Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases
Abstract
1. Introduction: Bone Structure and Cell Types
2. Pathways Involved in Bone Metabolism
3. The Bone Turnover Cycle
4. Epigenetics in Bone Remodeling Processes
4.1. DNA Methylation
4.2. Histone Post-Translational Modifications
4.3. Non-Coding RNAs
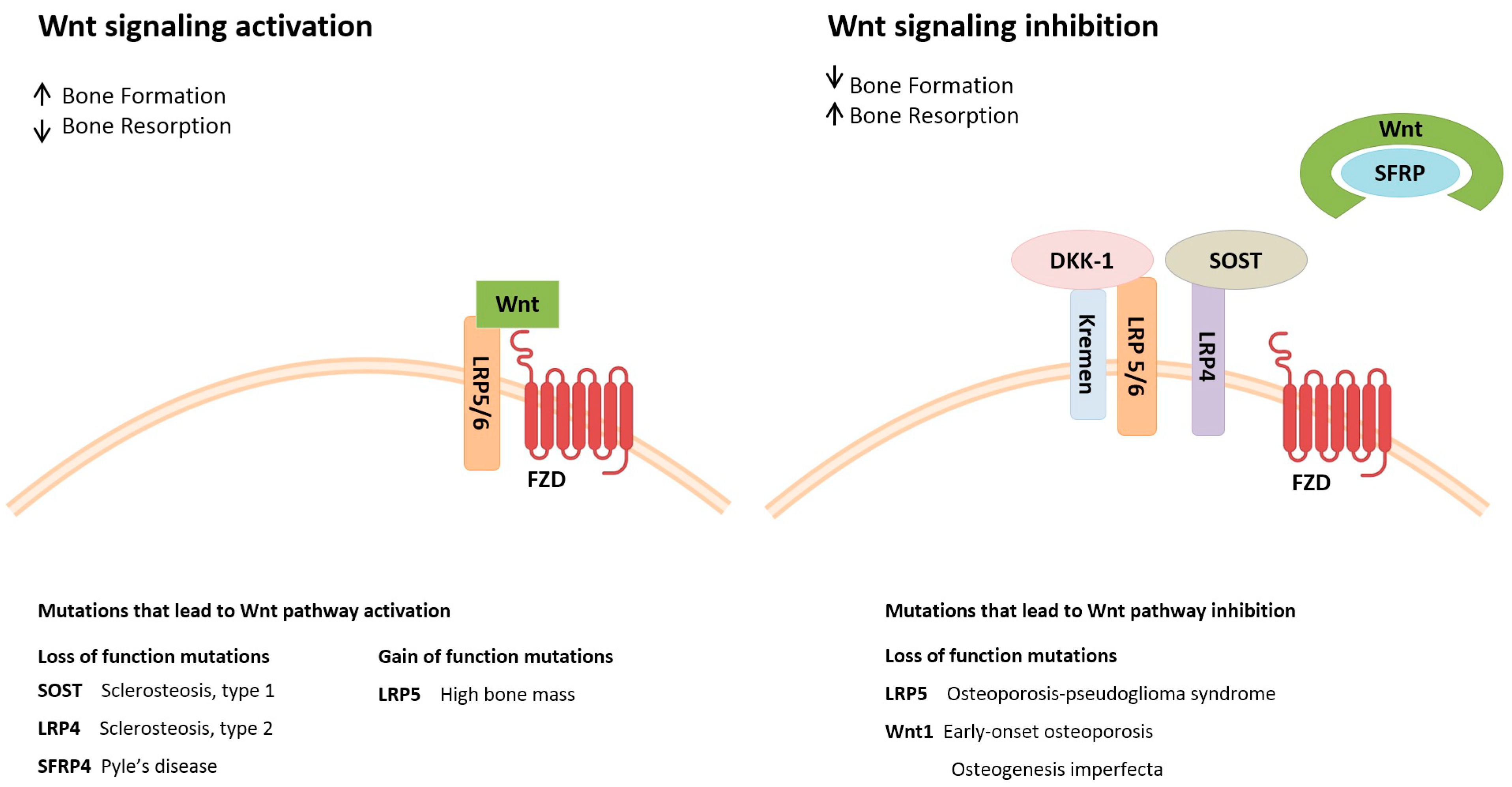
5. Genetics of Metabolic Bone Diseases
5.1. Osteoporosis
5.2. Rickets and Osteomalacia
5.3. Juvenile Paget’s Disease
5.4. Osteogenesis Imperfecta
5.5. Osteopetrosis
5.6. Fibrous Dysplasia
5.7. Pyle Disease
5.8. Additional Rare Metabolic Bone Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, F.; Li, W.; Yang, X.; Na, L.; Chen, L.; Liu, G. The Roles of Epigenetics Regulation in Bone Metabolism and Osteoporosis. Front. Cell Dev. Biol. 2021, 8, 1928. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotti, C.; De Mattei, M.; Mazziotta, C.; Taraballi, F.; Rotondo, J.C.; Tognon, M.; Martini, F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 646032. [Google Scholar] [CrossRef] [PubMed]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling signals between the osteoclast and osteoblast: How are messages transmitted between these temporary visitors to the bone surface? Front. Endocrinol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Taraballi, F.; Torreggiani, E.; Rotondo, J.C.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I.; et al. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef]
- Mazzoni, E.; Mazziotta, C.; Iaquinta, M.R.; Lanzillotti, C.; Fortini, F.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Barbanti-Brodano, G.; Mescola, A.; et al. Enhanced Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells by a Hybrid Hydroxylapatite/Collagen Scaffold. Front. Cell Dev. Biol. 2021, 8, 610570. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef]
- Mitxitorena, I.; Infante, A.; Gener, B.; Rodríguez, C.I. Suitability and limitations of mesenchymal stem cells to elucidate human bone illness. World J. Stem Cells 2019, 11, 578–593. [Google Scholar] [CrossRef]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Palumbo, C.; Ferretti, M. The Osteocyte: From “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol. 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Ficai, A.; Marques, C.; Ferreira, J.M.F.; Andronescu, E.; FICAI, D.; Sonmez, M. Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the Biological and Mechanical Performances of Sintered Hydroxyapatite by Multiple Ions Doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Torreggiani, E.; Mazziotta, C.; Ruffini, A.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F.; Mazzoni, E. In vitro osteoinductivity assay of hydroxylapatite scaffolds, obtained with biomorphic transformation processes, assessed using human adipose stem cell cultures. Int. J. Mol. Sci. 2021, 22, 7092. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired By Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Fantner, G.E.; Adams, J.; Turner, P.; Thurner, P.J.; Fisher, L.W.; Hansma, P.K. Nanoscale Ion Mediated Networks in Bone: Osteopontin Can Repeatedly Dissipate Large Amounts of Energy. Nano Lett. 2007, 7, 2491–2498. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef]
- Lambert, L.J.; Challa, A.K.; Niu, A.; Zhou, L.; Tucholski, J.; Johnson, M.S.; Nagy, T.R.; Eberhardt, A.W.; Estep, P.N.; Kesterson, R.A.; et al. Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. DMM Dis. Model. Mech. 2016, 9, 1169–1179. [Google Scholar] [CrossRef]
- Ninomiya, J.T.; Tracy, R.P.; Calore, J.D.; Gendreau, M.A.; Kelm, R.J.; Mann, K.G. Heterogeneity of human bone. J. Bone Miner. Res. 1990, 5, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573–6591. [Google Scholar] [CrossRef]
- Corazza, M.; Oton-Gonzalez, L.; Scuderi, V.; Rotondo, J.C.J.C.; Lanzillotti, C.; Di Mauro, G.; Tognon, M.; Martini, F.; Borghi, A. Tissue cytokine/chemokine profile in vulvar lichen sclerosus: An observational study on keratinocyte and fibroblast cultures. J. Dermatol. Sci. 2020, 100, 223–226. [Google Scholar] [CrossRef]
- Raut, N.; Wicks, S.M.; Lawal, T.O.; Mahady, G.B. Epigenetic regulation of bone remodeling by natural compounds. Pharmacol. Res. 2019, 147, 104350. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Weidner, D.; Kachler, K.; Faas, M.; Grüneboom, A.; Schlötzer-Schrehardt, U.; Muñoz, L.E.; Steffen, U.; Grötsch, B.; et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Investig. 2020, 130, 4811–4830. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Kindstedt, E.; Lundberg, P. The critical interplay between bone resorbing and bone forming cells. J. Clin. Periodontol. 2019, 46, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 1–16. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar]
- Cawley, K.M.; Bustamante-Gomez, N.C.; Guha, A.G.; MacLeod, R.S.; Xiong, J.; Gubrij, I.; Liu, Y.; Mulkey, R.; Palmieri, M.; Thostenson, J.D.; et al. Local Production of Osteoprotegerin by Osteoblasts Suppresses Bone Resorption. Cell Rep. 2020, 32, 108052. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 2014, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.J.; Lapsley, M.; Day, A.P.; Ayling, R.M. Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–932. [Google Scholar]
- McClung, M.R. Romosozumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 2018, 4, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, E.; Harinathbabu, M.; Thillaigovindan, R.; Prabhu, G. Marble Bone Disease: A Rare Bone Disorder. Cureus 2015, 7, e339. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Rotondo, J.C.; Lanzillotti, C.; Campione, G.; Martini, F.; Tognon, M. Cancer biology and molecular genetics of A3 adenosine receptor. Oncogene 2021, 8, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Oton-Gonzalez, L.; Rotondo, J.C.; Cerritelli, L.; Malagutti, N.; Lanzillotti, C.; Bononi, I.; Ciorba, A.; Bianchini, C.; Mazziotta, C.; De Mattei, M.; et al. Association between oncogenic human papillomavirus type 16 and Killian polyp. Infect. Agent. Cancer 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Tognon, M.; Tagliapietra, A.; Magagnoli, F.; Mazziotta, C.; Oton-Gonzalez, L.; Lanzillotti, C.; Vesce, F.; Contini, C.; Rotondo, J.C.; Martini, F.; et al. Investigation on Spontaneous Abortion and Human Papillomavirus Infection. Vaccines 2020, 8, 473. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bosi, S.; Bassi, C.; Ferracin, M.; Lanza, G.; Gafà, R.; Magri, E.; Selvatici, R.; Torresani, S.; Marci, R.; et al. Gene expression changes in progression of cervical neoplasia revealed by microarray analysis of cervical neoplastic keratinocytes. J. Cell. Physiol. 2015, 230, 806–812. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.L.; Chen, L.; Lin, X.; Xiong, D.; Xu, F.; Yuan, L.Q.; Liao, E.Y. Epigenetic mechanisms of bone regeneration and homeostasis. Prog. Biophys. Mol. Biol. 2016, 122, 85–92. [Google Scholar] [CrossRef]
- Feng, Q.; Zheng, S.; Zheng, J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci. Rep. 2018, 38, BSR20180453. [Google Scholar] [CrossRef]
- Patil, S.; Dang, K.; Zhao, X.; Gao, Y.; Qian, A. Role of LncRNAs and CircRNAs in Bone Metabolism and Osteoporosis. Front. Genet. 2020, 11, 584118. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Bosi, S.; Bazzan, E.; Di Domenico, M.; De Mattei, M.; Selvatici, R.; Patella, A.; Marci, R.; Tognon, M.; Martini, F. Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum. Reprod. 2012, 27, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Aquila, G.; Oton-Gonzalez, L.; Selvatici, R.; Rizzo, P.; De Mattei, M.; Pavasini, R.; Tognon, M.; Campo, G.C.; Martini, F. Methylation of SERPINA1 gene promoter may predict chronic obstructive pulmonary disease in patients affected by acute coronary syndrome. Clin. Epigenet. 2021, 13, 79. [Google Scholar] [CrossRef]
- Reppe, S.; Datta, H.; Gautvik, K.M. The Influence of DNA Methylation on Bone Cells. Curr. Genom. 2015, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Tognon, M.; Martini, F. Epigenetic Dysregulations in Merkel Cell Polyomavirus-Driven Merkel Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 11464. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Oton-Gonzalez, L.; Selvatici, R.; Rizzo, P.; Pavasini, R.; Campo, G.C.; Lanzillotti, C.; Mazziotta, C.; De Mattei, M.; Tognon, M.; et al. SERPINA1 gene promoter is differentially methylated in peripheral blood mononuclear cells of pregnant women. Front. Cell Dev. Biol. 2020, 8, 5505. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of male infertility: The role of DNA methylation. Front. Cell Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Selvatici, R.; Di Domenico, M.; Marci, R.; Vesce, F.; Tognon, M.; Martini, F. Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics 2013, 8, 990–997. [Google Scholar] [CrossRef]
- Schomacher, L. Mammalian DNA demethylation. Epigenetics 2013, 8, 679–684. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.P.; Shao, J.Z.; Xiang, L.X. GADD45A protein plays an essential role in active DNA demethylation during terminal osteogenic differentiation of adipose-derived mesenchymal stem cells. J. Biol. Chem. 2011, 286, 41083–41094. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Magri, E.; Bianchini, E.; Montinari, E.; Corazza, M.; Virgili, A.; Tognon, M.; Martini, F. Hypermethylation-induced inactivation of the IRF6 gene as a possible early event in progression of vulvar squamous cell carcinoma associated with lichen sclerosus. JAMA Dermatol. 2016, 152, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Lazarenko, O.P.; Kang, P.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Shankar, K. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012, 26, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Sañudo, C.; Sánchez-Verde, L.; García-Renedo, R.J.; Arozamena, J.; Riancho, J.A. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone 2011, 49, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Tarfiei, G.; Noruzinia, M.; Soleimani, M.; Kaviani, S.; Maymand, M.M.; Hagh, M.F.; Pujol, P. ROR2 Promoter Methylation Change in OsteoblasticDifferentiation of Mesenchymal Stem Cells. Cell J. 2011, 13, 11. [Google Scholar]
- Kitazawa, R.; Kitazawa, S. Methylation Status of a Single CpG Locus 3 Bases Upstream of TATA-Box of Receptor Activator of Nuclear Factor-κB Ligand (RANKL) Gene Promoter Modulates Cell- and Tissue-Specific RANKL Expression and Osteoclastogenesis. Mol. Endocrinol. 2007, 21, 148–158. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Chen, D.; Kim, D.J.; Shen, J.; Zou, Z.; O’Keefe, R.J. Runx2 plays a central role in Osteoarthritis development. J. Orthop. Transl. 2020, 23, 132–139. [Google Scholar] [CrossRef]
- Wakitani, S.; Yokoi, D.; Hidaka, Y.; Nishino, K. The differentially DNA-methylated region responsible for expression of runt-related transcription factor 2. J. Vet. Med. Sci. 2017, 79, 230–237. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Fu, B.; Wang, H.; Wang, J.; Barouhas, I.; Liu, W.; Shuboy, A.; Bushinsky, D.A.; Zhou, D.; Favus, M.J. Epigenetic regulation of BMP2 by 1,25-dihydroxyvitamin D3 through DNA methylation and histone modification. PLoS ONE 2013, 8, e61423. [Google Scholar] [CrossRef]
- Raje, M.M.; Ashma, R. Epigenetic regulation of BMP2 gene in osteoporosis: A DNA methylation study. Mol. Biol. Rep. 2019, 46, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Dhawan, S. DNA Methylation Patterning and the Regulation of Beta Cell Homeostasis. Front. Endocrinol. 2021, 12, 512. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Duan, X. Epigenetics, Bone Remodeling and Osteoporosis. Curr. Stem Cell Res. Ther. 2018, 13, 101–109. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Villagra, A.; Gutirrez, J.; Paredes, R.; Sierra, J.; Puchi, M.; Imschenetzky, M.; Van Wijnen, A.; Lian, J.; Stein, G.; Stein, J.; et al. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J. Cell. Biochem. 2002, 85, 112–122. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sañudo, C.; Fernández, A.F.; García-Renedo, R.; Fraga, M.F.; Riancho, J.A. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics 2012, 7, 83–91. [Google Scholar] [CrossRef]
- Kalkan, R.; Becer, E. RANK/RANKL/OPG pathway is an important for the epigenetic regulation of obesity. Mol. Biol. Rep. 2019, 46, 5425–5432. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Epigenetic Regulation of Bone Remodeling and Its Impacts in Osteoporosis. Int. J. Mol. Sci. 2016, 17, 1446. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Chen, Z.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Phipps-Green, A.; Liu-Bryan, R.; Ceponis, A.; Boyle, D.L.; Wang, J.; Merriman, T.R.; Wang, W.; Terkeltaub, R. Differential DNA Methylation of Networked Signaling, Transcriptional, Innate and Adaptive Immunity, and Osteoclastogenesis Genes and Pathways in Gout. Arthritis Rheumatol. 2020, 72, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Stomper, J.; Rotondo, J.C.; Greve, G.; Lübbert, M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: Mechanisms of resistance and novel HMA-based therapies. Leukemia 2021, 35, 1873–1889. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.-Q.; Liu, F. DNA Methyltransferase Inhibitors and their Therapeutic Potential. Curr. Top. Med. Chem. 2018, 18, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.S.; Zhang, X.L.; Wu, J.P.; Zhang, R.P.; Xiang, L.X.; Dai, L.C.; Shao, J.Z. 5-Azacytidine facilitates osteogenic gene expression and differentiation of mesenchymal stem cells by alteration in DNA methylation. Cytotechnology 2009, 60, 11–22. [Google Scholar] [CrossRef]
- Hagh, M.F.; Noruzinia, M.; Mortazavi, Y.; Soleimani, M.; Kaviani, S.; Abroun, S.; Fard, A.D.; Maymand, M.M. Different methylation patterns of RUNX2, OSX, DLX5 and BSP in osteoblastic differentiation of mesenchymal stem cells. Cell J. 2015, 17, 71–82. [Google Scholar]
- Nishikawa, K.; Iwamoto, Y.; Kobayashi, Y.; Katsuoka, F.; Kawaguchi, S.I.; Tsujita, T.; Nakamura, T.; Kato, S.; Yamamoto, M.; Takayanagi, H.; et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine–producing metabolic pathway. Nat. Med. 2015, 21, 281–287. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Du, J.; He, J.; Lin, P.; Amini, B.; Starbuck, M.W.; Novane, N.; Shah, J.J.; Davis, R.E.; et al. Thymidine phosphorylase exerts complex effects on bone resorption and formation in myeloma. Sci. Transl. Med. 2016, 8, 353ra113. [Google Scholar] [CrossRef]
- Yi, S.J.; Lee, H.; Lee, J.; Lee, K.; Kim, J.; Kim, Y.; Park, J.I.; Kim, K. Bone Remodeling: Histone Modifications as Fate Determinants of Bone Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3147. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Marc, J.; Ostanek, B. Epigenetic mechanisms in bone. Clin. Chem. Lab. Med. 2014, 52, 589–608. [Google Scholar] [CrossRef]
- Håkelien, A.M.; Bryne, J.C.; Harstad, K.G.; Lorenz, S.; Paulsen, J.; Sun, J.; Mikkelsen, T.S.; Myklebost, O.; Meza-Zepeda, L.A. The Regulatory Landscape of Osteogenic Differentiation. Stem Cells 2014, 32, 2780–2793. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Benkusky, N.A.; Sen, B.; Rubin, J.; Pike, J.W. Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J. Biol. Chem. 2016, 291, 17829–17847. [Google Scholar] [CrossRef]
- Shen, J.; Hovhannisyan, H.; Lian, J.B.; Montecino, M.A.; Stein, G.S.; Stein, J.L.; Van Wijnen, A.J. Transcriptional Induction of the Osteocalcin Gene During Osteoblast Differentiation Involves Acetylation of Histones H3 and H4. Mol. Endocrinol. 2003, 17, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Aguilar, R.; Henriquez, B.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Van Wijnen, A.J.; Van Zundert, B.; Allende, M.L.; Montecino, M. Epigenetic Control of the Bone-master Runx2 Gene during Osteoblast-lineage Commitment by the Histone Demethylase JARID1B/KDM5B. J. Biol. Chem. 2015, 290, 28329–28342. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Suh, J.H.; Kim, A.Y.; Lee, Y.S.; Park, S.Y.; Kim, J.B. Histone Deacetylase 1-Mediated Histone Modification Regulates Osteoblast Differentiation. Mol. Endocrinol. 2006, 20, 2432–2443. [Google Scholar] [CrossRef]
- Shen, J.; Montecino, M.; Lian, J.B.; Stein, G.S.; Van Wijnen, A.J.; Stein, J.L. Histone Acetylation in Vivo at the Osteocalcin Locus Is Functionally Linked to Vitamin D-dependent, Bone Tissue-specific Transcription. J. Biol. Chem. 2002, 277, 20284–20292. [Google Scholar] [CrossRef]
- Montecino, M.; Frenkel, B.; Van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Stein, J.L. Chromatin Hyperacetylation Abrogates Vitamin D-Mediated Transcriptional Upregulation of the Tissue-Specific Osteocalcin Gene in Vivo. Biochemistry 1998, 38, 1338–1345. [Google Scholar] [CrossRef]
- Sierra, J.; Villagra, A.; Paredes, R.; Cruzat, F.; Gutierrez, S.; Javed, A.; Arriagada, G.; Olate, J.; Imschenetzky, M.; van Wijnen, A.J.; et al. Regulation of the Bone-Specific Osteocalcin Gene by p300 Requires Runx2/Cbfa1 and the Vitamin D3 Receptor but Not p300 Intrinsic Histone Acetyltransferase Activity. Mol. Cell. Biol. 2003, 23, 3339–3351. [Google Scholar] [CrossRef]
- Choo, M.K.; Yeo, H.; Zayzafoon, M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone 2009, 45, 579–589. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Kahler, R.A.; Li, X.; Westendorf, J.J. Histone Deacetylase 3 Interacts with Runx2 to Repress the Osteocalcin Promoter and Regulate Osteoblast Differentiation. J. Biol. Chem. 2004, 279, 41998–42007. [Google Scholar] [CrossRef]
- Hesse, E.; Saito, H.; Kiviranta, R.; Correa, D.; Yamana, K.; Neff, L.; Toben, D.; Duda, G.; Atfi, A.; Geoffroy, V.; et al. Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. J. Cell Biol. 2010, 191, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Lamour, V.; Detry, C.; Sanchez, C.; Henrotin, Y.; Castronovo, V.; Bellahcène, A. Runx2- and Histone Deacetylase 3-mediated Repression Is Relieved in Differentiating Human Osteoblast Cells to Allow High Bone Sialoprotein Expression. J. Biol. Chem. 2007, 282, 36240–36249. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballo, E.; Ulsamer, A.; Susperregui, A.R.G.; Manzanares-Céspedes, C.; Sánchez-García, E.; Bartrons, R.; Rosa, J.L.; Ventura, F. Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J. Bone Miner. Res. 2011, 26, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, N.; Champagne, N.; Stifani, S.; Yang, X.J. MOZ and MORF histone acetyltransferases interact with the Runt-domain transcription factor Runx2. Oncogene 2002, 21, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Nimura, K.; Lo, W.N.; Saga, K.; Kaneda, Y. Histone H3 Lysine 36 Methyltransferase Whsc1 Promotes the Association of Runx2 and p300 in the Activation of Bone-Related Genes. PLoS ONE 2014, 9, e106661. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.H.; Li, L.Y.; Lang, J.; Yeh, S.P.; Shi, B.; Yang, C.C.; Yang, J.Y.; Lin, C.Y.; Lai, C.C.; et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 2010, 13, 87–94. [Google Scholar] [CrossRef]
- Hemming, S.; Cakouros, D.; Isenmann, S.; Cooper, L.; Menicanin, D.; Zannettino, A.; Gronthos, S. EZH2 and KDM6A Act as an Epigenetic Switch to Regulate Mesenchymal Stem Cell Lineage Specification. Stem Cells 2014, 32, 802–815. [Google Scholar] [CrossRef]
- Dudakovic, A.; Camilleri, E.T.; Paradise, C.R.; Samsonraj, R.M.; Gluscevic, M.; Paggi, C.A.; Begun, D.L.; Khani, F.; Pichurin, O.; Ahmed, F.S.; et al. Enhancer of zeste homolog 2 (Ezh2) controls bone formation and cell cycle progression during osteogenesis in mice. J. Biol. Chem. 2018, 293, 12894–12907. [Google Scholar] [CrossRef]
- Hemming, S.; Cakouros, D.; Vandyke, K.; Davis, M.J.; Zannettino, A.C.W.; Gronthos, S. Identification of Novel EZH2 Targets Regulating Osteogenic Differentiation in Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 909–921. [Google Scholar] [CrossRef]
- Khani, F.; Thaler, R.; Paradise, C.R.; Deyle, D.R.; Kruijthof-de Julio, M.; Galindo, M.; Gordon, J.A.; Stein, G.S.; Dudakovic, A.; van Wijnen, A.J. Histone H4 Methyltransferase Suv420h2 Maintains Fidelity of Osteoblast Differentiation. J. Cell. Biochem. 2017, 118, 1262–1272. [Google Scholar] [CrossRef]
- Sun, J.; Ermann, J.; Niu, N.; Yan, G.; Yang, Y.; Shi, Y.; Zou, W. Histone demethylase LSD1 regulates bone mass by controlling WNT7B and BMP2 signaling in osteoblasts. Bone Res. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.H.; Lebedeva, S.; Hirsekorn, A.; Wurmus, R.; Akalin, A.; Mukherjee, N.; Ohler, U. Global identification of functional microRNA-mRNA interactions in Drosophila. Nat. Commun. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, Y.; Yang, S.; Zhang, H.; Chen, F. Integrative analysis of miRNA-mRNA and miRNA-miRNA interactions. BioMed Res. Int. 2014, 2014, 907420. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, J.; Ji, X.; Yao, Z.; Wang, X. MiR-21 promotes osteoclastogenesis through activation of PI3K/Akt signaling by targeting Pten in RAW264.7 cells. Mol. Med. Rep. 2020, 21, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Liu, H.; Chen, S.R. Mechanisms of Long Non-Coding RNAs in Cancers and Their Dynamic Regulations. Cancers 2020, 12, 1245. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Z.; Bai, Y.; Dou, C.; Gong, X.; Liang, M.; Dong, R.; Quan, H.; Li, J.; Dai, J.; et al. LncRNA AK077216 promotes RANKL-induced osteoclastogenesis and bone resorption via NFATc1 by inhibition of NIP45. J. Cell. Physiol. 2019, 234, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yu, D.; Chu, W.; Liu, Z.; Li, H.; Zhai, Z. LncRNA expression profiles and the negative regulation of lncRNA-NOMMUT037835.2 in osteoclastogenesis. Bone 2020, 130, 115072. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.; Cai, Z.; Xie, Z.; Li, J.; Li, M.; Cen, S.; Tang, S.; Zheng, G.; Ye, G.; et al. LncRNA-mRNA expression profiles and functional networks in osteoclast differentiation. J. Cell. Mol. Med. 2020, 24, 9786–9797. [Google Scholar] [CrossRef]
- Barrett, S.P.; Salzman, J. Circular RNAs: Analysis, expression and potential functions. Development 2016, 143, 1838–1847. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, L.; Zheng, Y. circRNA Expression Profiles in Human Bone Marrow Stem Cells Undergoing Osteoblast Differentiation. Stem Cell Rev. Rep. 2019, 15, 126–138. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Huang, Y.; Jia, L.; Li, W. The Circular RNA Landscape of Periodontal Ligament Stem Cells During Osteogenesis. J. Periodontol. 2017, 88, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, E.; Menzel, U.; Basoli, V.; Tourbier, C.; Alini, M.; Stoddart, M.J. Differential Regulation of circRNA, miRNA, and piRNA during Early Osteogenic and Chondrogenic Differentiation of Human Mesenchymal Stromal Cells. Cells 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Cao, Z.; Yang, B.; Ding, N.; Hou, T.; Luo, F.; Kang, F.; Li, J.; Yang, X.; Jiang, H.; et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci. Rep. 2016, 6, 21499. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Tan, T.; Zhang, X.; Wan, J.; Zhou, Y.; Jiang, G.; Yang, D.; Guo, X.; Liu, T. CircRNA hsa_circ_0074834 promotes the osteogenesis-angiogenesis coupling process in bone mesenchymal stem cells (BMSCs) by acting as a ceRNA for miR-942-5p. Cell Death Dis. 2019, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Li, P.B.; Guo, S.F.; Yang, Q.S.; Chen, Z.X.; Wang, D.; Shi, S.B. CircRNA_0006393 promotes osteogenesis in glucocorticoid-induced osteoporosis by sponging miR-145-5p and upregulating FOXO1. Mol. Med. Rep. 2019, 20, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Yin, B.H.; Zhang, X.; Xue, D.D.; Ma, C. CircRNA-009934 induces osteoclast bone resorption via silencing miR-5107. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7580–7588. [Google Scholar]
- Chen, X.; Ouyang, Z.; Shen, Y.; Liu, B.; Zhang, Q.; Wan, L.; Yin, Z.; Zhu, W.; Li, S.; Peng, D. CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. 2019, 16, 1249–1262. [Google Scholar] [CrossRef]
- Hannan, F.M.; Newey, P.J.; Whyte, M.P.; Thakker, R.V. Genetic approaches to metabolic bone diseases. Br. J. Clin. Pharmacol. 2019, 85, 1147–1160. [Google Scholar] [CrossRef]
- Bhansali, A. Metabolic bone disease: Newer perspectives. Indian J. Endocrinol. Metab. 2012, 16, S140. [Google Scholar]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Craviari, T.; Pettifor, J.M.; Thacher, T.D.; Meisner, C.; Arnaud, J.; Fischer, P.R. Rickets: An Overview and Future Directions, with Special Reference to Bangladesh: A Summary of the Rickets Convergence Group Meeting, Dhaka, 26–27 January 2006. J. Health Popul. Nutr. 2008, 26, 112. [Google Scholar]
- Campbell, G.A.; Hosking, D.J.; Kemm, J.R.; Boyd, R.V. How common is osteomalacia in the elderly? Lancet 1984, 324, 386–388. [Google Scholar] [CrossRef]
- Nebot Valenzuela, E.; Pietschmann, P. Epidemiologie und Pathologie des Morbus Pagetein Überblick. Wien. Med. Wochenschr. 2017, 167, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.; Agnusdei, D.; Bilezikian, J.; Chappard, D.; Chapurlat, R.; Cianferotti, L.; Devolgelaer, J.P.; El Maghraoui, A.; Ferrari, S.; Javaid, M.K.; et al. Taxonomy of rare genetic metabolic bone disorders. Osteoporos. Int. 2015, 26, 2529–2558. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Lane, N.E. Metabolic Bone Disease. In Kelley and Firestein’s Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1730–1750.e4. [Google Scholar]
- Kemp, J.P.; Morris, J.A.; Medina-Gomez, C.; Forgetta, V.; Warrington, N.M.; Youlten, S.E.; Zheng, J.; Gregson, C.L.; Grundberg, E.; Trajanoska, K.; et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat. Genet. 2017, 49, 1468–1475. [Google Scholar] [CrossRef]
- Ferrari, S.; Rizzoli, R.; Jean-Philippe, B. Genetic aspects of osteoporosis: Current Opinion in Rheumatology. Curr. Opin. Rheumatol. 1999, 11, 294–300. [Google Scholar] [CrossRef]
- Stewart, T.L.; Ralston, S.H. Role of genetic factors in the pathogenesis of osteoporosis. J. Endocrinol. 2000, 166, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.A.; Qi, J.C.; Tokita, A.; Kelly, P.J.; Crofts, L.; Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Prediction of bone density from vitamin D receptor alleles. Nature 1994, 367, 284–287. [Google Scholar] [CrossRef]
- Yamada, Y.; Ando, F.; Niino, N.; Shimokata, H. Association of Polymorphisms of Interleukin-6, Osteocalcin, and Vitamin D Receptor Genes, Alone or in Combination, with Bone Mineral Density in Community-Dwelling Japanese Women and Men. J. Clin. Endocrinol. Metab. 2003, 88, 3372–3378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awasthi, N.; Awasthi, S.; Pandey, S. Role of VDR gene polymorphisms with community acquired pneumonia in North Indian children: A case-control study. Int. J. Mol. Epidemiol. Genet. 2021, 12, 1–8. [Google Scholar] [PubMed]
- Chen, J.F.; Lin, P.W.; Tsai, Y.R.; Yang, Y.C.; Kang, H.Y. Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy. Cells 2019, 8, 1318. [Google Scholar] [CrossRef] [PubMed]
- Emmanuelle, N.E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [PubMed]
- Sano, M.; Inoue, S.; Hosoi, T.; Ouchi, Y.; Emi, M.; Shiraki, M.; Orimo, H. Association of Estrogen Receptor Dinucleotide Repeat Polymorphism with Osteoporosis. Biochem. Biophys. Res. Commun. 1995, 217, 378–383. [Google Scholar] [CrossRef]
- Ferrari, S.L.; Rizzoli, R. Gene variants for osteoporosis and their pleiotropic effects in aging. Mol. Aspects Med. 2005, 26, 145–167. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Jiang, X.; Hu, J.; Li, R.; Liu, Y.; Zhu, G.; Rong, X. Glucocorticoids induce osteoporosis mediated by glucocorticoid receptor-dependent and -independent pathways. Biomed. Pharmacother. 2020, 125, 109979. [Google Scholar] [CrossRef]
- Çakmak, B.; Yiğit, S.; Karakuş, N.; Yıldırı, E.; İnanır, A. Relationship between postmenopausal osteoporosis and glucocorticoid receptor gene (NR3C1) polymorphism in a Turkish population. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Peng, Y.M.; Lei, S.F.; Guo, Y.; Xiong, D.H.; Yan, H.; Wang, L.; Guo, Y.F.; Deng, H.W. Sex-specific association of the glucocorticoid receptor gene with extreme BMD. J. Bone Miner. Res. 2008, 23, 247–252. [Google Scholar] [CrossRef]
- Pluijm, S.M.F.; Van Essen, H.W.; Bravenboer, N.; Uitterlinden, A.G.; Smit, J.H.; Pols, H.A.P.; Lips, P. Collagen type I α1 Sp1 polymorphism, osteoporosis, and intervertebral disc degeneration in older men and women. Ann. Rheum. Dis. 2004, 63, 71–77. [Google Scholar] [CrossRef]
- Xie, P.; Liu, B.; Zhang, L.; Chen, R.; Yang, B.; Dong, J.; Rong, L. Association of COL1A1 polymorphisms with osteoporosis: A meta-analysis of clinical studies. Int. J. Clin. Exp. Med. 2015, 8, 14764–14781. [Google Scholar] [PubMed]
- Trefilova, V.V.; Shnayder, N.A.; Petrova, M.M.; Kaskaeva, D.S.; Tutynina, O.V.; Petrov, K.V.; Popova, T.E.; Balberova, O.V.; Medvedev, G.V.; Nasyrova, R.F. The Role of Polymorphisms in Collagen-Encoding Genes in Intervertebral Disc Degeneration. Biomolecules 2021, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Mio, F.; Chiba, K.; Hirose, Y.; Kawaguchi, Y.; Mikami, Y.; Oya, T.; Mori, M.; Kamata, M.; Matsumoto, M.; Ozaki, K.; et al. A Functional Polymorphism in COL11A1, Which Encodes the α1 Chain of Type XI Collagen, Is Associated with Susceptibility to Lumbar Disc Herniation. Am. J. Hum. Genet. 2007, 81, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Gort-Van Dijk, D.; Weerink, L.B.M.; Milovanovic, M.; Haveman, J.W.; Hemmer, P.H.J.; Dijkstra, G.; Lindeboom, R.; Campmans-Kuijpers, M.J.E. Bioelectrical Impedance Analysis and Mid-Upper Arm Muscle Circumference Can Be Used to Detect Low Muscle Mass in Clinical Practice. Nutrients 2021, 13, 2350. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jia, H.; Xing, W.; Li, F.; Li, M.; Sun, K.; Zhu, Y. Genetic variants in COL11A2 of lumbar disk degeneration among Chinese Han population. Mol. Genet. Genom. Med. 2019, 7, e00524. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H. Type I Collagen Polymorphisms and Osteoporosis. In The Genetics of Osteoporosis and Metabolic Bone Disease; Humana Press: Totowa, NJ, USA, 2000; pp. 61–74. [Google Scholar]
- Majchrzycki, M.; Bartkowiak-Wieczorek, J.; Bogacz, A.; Szyfter-Harris, J.; Wolski, H.; Klejewski, A.; Goch, M.; Drews, K.; Barlik, M.; Ozarowski, M.; et al. The importance of polymorphic variants of collagen 1A2 gene (COL1A2) in the development of osteopenia and osteoporosis in postmenopausal women. Ginekol. Pol. 2017, 88, 414–420. [Google Scholar] [CrossRef]
- Rocha-Braz, M.G.M.; França, M.M.; Fernandes, A.M.; Lerario, A.M.; Zanardo, E.A.; de Santana, L.S.; Kulikowski, L.D.; Martin, R.M.; Mendonca, B.B.; Ferraz-De-Souza, B. Comprehensive Genetic Analysis of 128 Candidate Genes in a Cohort with Idiopathic, Severe, or Familial Osteoporosis. J. Endocr. Soc. 2020, 4, bvaa148. [Google Scholar] [CrossRef]
- Rolvien, T.; Yorgan, T.A.; Kornak, U.; Hermans-Borgmeyer, I.; Mundlos, S.; Schmidt, T.; Niemeier, A.; Schinke, T.; Amling, M.; Oheim, R. Skeletal deterioration in COL2A1-related spondyloepiphyseal dysplasia occurs prior to osteoarthritis. Osteoarthr. Cartil. 2020, 28, 334–343. [Google Scholar] [CrossRef]
- Liang, C.; Wang, P.; Liu, X.; Yang, C.; Ma, Y.; Yong, L.; Zhu, B.; Liu, X.; Liu, Z. Whole-genome sequencing reveals novel genes in ossification of the posterior longitudinal ligament of the thoracic spine in the Chinese population. J. Orthop. Surg. Res. 2018, 13, 324. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Lin, H.; Li, Z.; Xue, H.; Zhang, Y.; Lu, J. Relationship of COL9A1 and SOX9 Genes with Genetic Susceptibility of Postmenopausal Osteoporosis. Calcif. Tissue Int. 2020, 106, 248–255. [Google Scholar] [CrossRef]
- Wang, L.; Bian, X.; Cheng, G.; Zhao, P.; Xiang, X.; Tian, W.; Li, T.; Zhai, Q. A novel nonsense variant in PLS3 causes X-linked osteoporosis in a Chinese family. Ann. Hum. Genet. 2020, 84, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gowda; Vegda, H.; Shivappa, S.K.; Benakappa, N. Osteoporosis pseudoglioma syndrome. J. Pediatr. Neurosci. 2020, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Mota, A.S.; Misra, M.; Ackerman, K.E. LRP5, bone density, and mechanical stress: A case report and literature review. Front. Endocrinol. 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.T. Osteoprotegerin as a potential therapy for osteoporosis. Curr. Osteoporos. Rep. 2005, 3, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tuñón-Le Poultel, D.; Cannata-Andía, J.B.; Román-García, P.; Díaz-López, J.B.; Coto, E.; Gómez, C.; Naves-Díaz, M.; Rodríguez, I. Association of matrix Gla protein gene functional polymorphisms with loss of bone mineral density and progression of aortic calcification. Osteoporos. Int. 2014, 25, 1237–1246. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Liang, F.; Zhou, Y. Association of insulin-like growth factor I gene polymorphisms with the risk of osteoporosis in a Chinese population. Int. J. Clin. Exp. Pathol. 2017, 10, 8443. [Google Scholar]
- Gao, S.T.; Lv, Z.T.; Zhou, C.K.; Mao, C.; Sheng, W. Bin Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: A meta-analysis. BMC Musculoskelet. Disord. 2018, 19, 141. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.K.; Liu, J.; Yang, R.F.; Shao, F.F.; Tian, L.M. Association of insulin-like growth factor 1 receptor gene rs2229765 polymorphism with osteoporosis in postmenopausal women. Chin. J. Tissue Eng. Res. 2017, 21, 1813–1818. [Google Scholar]
- Paradowska, A.; Łącki, J. Genetic aspects of osteoporosis. Cent. Eur. J. Immunol. 2007, 32, 3039. [Google Scholar]
- Rotondo, J.C.; Giari, L.; Guerranti, C.; Tognon, M.; Castaldelli, G.; Fano, E.A.; Martini, F. Environmental doses of perfluorooctanoic acid change the expression of genes in target tissues of common carp. Environ. Toxicol. Chem. 2018, 37, 942–948. [Google Scholar] [CrossRef]
- Napoli, N.; Villareal, D.T.; Mumm, S.; Halstead, L.; Sheikh, S.; Cagaanan, M.; Rini, G.B.; Armamento-Villareal, R.C. Effect of CYP1A1 Gene Polymorphisms on Estrogen Metabolism and Bone Density. J. Bone Miner. Res. 2005, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; Ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming Growth Factor-β1 to the Bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [PubMed]
- Dohi, Y.; Iki, M.; Ohgushi, H.; Gojo, S.; Tabata, S.; Kajita, E.; Nishino, H.; Yonemasu, K. A Novel Polymorphism in the Promoter Region for the Human Osteocalcin Gene: The Possibility of a Correlation with Bone Mineral Density in Postmenopausal Japanese Women. J. Bone Miner. Res. 1998, 13, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Lu, Y.; Xi, X.E.; Huang, X.L.; Lu, Q.; Huang, X.; Li, S.; Qin, X. Relationships between the Osteocalcin Gene Polymorphisms, Serum Osteocalcin Levels, and Hepatitis B Virus-Related Hepatocellular Carcinoma in a Chinese Population. PLoS ONE 2015, 10, e0116479. [Google Scholar] [CrossRef]
- Zajíčková, K.; Žfková, I.; Hill, M.; Hořínek, A.; Nováková, A. Apolipoprotein E 4 allele is associated with low bone density in postmenopausal women. J. Endocrinol. Investig. 2014, 26, 312–315. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Arp, P.P.; Paeper, B.W.; Charmley, P.; Proll, S.; Rivadeneira, F.; Fang, Y.; Van Meurs, J.B.J.; Britschgi, T.B.; Latham, J.A.; et al. Polymorphisms in the Sclerosteosis/van Buchem Disease Gene (SOST) Region Are Associated with Bone-Mineral Density in Elderly Whites. Am. J. Hum. Genet. 2004, 75, 1032–1045. [Google Scholar] [CrossRef]
- Keen, R.W.; Woodford-Richens, K.L.; Lanchbury, J.S.; Spector, T.D. Allelic variation at the interleukin-1 receptor antagonist gene is associated with early postmenopausal bone loss at the spine. Bone 1998, 23, 367–371. [Google Scholar] [CrossRef]
- Delany, A.M.; McMahon, D.J.; Powell, J.S.; Greenberg, D.A.; Kurland, E.S. Osteonectin/SPARC polymorphisms in Caucasian men with idiopathic osteoporosis. Osteoporos. Int. 2008, 19, 969–978. [Google Scholar] [CrossRef]
- Acar, S.; Demir, K.; Shi, Y. Genetic Causes of Rickets. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 88. [Google Scholar] [CrossRef]
- Cuadrado-Soto, E.; López-Sobaler, A.M.; Jiménez-Ortega, A.I.; Aparicio, A.; Bermejo, L.M.; Hernández-Ruiz, Á.; Villoslada, F.L.; Leis, R.; de Victoria, E.M.; Moreno, J.M.; et al. Usual Dietary Intake, Nutritional Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D of Spanish Children Aged One to <10 Years. Findings from the EsNuPI Study. Nutrients 2020, 12, 1787. [Google Scholar] [CrossRef]
- Chanchlani, R.; Nemer, P.; Sinha, R.; Nemer, L.; Krishnappa, V.; Sochett, E.; Safadi, F.; Raina, R. An Overview of Rickets in Children. Kidney Int. Rep. 2020, 5, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Shaw, N.J.; Portale, A.A.; Ward, L.M.; Abrams, S.A.; Pettifor, J.M. Rickets. Nat. Rev. Dis. Prim. 2017, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- White, K.E.; Evans, W.E.; O’Riordan, J.L.H.; Speer, M.C.; Econs, M.J.; Lorenz-Depiereux, B.; Grabowski, M.; Meitinger, T.; Strom, T.M. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.R.; De Schepper, J.; Trouet, D.; Godefroid, N.; Boros, E.; Heinrichs, C.; Bravenboer, B.; Velkeniers, B.; Lammens, J.; Harvengt, P.; et al. Consensus Recommendations for the Diagnosis and Management of X-Linked Hypophosphatemia in Belgium. Front. Endocrinol. 2021, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- González-Lamuño, D. Hypophosphataemic Rickets: Diagnosis Algorithm—How Not to Make a Mistake. Adv. Ther. 2020, 37, 95–104. [Google Scholar] [CrossRef]
- Sitta, M.D.C.; Cassis, S.V.A.; Horie, N.C.; Moyses, R.M.A.; Jorgetti, V.; Garcez-Leme, L.E. Osteomalacia and vitamin D deficiency in the elderly. Clinics 2009, 64, 156–158. [Google Scholar] [CrossRef]
- Minisola, S.; Colangelo, L.; Pepe, J.; Diacinti, D.; Cipriani, C.; Rao, S.D. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR Plus 2021, 5, e10447. [Google Scholar] [CrossRef]
- Insogna, K.L.; Rauch, F.; Kamenický, P.; Ito, N.; Kubota, T.; Nakamura, A.; Zhang, L.; Mealiffe, M.; San Martin, J.; Portale, A.A. Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J. Bone Miner. Res. 2019, 34, 2183–2191. [Google Scholar] [CrossRef]
- Folpe, A.L. Phosphaturic mesenchymal tumors: A review and update. Semin. Diagn. Pathol. 2019, 36, 260–268. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Cundy, T.; Mantzoros, C.S. Juvenile Paget disease. Metab. Clin. Exp. 2018, 80, 15–26. [Google Scholar] [CrossRef]
- Rossi, V.; Lee, B.; Marom, R. Osteogenesis imperfecta: Advancements in genetics and treatment. Curr. Opin. Pediatr. 2019, 31, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Palomo, T.; Vilacą, T.; Lazaretti-Castro, M. Osteogenesis imperfecta: Diagnosis and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Palagano, E.; Menale, C.; Sobacchi, C.; Villa, A. Genetics of Osteopetrosis. Curr. Osteoporos. Rep. 2018, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, W.; Guo, J.; Zhao, L.; Tu, M.; Zheng, Y.; Li, L. CLCN7 and TCIRG1 mutations in a single family: Evidence for digenic inheritance of osteopetrosis. Mol. Med. Rep. 2019, 19, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Penna, S.; Capo, V.; Palagano, E.; Sobacchi, C.; Villa, A. One disease, many genes: Implications for the treatment of osteopetroses. Front. Endocrinol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Bollerslev, J.; Henriksen, K.; Nielsen, M.F.; Brixen, K.; Van Hul, W. Genetics in Endocrinology: Autosomal dominant osteopetrosis revisited: Lessons from recent studies. Eur. J. Endocrinol. 2013, 169, R39–R57. [Google Scholar] [CrossRef]
- Kang, S.; Kang, Y.K.; Lee, J.A.; Kim, D.H.; Lim, J.S. A Case of Autosomal Dominant Osteopetrosis Type 2 with a CLCN7 Gene Mutation. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 439. [Google Scholar] [CrossRef]
- Döffinger, R.; Smahi, A.; Bessia, C.; Geissmann, F.; Feinberg, J.; Durandy, A.; Bodemer, C.; Kenwrick, S.; Dupuis-Girod, S.; Blanche, S.; et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat. Genet. 2001, 27, 277–285. [Google Scholar] [CrossRef]
- Hartley, I.; Zhadina, M.; Collins, M.T.; Boyce, A.M. Fibrous Dysplasia of Bone and McCune–Albright Syndrome: A Bench to Bedside Review. Calcif. Tissue Int. 2019, 104, 517–529. [Google Scholar] [CrossRef]
- Lietman, S.A.; Levine, M.A. Fibrous dysplasia. Pediatr. Endocrinol. Rev. 2013, 10 (Suppl. 2), 389–396. [Google Scholar]
- Regard, J.B.; Cherman, N.; Palmer, D.; Kuznetsov, S.A.; Celi, F.S.; Guettier, J.M.; Chen, M.; Bhattacharyya, N.; Wess, J.; Coughlin, S.R.; et al. Wnt/β-catenin signaling is differentially regulated by Gα proteins and contributes to fibrous dysplasia. Proc. Natl. Acad. Sci. USA 2011, 108, 20101–20106. [Google Scholar] [CrossRef] [PubMed]
- Galada, C.; Shah, H.; Shukla, A.; Girisha, K.M. A novel sequence variant in SFRP4 causing Pyle disease. J. Hum. Genet. 2017, 62, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M.; Florenzano, P.; de Castro, L.F.; Collins, M.T. Fibrous Dysplasia/McCune-Albright Syndrome. In GeneReviews®; University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Hannan, F.M.; Babinsky, V.N.; Thakker, R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2016, 57, R127–R142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shoback, D.M. Familial hypocalciuric hypercalcemia and related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 609–619. [Google Scholar] [CrossRef]
- Roszko, K.L.; Bi, R.D.; Mannstadt, M. Autosomal dominant hypocalcemia (Hypoparathyroidism) types 1 and 2. Front. Physiol. 2016, 7, 458. [Google Scholar] [CrossRef]
- Picard, C.; Decrequy, A.; Guenet, D.; Bursztejn, A.C.; Toledano, D.; Richard, N.; Kottler, M.L. Diagnosis and Management of Congenital Hypothyroidism Associated with Pseudohypoparathyroidism. Horm. Res. Paediatr. 2015, 83, 111–117. [Google Scholar] [CrossRef]
- Whyte, M.P.; Deepak Amalnath, S.; McAlister, W.H.; Pedapati, R.; Muthupillai, V.; Duan, S.; Huskey, M.; Bijanki, V.N.; Mumm, S. Sclerosteosis: Report of type 1 or 2 in three Indian Tamil families and literature review. Bone 2018, 116, 321–332. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oton-Gonzalez, L.; Mazziotta, C.; Iaquinta, M.R.; Mazzoni, E.; Nocini, R.; Trevisiol, L.; D’Agostino, A.; Tognon, M.; Rotondo, J.C.; Martini, F. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. Int. J. Mol. Sci. 2022, 23, 1500. https://doi.org/10.3390/ijms23031500
Oton-Gonzalez L, Mazziotta C, Iaquinta MR, Mazzoni E, Nocini R, Trevisiol L, D’Agostino A, Tognon M, Rotondo JC, Martini F. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. International Journal of Molecular Sciences. 2022; 23(3):1500. https://doi.org/10.3390/ijms23031500
Chicago/Turabian StyleOton-Gonzalez, Lucia, Chiara Mazziotta, Maria Rosa Iaquinta, Elisa Mazzoni, Riccardo Nocini, Lorenzo Trevisiol, Antonio D’Agostino, Mauro Tognon, John Charles Rotondo, and Fernanda Martini. 2022. "Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases" International Journal of Molecular Sciences 23, no. 3: 1500. https://doi.org/10.3390/ijms23031500
APA StyleOton-Gonzalez, L., Mazziotta, C., Iaquinta, M. R., Mazzoni, E., Nocini, R., Trevisiol, L., D’Agostino, A., Tognon, M., Rotondo, J. C., & Martini, F. (2022). Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. International Journal of Molecular Sciences, 23(3), 1500. https://doi.org/10.3390/ijms23031500









