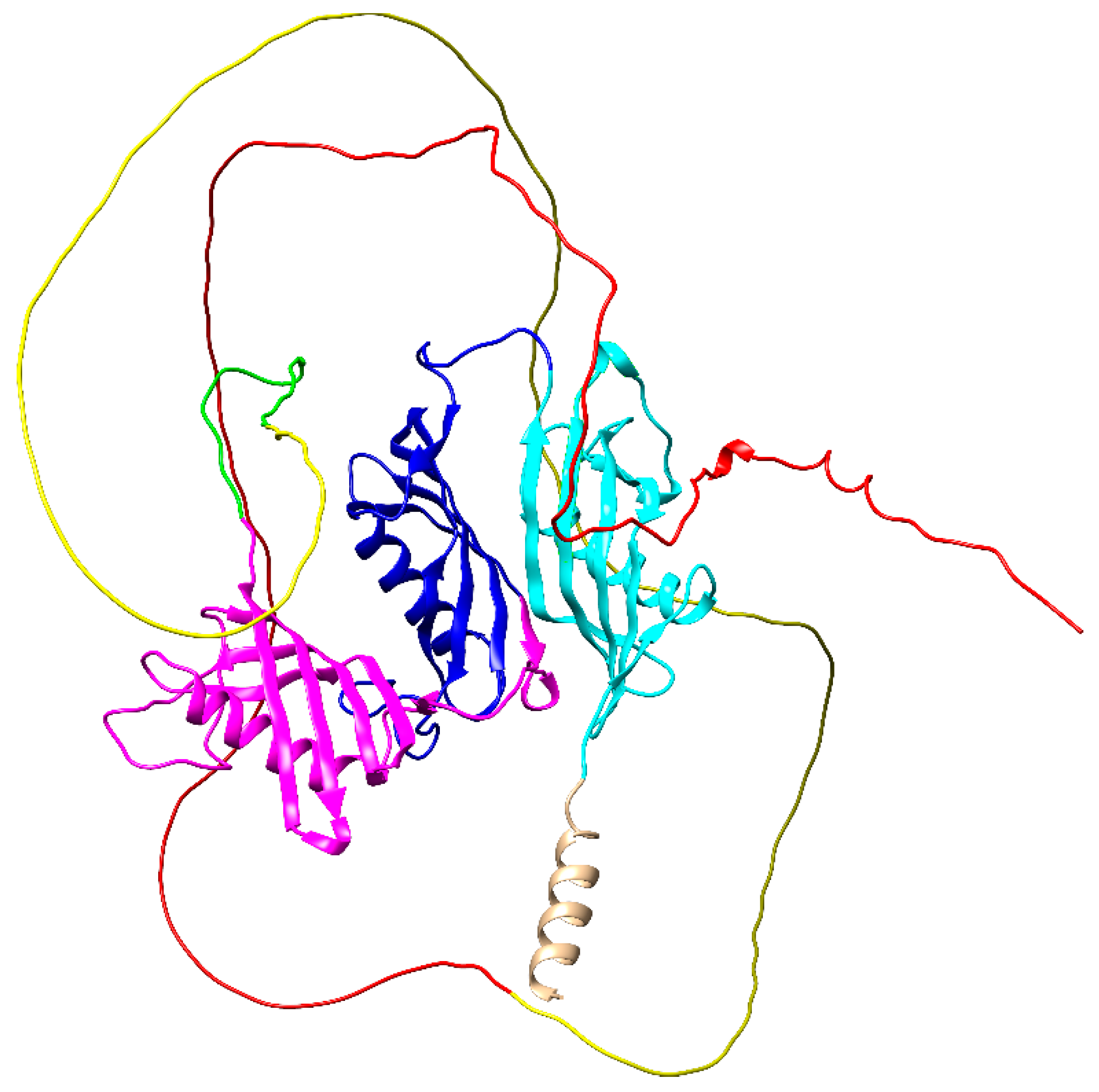
| Description | Percent Identity 1 | PDB Accession and Chain 2 |
|---|---|---|
| Cleaved human fetuin-b in complex with crayfish astacin [Homo sapiens] | 26.20 | 6SAZ B |
| Crystal structure of L68V mutant of human cystatin C [Homo sapiens] | 29.73 | 3PS8 A |
| Human Cystatin C; Dimeric Form With 3d Domain Swapping [Homo sapiens] | 30.63 | 4N6M A |
| Crystal structure of human cystatin E/M [Homo sapiens] | 29.73 | 1G96 A |
| Crystal structure of monomeric human cystatin C stabilized against aggregation [Homo sapiens] | 31.48 | 4N6L A |
| N-Truncated Human Cystatin C; Dimeric Form With 3D Domain Swapping [Homo sapiens] | 28.57 | 3GAX A |
| Crystal structure of V57G mutant of human cystatin C [Homo sapiens] | 27.84 | 1R4C A |
| Crystal structure of V57P mutant of human cystatin C [Homo sapiens] | 28.83 | 6ROA A |
| Hinge-loop mutation can be used to control 3D domain swapping and amyloidogenesis of human cystatin C [Homo sapiens] | 28.83 | 3S67 A |
| ID | Title | Released |
|---|---|---|
| 4ECC | Chimeric GST Containing Inserts of Kininogen Peptides | 16 May 2012 |
| 4ECB | Chimeric GST Containing Inserts of Kininogen Peptides | 16 May 2012 |
| 6BFP | Bovine trypsin bound to potent inhibitor | 31 October 2018 |
| 4JD9 | Contact pathway inhibitor from a sand fly | 16 October 2013 |
| 6F3Y | Backbone structure of Des-Arg10-Kallidin (DAKD) peptide bound to human Bradykinin 1 Receptor (B1R) determined by DNP-enhanced MAS SSNMR | 10 January 2018 |
| 6F3X | Backbone structure of Des-Arg10-Kallidin (DAKD) peptide in frozen DDM/CHS detergent micelle solution determined by DNP-enhanced MAS SSNMR | 10 January 2018 |
| 6F3W | Backbone structure of free bradykinin (BK) in DDM/CHS detergent micelle determined by MAS SSNMR | 10 January 2018 |
| 6O1S | Structure of human plasma kallikrein protease domain with inhibitor | 6 March 2019 |
| 6O1G | Full length human plasma kallikrein with inhibitor | 6 March 2019 |
| 6F27 | NMR solution structure of non-bound [des-Arg10]-kallidin (DAKD) | 10 January 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponczek, M.B. High Molecular Weight Kininogen: A Review of the Structural Literature. Int. J. Mol. Sci. 2021, 22, 13370. https://doi.org/10.3390/ijms222413370
Ponczek MB. High Molecular Weight Kininogen: A Review of the Structural Literature. International Journal of Molecular Sciences. 2021; 22(24):13370. https://doi.org/10.3390/ijms222413370
Chicago/Turabian StylePonczek, Michał B. 2021. "High Molecular Weight Kininogen: A Review of the Structural Literature" International Journal of Molecular Sciences 22, no. 24: 13370. https://doi.org/10.3390/ijms222413370
APA StylePonczek, M. B. (2021). High Molecular Weight Kininogen: A Review of the Structural Literature. International Journal of Molecular Sciences, 22(24), 13370. https://doi.org/10.3390/ijms222413370






