Novel Criteria for Intratumoral Budding with Prognostic Relevance for Colon Cancer and Its Histological Subtypes
Abstract
:1. Introduction
2. Results
2.1. The Cohort of Patients with Adenocarcinoma
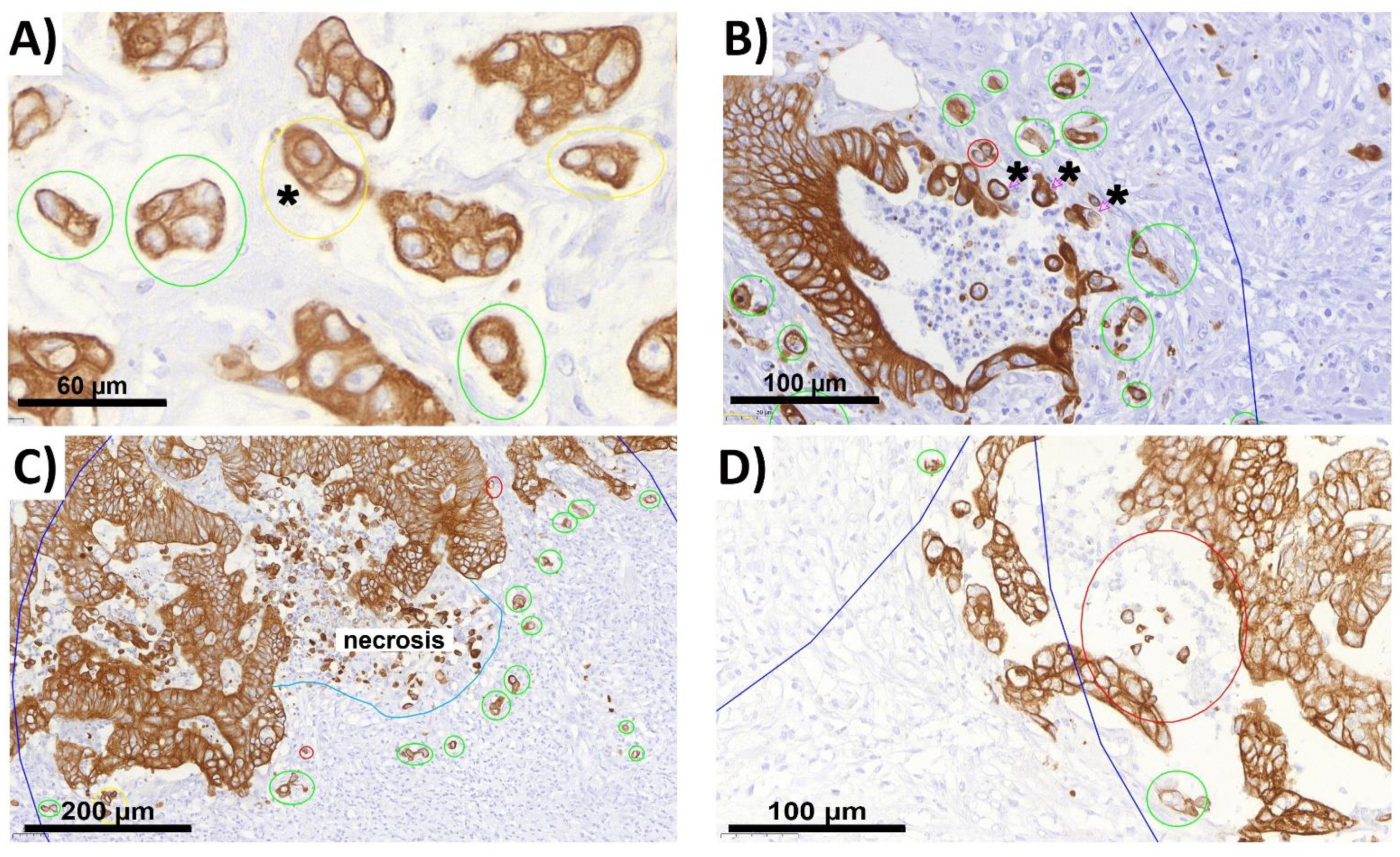
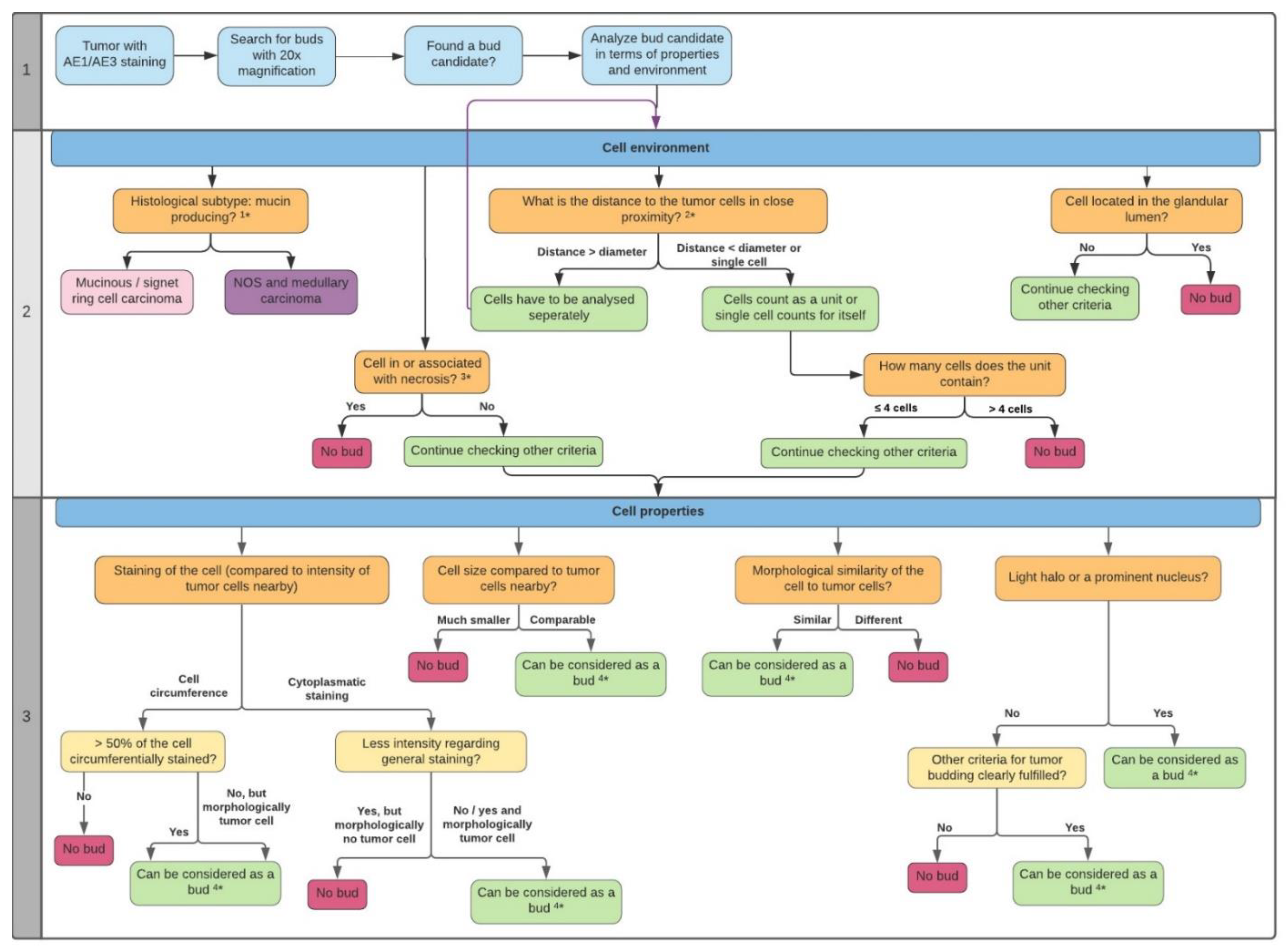
2.2. ITB Evaluation and the Criteria Definition
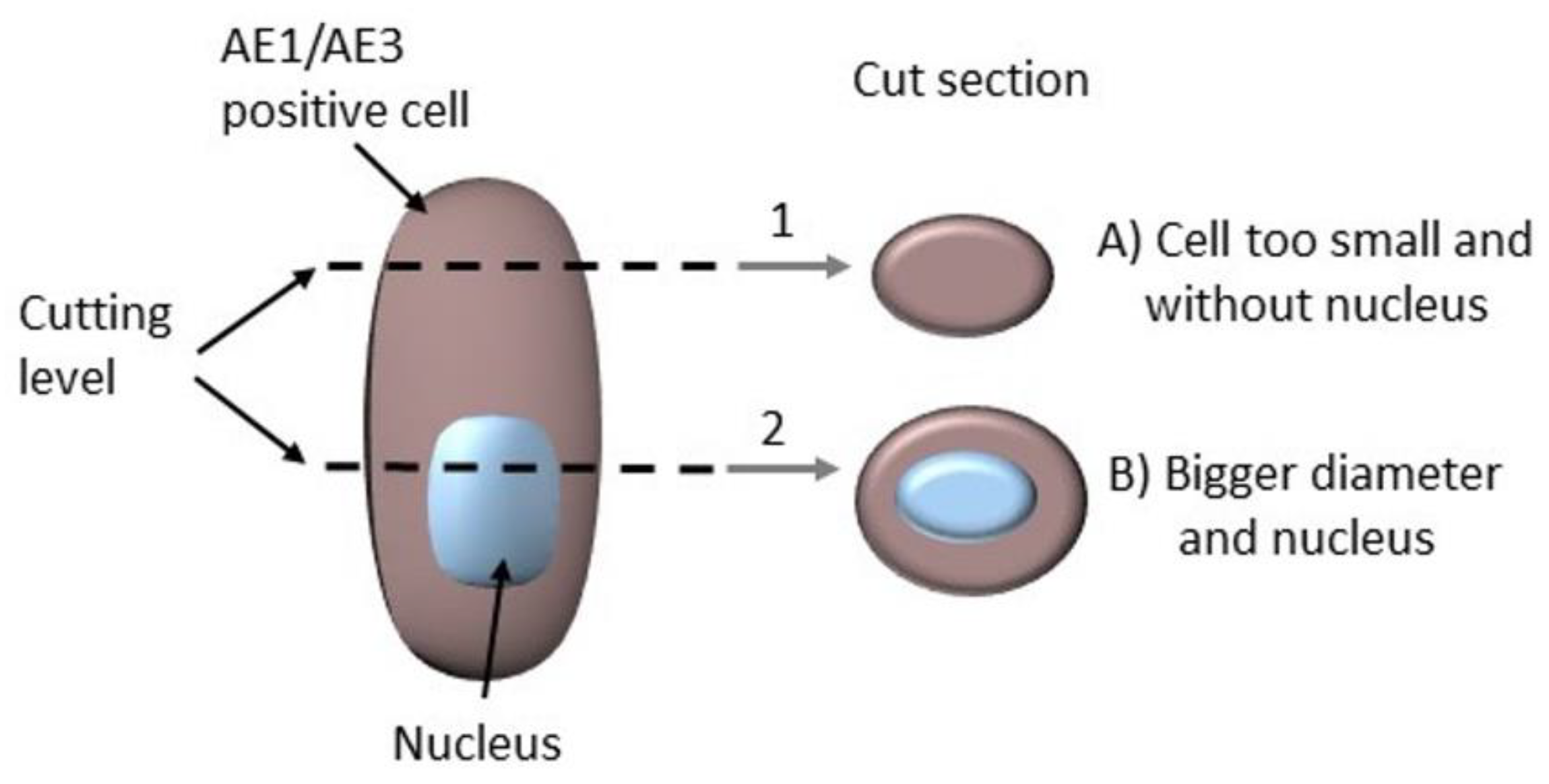
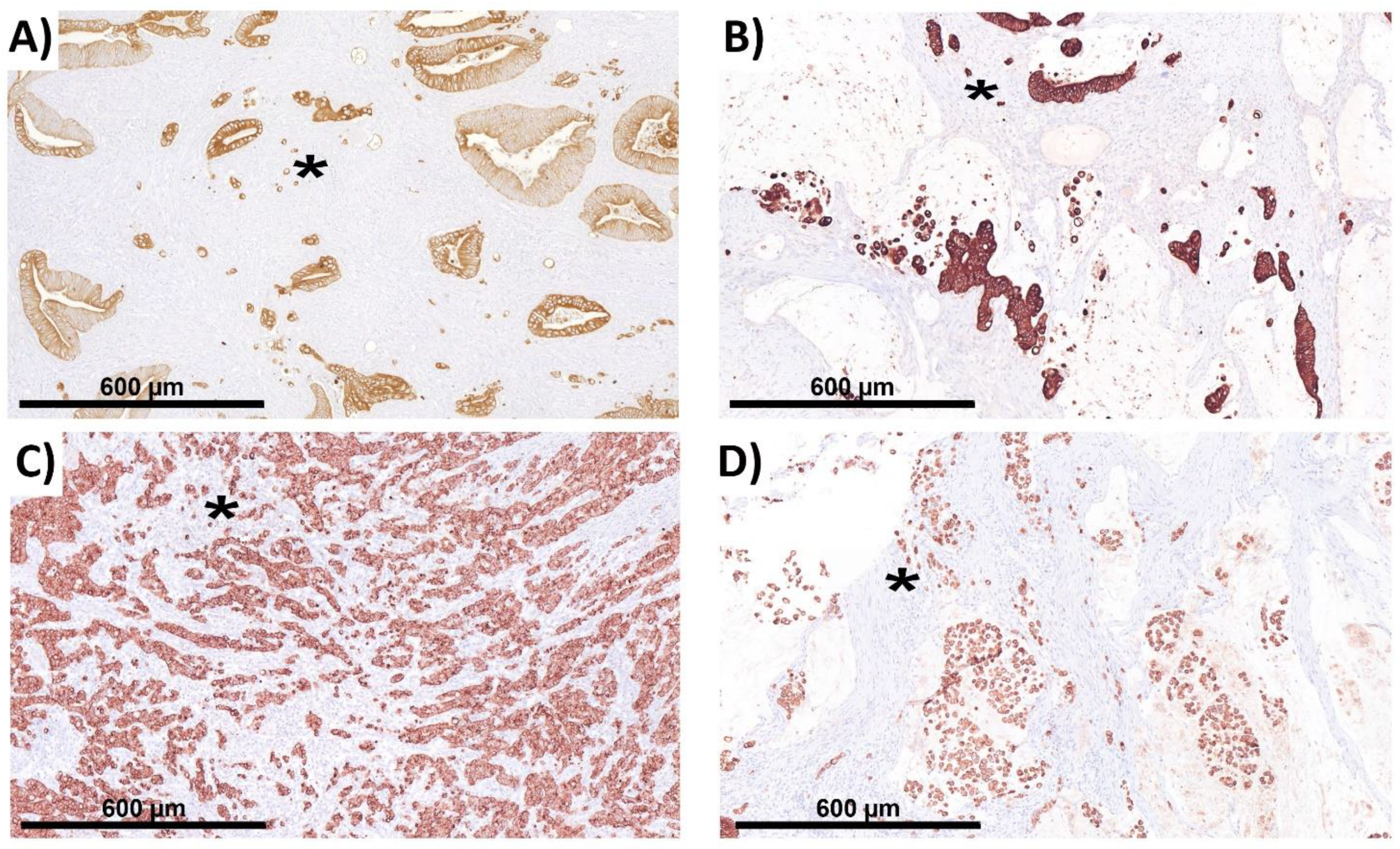
2.3. The Complicating Aspects in Budding Evaluations: Cell Truncation
2.4. Peritumoral Budding (PTB) in NOS Adenocarcinoma
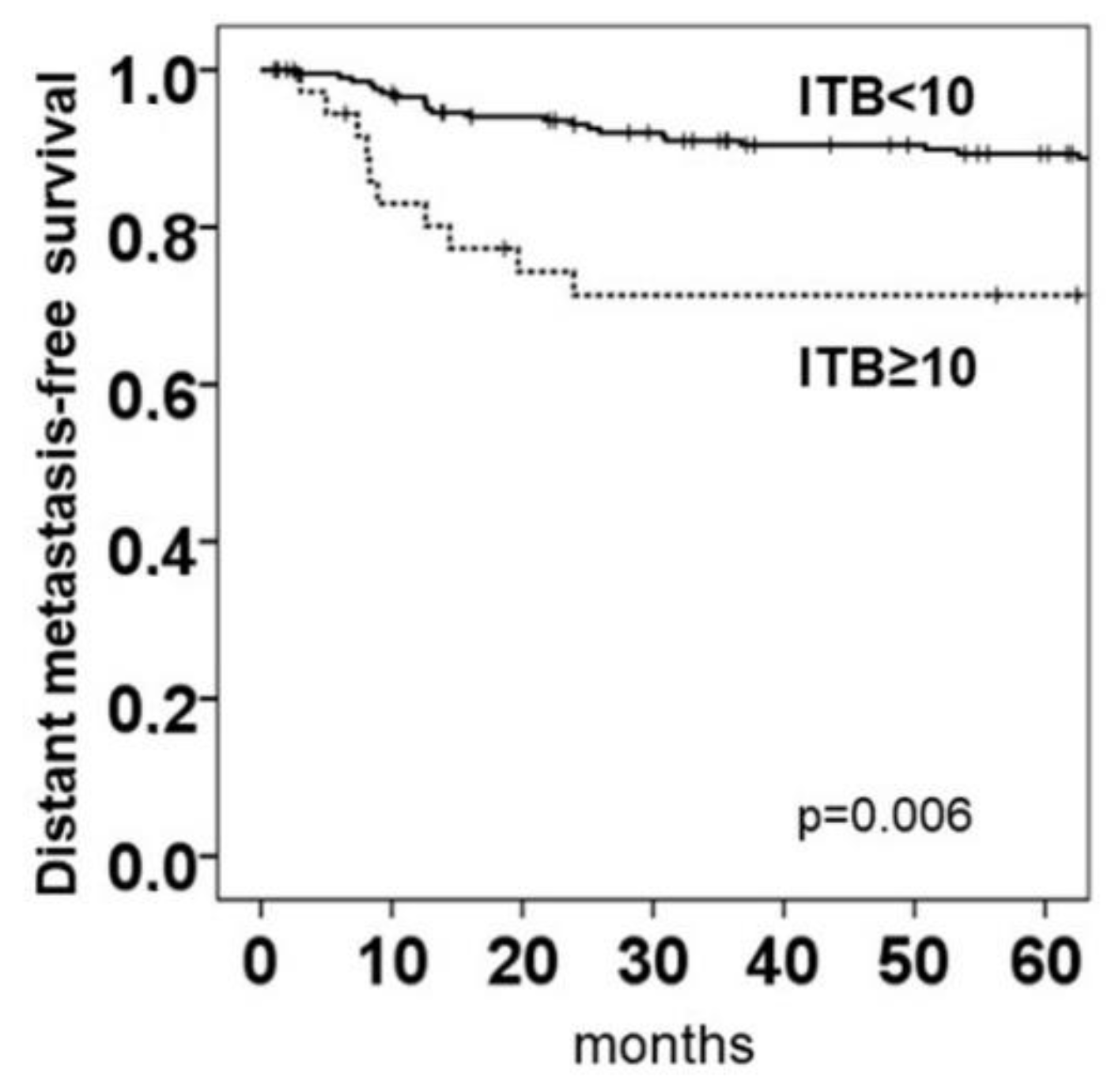
2.5. Intratumoral Budding in NOS Adenocarcinoma and Clinical Outcomes
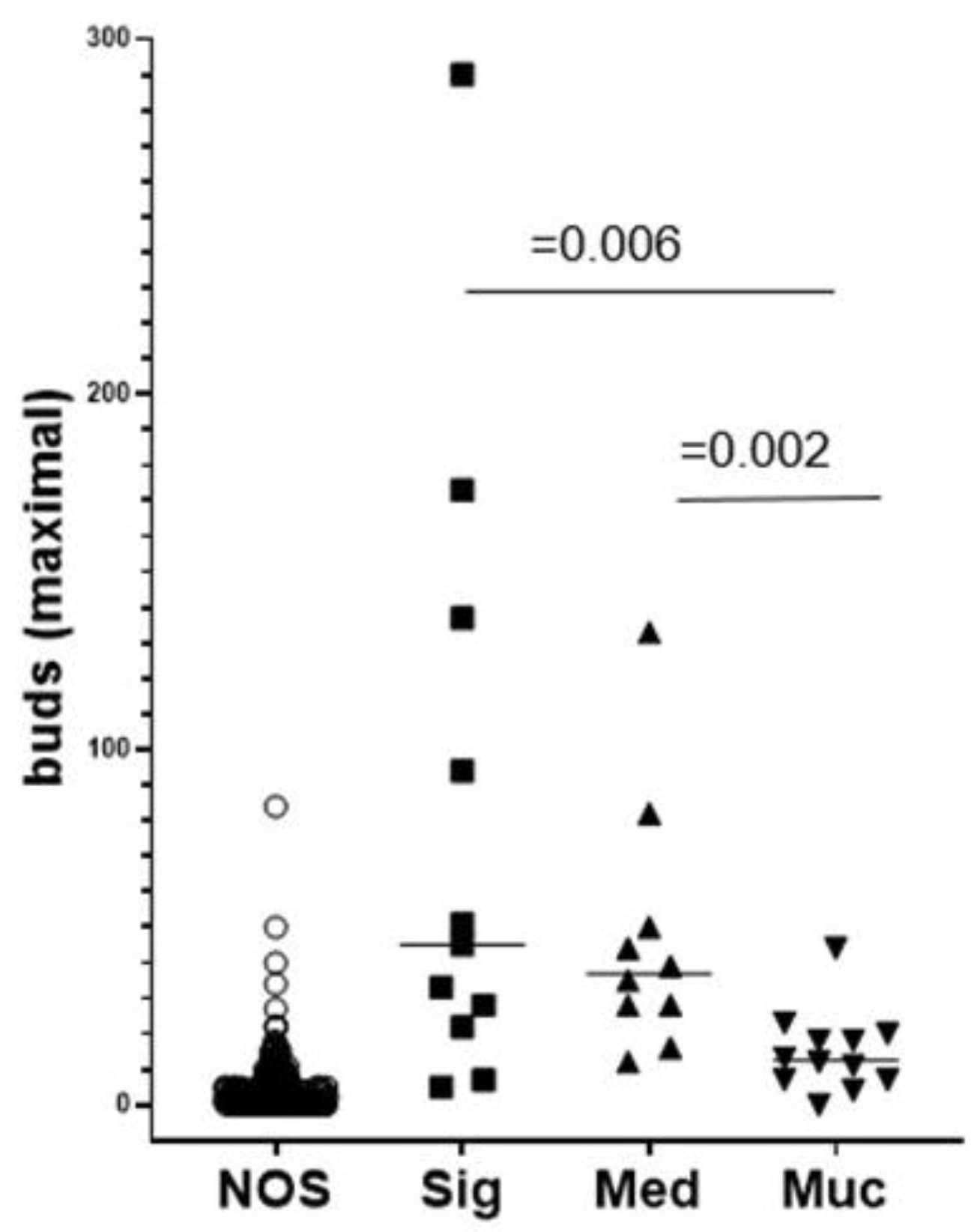
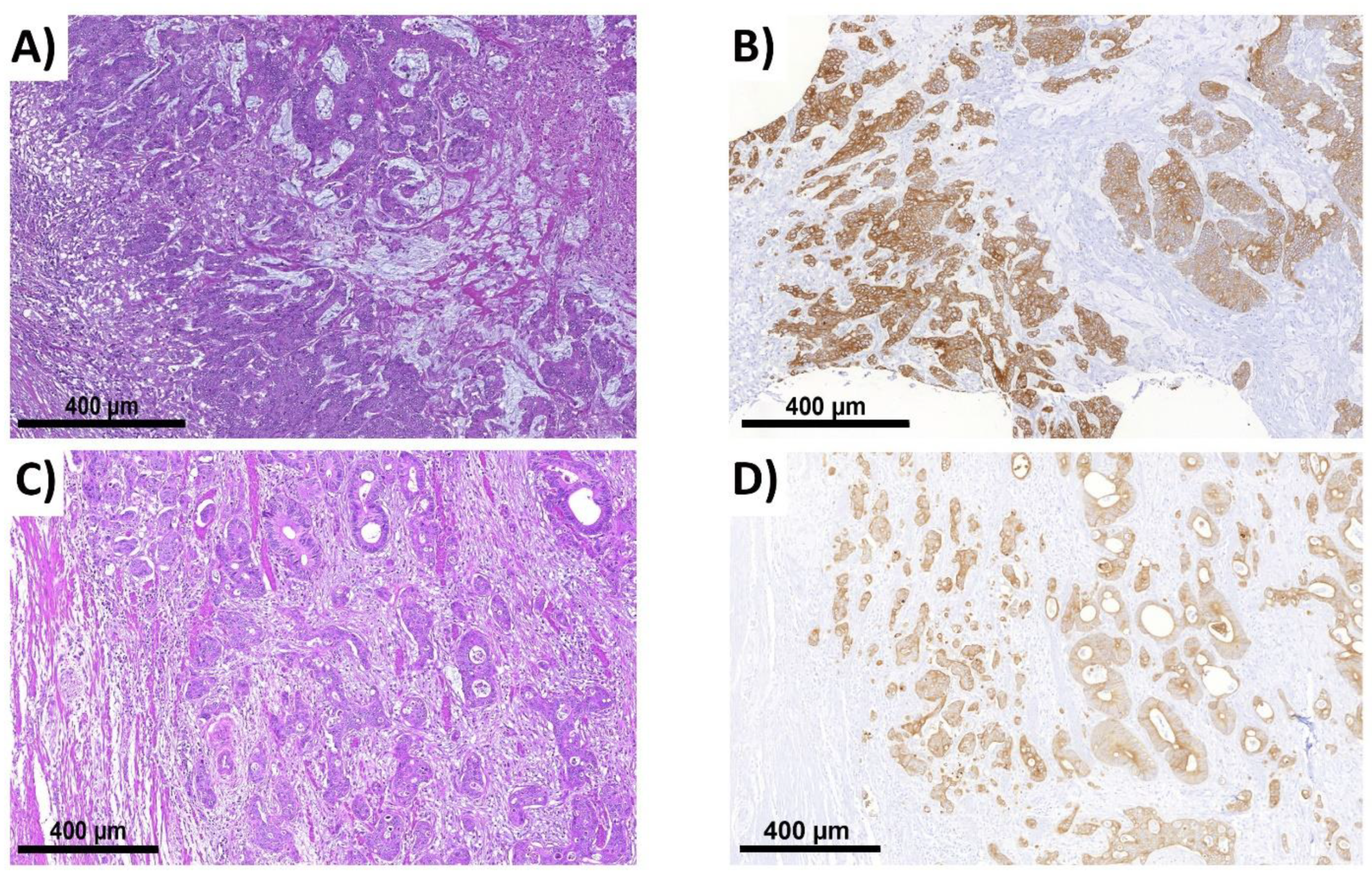
2.6. ITB Evaluation in Histological Subtypes and the Correlation with Prognoses
2.7. Intratumoral Budding in Histological Subtypes and Clinical Outcomes
2.8. The Comparison of the Mean Survival Rates between Histological Subtypes
3. Discussion
4. Materials and Methods
4.1. Cohort of Patients with Adenocarcinoma
4.2. Next-Generation Tissue Microarray Construction (ngTMA®)
4.3. Immunohistochemistry (IHC)
4.4. ITB Analysis Criteria
4.4.1. General Information
4.4.2. Preparatory Steps
4.4.3. Cell Environment
4.4.4. Cell Properties
4.5. TMA Analysis
4.6. The ITB Analysis
4.7. The WSI Analysis
4.8. Patients with Histological Subtypes Other Than Adenocarcinoma NOS
4.9. The ITB Analysis in Histological Subtypes
4.9.1. Medullary Carcinoma
4.9.2. Mucinous and Signet Ring Cell Carcinoma
4.10. The Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE1/AE3 | cytokeratin cocktail for detecting different cytokeratins (CK1-8,10,14-16 and 19) |
| AI | artificial intelligence |
| CDX2 | caudal-related homeobox gene 2 |
| CK | cytokeratin |
| COST | Cooperation in Science and Technology |
| CRC | colorectal cancer |
| EMT | epithelial-to-mesenchymal transition |
| FAU | Friedrich-Alexander-University |
| FFPE | formalin-fixed paraffin-embedded |
| FISH | fluorescence in situ hybridization |
| HE | hematoxylin-eosin |
| ICCR | International Collaboration on Cancer Reporting |
| IHC | immunohistochemistry |
| ITB | intratumoral budding |
| ITBCC | International Tumor Budding Consensus Conference |
| MSS/MSI | microsatellite stability/instability |
| ngTMA | next-generation tissue microarray |
| NOS | no other specified |
| OS | overall-survival |
| PDC | poorly differentiated clusters |
| pH | pondus hydrogenii |
| PTB | peritumoral budding |
| ROI | region of interest |
| UICC | Union internationale contre le cancer |
| WHO | World Health Organization |
| WSI | whole slide image |
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedzwiedzka, E.; Arlukowicz, T.; Przybylowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Schmitt, M.; Lang, C.; Reiser, M.; Scheiter, A.; Konukiewitz, B.; Steiger, K.; Silva, M.; Tschurtschenthaler, M.; Lange, S.; et al. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am. J. Surg. Pathol. 2021, 45, 969–978. [Google Scholar] [CrossRef]
- Imai, T. Histological comparison of cancer of the stomach in autopsy and operation cases. Jpn. J. Cancer Res. 1949, 40, 199–201. [Google Scholar]
- Choi, H.J.; Park, K.J.; Shin, J.S.; Roh, M.S.; Kwon, H.C.; Lee, H.S. Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int. J. Colorectal. Dis. 2007, 22, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Karamitopoulou, E.; Panayiotides, I.; Karakitsos, P.; Rallis, G.; Peros, G.; Iezzi, G.; Spagnoli, G.; Bihl, M.; Terracciano, L.; et al. CD8+ lymphocytes/tumour-budding index: An independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br. J. Cancer 2009, 101, 1382–1392. [Google Scholar] [CrossRef]
- Prall, F.; Nizze, H.; Barten, M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology 2005, 47, 17–24. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, H.C.; Roseweir, A.; Alexander, P.; Park, J.H.; Horgan, P.G.; McMillan, D.C.; Edwards, J. The Relationship Between Tumor Budding, Tumor Microenvironment, and Survival in Patients with Primary Operable Colorectal Cancer. Ann. Surg. Oncol. 2019, 26, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Molinari, F.; Martin, V.; Mazzucchelli, L.; Saletti, P.; Trezzi, R.; De Dosso, S.; Vlajnic, T.; Frattini, M.; Lugli, A. Tumor budding predicts response to anti-EGFR therapies in metastatic colorectal cancer patients. World J. Gastroenterol. 2010, 16, 4823–4831. [Google Scholar] [CrossRef]
- Okuyama, T.; Nakamura, T.; Yamaguchi, M. Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis. Colon. Rectum. 2003, 46, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, H.; Eishi, Y.; Ueno, H.; Nemoto, T.; Fujimori, T.; Iwashita, A.; Ajioka, Y.; Ochiai, A.; Ishiguro, S.; Shimoda, T.; et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: A retrospective multicenter study. Mod. Pathol. 2015, 28, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Ekmekci, S.; Kucuk, U.; Kokkoz, S.; Cakir, E.; Gumussoy, M. Tumor budding in laryngeal carcinoma. Indian J. Pathol. Microbiol. 2019, 62, 7–10. [Google Scholar] [CrossRef]
- Hong, K.O.; Oh, K.Y.; Shin, W.J.; Yoon, H.J.; Lee, J.I.; Hong, S.D. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum. Pathol. 2018, 80, 123–129. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Prognostic role of tumor budding in breast cancer. World J. Exp. Med. 2018, 8, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pr. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef]
- Mitrovic, B.; Schaeffer, D.F.; Riddell, R.H.; Kirsch, R. Tumor budding in colorectal carcinoma: Time to take notice. Mod. Pathol. 2012, 25, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- Lugli, A.; Vlajnic, T.; Giger, O.; Karamitopoulou, E.; Patsouris, E.S.; Peros, G.; Terracciano, L.M.; Zlobec, I. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum. Pathol. 2011, 42, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Aishima, S.; Aoki, S.; Takase, Y.; Uchihashi, K.; Masuda, M.; Nishijima-Matsunobu, A.; Yamamoto, M.; Ide, K.; Nakayama, A.; et al. Cytokeratin immunohistochemistry improves interobserver variability between unskilled pathologists in the evaluation of tumor budding in T1 colorectal cancer. Pathol. Int. 2016, 66, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Geppert, C.I.; Rummele, P.; Sarbia, M.; Langer, R.; Feith, M.; Morrison, L.; Pestova, E.; Schneider-Stock, R.; Hartmann, A.; Rau, T.T. Multi-colour FISH in oesophageal adenocarcinoma-predictors of prognosis independent of stage and grade. Br. J. Cancer 2014, 110, 2985–2995. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.V.; Ejlertsen, B.; Moller, S.; Jensen, M.B.; Balslev, E.; Muller, S.; Knoop, A.; Mouridsen, H.T. Lack of independent prognostic and predictive value of centromere 17 copy number changes in breast cancer patients with known HER2 and TOP2A status. Mol. Oncol. 2012, 6, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Fujiyoshi, K.; Väyrynen, J.P.; Borowsky, J.; Papke, D.J., Jr.; Arima, K.; Haruki, K.; Kishikawa, J.; Akimoto, N.; Ugai, T.; Lau, M.C.; et al. Tumour budding, poorly differentiated clusters, and T-cell response in colorectal cancer. EBioMedicine 2020, 57, 102860. [Google Scholar] [CrossRef]
- Nitsche, U.; Zimmermann, A.; Spath, C.; Muller, T.; Maak, M.; Schuster, T.; Slotta-Huspenina, J.; Kaser, S.A.; Michalski, C.W.; Janssen, K.P.; et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013, 258, 775–782; discussion 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Classification of Tumours Editorial Board (Ed.) WHO Classification of Tumours. Digestive System Tumours, 5th ed.; WHO: Geneva, Switzerland, 2019; ISBN 9283244990/978-9283244998. [Google Scholar]
- Marx, A.H.; Mickler, C.; Sauter, G.; Simon, R.; Terracciano, L.M.; Izbicki, J.R.; Clauditz, T.S. High-grade intratumoral tumor budding is a predictor for lymphovascular invasion and adverse outcome in stage II colorectal cancer. Int. J. Colorectal. Dis. 2020, 35, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bokhorst, J.M.; Blank, A.; Lugli, A.; Zlobec, I.; Dawson, H.; Vieth, M.; Rijstenberg, L.L.; Brockmoeller, S.; Urbanowicz, M.; Flejou, J.F.; et al. Assessment of individual tumor buds using keratin immunohistochemistry: Moderate interobserver agreement suggests a role for machine learning. Mod. Pathol. 2020, 33, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enderle-Ammour, K.; Wellner, U.; Kocsmar, E.; Kiss, A.; Lotz, G.; Csanadi, A.; Bader, M.; Schilling, O.; Werner, M.; Bronsert, P. Three-dimensional reconstruction of solid tumors: Morphological evidence for tumor heterogeneity. Pathologe 2018, 39, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lino-Silva, L.S.; Gamboa-Dominguez, A.; Zuniga-Tamayo, D.; Salcedo-Hernandez, R.A.; Cetina, L.; Cantu-de-Leon, D. Mismatch repair protein expression and intratumoral budding in rectal cancer are associated with an increased pathological complete response to preoperative chemoradiotherapy: A case-control study. World J. Clin. Oncol. 2018, 9, 133–139. [Google Scholar] [CrossRef]
- Haddad, T.S.; Lugli, A.; Aherne, S.; Barresi, V.; Terris, B.; Bokhorst, J.M.; Brockmoeller, S.F.; Cuatrecasas, M.; Simmer, F.; El-Zimaity, H.; et al. Improving tumor budding reporting in colorectal cancer: A Delphi consensus study. Virchows Arch. 2021, 479, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Lugli, A. Invasive front of colorectal cancer: Dynamic interface of pro-/anti-tumor factors. World J. Gastroenterol. 2009, 15, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, M.; Kawachi, H.; Yamamoto, N.; Kobayashi, M.; Toyama, Y.; Maekawa, T.; Chino, A.; Saito, S.; Ueno, M.; Takazawa, Y.; et al. Immunohistochemical evaluation of tumor budding for stratifying T1 colorectal cancer: Optimal cut-off value and a novel computer-assisted semiautomatic method. Mod. Pathol. 2019, 32, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, M.B.; Arends, M.; Brown, I.; Burgart, L.J.; Cunningham, C.; Flejou, J.F.; Kakar, S.; Kirsch, R.; Kojima, M.; Lugli, A.; et al. Colorectal Cancer Histopathology Reporting Guide; Version 1.0; International Collaboration on Cancer Reporting Limited (ICCR): Sydney, Australia, 2020; pp. 12–13. ISBN 978-1-922324-01-6. [Google Scholar]
- Sawayama, H.; Miyamoto, Y.; Ogawa, K.; Yoshida, N.; Baba, H. Investigation of colorectal cancer in accordance with consensus molecular subtype classification. Ann. Gastroenterol. Surg. 2020, 4, 528–539. [Google Scholar] [CrossRef]
- Bergler, M.; Benz, M.; Rauber, D.; Hartmann, D.; Kötter, M.; Eckstein, M.; Schneider-Stock, R.; Hartmann, A.; Merkel, S.; Bruns, V.; et al. Automatic Detection of Tumor Buds in Pan-Cytokeratin stained Colorectal Cancer Sections by a Hybrid Image Analysis Approach. In Proceedings of the 15th European Congress on Digital Pathology (ECDP 2019), Warwick, UK, 10–13 April 2019; pp. 83–90. [Google Scholar]
- Nolte, S.; Zlobec, I.; Lugli, A.; Hohenberger, W.; Croner, R.; Merkel, S.; Hartmann, A.; Geppert, C.I.; Rau, T.T. Construction and analysis of tissue microarrays in the era of digital pathology: A pilot study targeting CDX1 and CDX2 in a colon cancer cohort of 612 patients. J. Pathol Clin. Res. 2017, 3, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouse anti-Cytokeratin Broad Spectrum (AE1 & AE3), Cat. No.: MSK019 (1 mL Konzentrat); MSK019-05 (0,5 mL Konzentrat); MSG019 (6 mL gebrauchsfertig). ZYTOMED Syst. Rev: A1015, Doc: DB_BMS006. 2015. Available online: https://www.zytomed-systems.de/storage/uploads/datasheets/de/BMS006_Gef.pdf (accessed on 1 March 2021).
- Zlobec, I.; Lugli, A.; Baker, K.; Roth, S.; Minoo, P.; Hayashi, S.; Terracciano, L.; Jass, J.R. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J. Pathol. 2007, 212, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Sugai, T.; Eizuka, M.; Tsuchida, K.; Sugimoto, R.; Mue, Y.; Suzuki, M.; Osakabe, M.; Uesugi, N.; Ishida, K.; et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum. Patho.l 2017, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Pages, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Pages, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Ascierto, M.L.; De Giorgi, V.; Liu, Q.; Bedognetti, D.; Spivey, T.L.; Murtas, D.; Uccellini, L.; Ayotte, B.D.; Stroncek, D.F.; Chouchane, L.; et al. An immunologic portrait of cancer. J. Transl. Med. 2011, 9, 146. [Google Scholar] [CrossRef] [Green Version]


| Feature | Frequency n (%) | ITB < 10 | ITB ≥ 10 | p-Value | |
|---|---|---|---|---|---|
| Sex | Male Female | 152 93 | 126 (82.9) 83 (89.2) | 26 (17.1) 10 (10.8) | 0.173 |
| Age | ≤65 years >65 years | 94 151 | 79 (84) 130 (86.1) | 15 (16) 21 (13.9) | 0.659 |
| Localization | Left-sided Right-sided | 108 137 | 94 (87.0) 115 (83.9) | 14 (13.0) 22 (16.1) | 0.497 |
| Tumor grading | G1/2 G3/4 | 189 56 | 169 (89.4) 40 (71.4) | 20 (10.6) 16 (28.6) | 0.001 |
| Stage UICC | I/II III/IV | 152 93 | 141 (92.8) 68 (73) | 11 (7.2) 25 (27) | <0.001 |
| pT category | pT1/2 pT3/4 | 70 175 | 66 (94) 143 (81.7) | 4 (6) 32 (18.3) | 0.012 |
| Primary metastasis * | pM0 pM1 | 231 14 | 198 (85.7) 11 (79) | 33 (14.3) 3 (21) | 0.464 |
| pN category | pN0 pN1,2 | 154 91 | 143 (92.9) 66 (73) | 11 (7.1) 25 (28) | <0.001 |
| Lymphatic invasion | No | 176 | 157 (89.2) | 19 (10.8) | |
| Yes | 69 | 52 (75) | 17 (25) | 0.006 | |
| Venous invasion | No | 235 | 201 (85.5) | 34 (14.5) | |
| Yes | 10 | 8 (80) | 2 (20) | 0.628 | |
| Perineural invasion | No | 224 | 196 (87.5) | 28 (12.5) | |
| Yes | 14 | 8 (57.1) | 6 (42.9) | 0.010 | |
| MSS/MSI status | MSS | 198 | 169 (85.4) | 29 (14.6) | |
| MSI | 47 | 40 (85) | 7 (15) | 0.966 |
| Parameter | Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Age (years) | >65 | 0.932 | 0.498–1.746 | 0.826 |
| pT category | pT3/4 | 4.167 | 1.483–11.708 | 0.007 |
| Tumor grade | G3/4 | 1.956 | 1.021–3.747 | 0.043 |
| Lymphatic invasion | L1 | 4.824 | 2.558–9.098 | <0.001 |
| Venous invasion | V1 | 3.344 | 1.188–9.409 | 0.022 |
| Tumor localization | Left-sided | 1.476 | 0.771–2.828 | 0.240 |
| Lymph node metastases | pN1,2 | 2.686 | 1.434–5.032 | 0.002 |
| ITB score | ≥10 | 2.542 | 1.269–5.090 | 0.008 |
| Multivariate analysis | ||||
| pT category | pT3,4 | 2.354 | 0.802–6.906 | 0.169 |
| Lymphatic invasion | L1 | 3.519 | 1.701–7.282 | 0.001 |
| Venous invasion | V1 | 2.095 | 0.731–6.009 | 0.169 |
| Lymph node metastases | pN1,2 | 1.099 | 0.524–2.307 | 0.802 |
| ITB score | ≥10 | 1.972 | 0.949–4.098 | 0.069 |
| Subtype | ITB Score * | Number of Patients (n) 1 | Survival (Months) 2 | Delta (Months) 3 | Ranking 4 |
|---|---|---|---|---|---|
| NOS | Low | 210 | 114.0 | 11.0 | 2 |
| NOS | High | 35 | 103.0 | 3 | |
| Medullary Ca | Low | 2 | 116.3 | 48.6 | 1 |
| Medullary Ca | High | 8 | 67.7 | 5 | |
| Signet ring cell Ca | Low | 2 | 74.1 | 47.6 | 4 |
| Signet ring cell Ca | High | 9 | 26.4 | 8 | |
| Mucinous Ca | Low | 9 | 65.3 | 33.7 | 6 |
| Mucinous Ca | High | 3 | 31.6 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pour Farid, P.; Eckstein, M.; Merkel, S.; Grützmann, R.; Hartmann, A.; Bruns, V.; Benz, M.; Schneider-Stock, R.; Geppert, C.I. Novel Criteria for Intratumoral Budding with Prognostic Relevance for Colon Cancer and Its Histological Subtypes. Int. J. Mol. Sci. 2021, 22, 13108. https://doi.org/10.3390/ijms222313108
Pour Farid P, Eckstein M, Merkel S, Grützmann R, Hartmann A, Bruns V, Benz M, Schneider-Stock R, Geppert CI. Novel Criteria for Intratumoral Budding with Prognostic Relevance for Colon Cancer and Its Histological Subtypes. International Journal of Molecular Sciences. 2021; 22(23):13108. https://doi.org/10.3390/ijms222313108
Chicago/Turabian StylePour Farid, Pantea, Markus Eckstein, Susanne Merkel, Robert Grützmann, Arndt Hartmann, Volker Bruns, Michaela Benz, Regine Schneider-Stock, and Carol I. Geppert. 2021. "Novel Criteria for Intratumoral Budding with Prognostic Relevance for Colon Cancer and Its Histological Subtypes" International Journal of Molecular Sciences 22, no. 23: 13108. https://doi.org/10.3390/ijms222313108
APA StylePour Farid, P., Eckstein, M., Merkel, S., Grützmann, R., Hartmann, A., Bruns, V., Benz, M., Schneider-Stock, R., & Geppert, C. I. (2021). Novel Criteria for Intratumoral Budding with Prognostic Relevance for Colon Cancer and Its Histological Subtypes. International Journal of Molecular Sciences, 22(23), 13108. https://doi.org/10.3390/ijms222313108










