Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes
Abstract
:1. Introduction
1.1. Gut-Microbial Metabolites and Their Roles in the Development of T2D
1.1.1. Bacterial Toxins and LPS
1.1.2. Carbohydrate Metabolites: Short-Chain Fatty Acids (SCFAs)
1.1.3. Primary and Secondary Bile Acid Metabolites
1.1.4. Protein and Peptide Metabolites
1.1.5. Lecithin, Choline and L-Carnitine Products: TMA and TMAO
1.1.6. Gases Products: Methane and Hydrogen Sulfide
1.2. Treatment of T2D by Probiotics
1.2.1. Probiotics Interventions in Animal Models of Diabetes
1.2.2. Probiotics Randomized Control Trial (RCT) Interventions Studies in Human with T2D
- i.
- Single-strain probiotic intervention in T2D patients
- ii.
- Multi-strain probiotic intervention on T2D patients
1.2.3. Molecular Mechanism of Probiotics Intervention on T2D
2. Discussion
2.1. Diagnostic Applications in the Clinic
2.2. Drug Discovery Based on Gut Microbiota-Derived Metabolites
2.3. Alternative Therapeutic Options
2.3.1. Gut Microbiota-Derived Probiotics (GPs)
2.3.2. Prebiotics Supplement
2.3.3. Fecal Microbiota Transplantation (FMT)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| AAAs | Aromatic amino acids |
| ASBT | Apical-sodium-dependent BA transporter |
| BA | Bile acids |
| BCAAs | Branched-chain amino acids |
| BCFA | Branched-chain fatty acids |
| BSH | Bile salt hydrolase |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| CKD | Chronic kidney diseases |
| DCA | Deoxycholic acid |
| DMA | Dimethylamine |
| EPS | 4-ethyl phenyl sulfate |
| FGF15 | Fibroblast growth factor 15 |
| FGFR4 | Fibroblast growth factor receptor 4 |
| FIL | Fasting insulin levels |
| FMO3 | Flavin monooxygenase 3 |
| FMT | Fecal microbiota transplantation |
| FPG | Fasting plasma glucose |
| FXR | Farnesoid X receptor |
| GCA | Glycocholic acid |
| GCDCA | Glycine chenodeoxycholic acid |
| GLP-1 | Glucagon-like peptide-1 |
| GPCR | G-protein coupled receptor |
| GPR43/FFA2 and GPR41/FFA3 | G-protein-coupled receptors 43 and 41 |
| GPs | Gut microbiota-derived probiotics |
| HFD | High fat diet |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| IA | Indole acrylic acid |
| IAA | Indole-3-acid-acetic |
| IAld | Indole-3-aldehyde |
| IPA | Indole-3-propionic acid |
| IR | Insulin resistance |
| IRI | Insulin resistance index |
| IRS | Insulin receptor substrate |
| ITT | Insulin tolerance test |
| LCA | Lithocholic acid |
| LPS | Lipopolysaccharides |
| OGTT | Oral glucose tolerance test |
| OST alpha/beta | Organic solute transporter alpha/beta |
| PCS | Para-cresyl sulfate |
| PXR | Pregnane X receptor |
| PYY | Peptide YY |
| QUICKI | Quantitative insulin sensitivity check index |
| S1PR | Sphingosine-1-phosphate receptor |
| SCFAs | Short-chain fatty acids |
| SHP | Small heterodimer partner |
| STZ | Streptomycin |
| T2D | Type 2 diabetes |
| TCA | Taurocholic acid |
| TCDCA | Taurine chenodeoxycholic acid |
| TLRs | Toll-like receptors |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| VDR | Vitamin D receptor |
| WAT | White adipose tissue |
References
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palau-Rodriguez, M.; Tulipani, S.; Isabel Queipo-Ortuño, M.; Urpi-Sarda, M.; Tinahones, F.J.; Andres-Lacueva, C. Metabolomic insights into the intricate gut microbial–host interaction in the development of obesity and type 2 diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Ren, H.; Lu, Y.; Fang, C.; Hou, G.; Yang, Z.; Chen, B.; Yang, F.; Zhao, Y.; Shi, Z.; et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine 2019, 47, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: A systematic review and meta-analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, H.; Zhu, M.-J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Skoglund, J. Quantification of Short Chain Fatty Acids in Serum and Plasma; Swedish University of Agricultural Science: Uppsala, Sweden, 2016. [Google Scholar]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.-P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Lewindon, P.J.; Hoskins, A.C.; Pereira, T.N.; Setchell, K.D.R.; O’Connell, N.C.; Shepherd, R.W.; Ramm, G.A. Endogenous ursodeoxycholic acid and cholic acid in liver disease due to cystic fibrosis. Hepatology 2004, 39, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S.; et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 2015, 10, e0124844. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, M.; Haigh, R.; Tarr, J.M.; Gooding, K.M.; Shore, A.C.; Winyard, P.G. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: Relation to clinical and laboratory measures of inflammation. Ann. N. Y. Acad. Sci. 2010, 1203, 146–150. [Google Scholar] [CrossRef]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Ong, C.-N.; Subramaniam, T.; Choi, H.W.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: An untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016, 59, 2349–2359. [Google Scholar] [CrossRef] [Green Version]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; LaPoint, K.; Martinez, K.; Kennedy, A.; Boysen Sandberg, M.; McIntosh, M.K. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 2006, 147, 5340–5351. [Google Scholar] [CrossRef]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serino, M.; Luche, E.; Waget, A.; Pardo, G.; Salvador, J.; Ricart, W.; Frühbeck, G.; Burcelin, R. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int. J. Obes. 2012, 36, 1442–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Zhang, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef]
- Camargo, A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Garcia-Carpintero, S.; Lopez-Moreno, J.; Blanco-Rojo, R.; Delgado-Lista, J.; Perez-Martinez, P.; van Ommen, B.; et al. Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: From the CORDIOPREV study. Clin. Nutr. 2019, 38, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzi, S.; Neumann, S.; Gershengorn, M.C. Seven transmembrane-spanning receptors for free fatty acids as therapeutic targets for diabetes mellitus: Pharmacological, phylogenetic, and drug discovery aspects. J. Biol. Chem. 2008, 283, 16269–16273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognini, D.; Dedeo, D.; Milligan, G. Metabolic and inflammatory functions of short-chain fatty acid receptors. Curr. Opin. Endocr. Metab. Res. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, T.F.S.; Grześkowiak, Ł.; Franceschini, S.C.C.; Bressan, J.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Prawitt, J.; Caron, S.; Staels, B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 2011, 11, 160. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Distrutti, E. The Pharmacology of Bile Acids and Their Receptors. Bile Acids Recept. 2019, 3–18. [Google Scholar]
- van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017, 5, 224–233. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front. Cell. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, H.; Zhang, M.; Guo, G.L. Fatty liver diseases, bile acids, and FXR. Acta Pharm. Sin. B 2016, 6, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Li, H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother. 2020, 121, 109609. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017, 152, 1679–1694. [Google Scholar] [CrossRef]
- Chatterjee, I.; Lu, R.; Zhang, Y.; Zhang, J.; Dai, Y.; Xia, Y.; Sun, J. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci. Rep. 2020, 10, 7340. [Google Scholar] [CrossRef]
- Han, S.; Li, T.; Ellis, E.; Strom, S.; Chiang, J.Y.L. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol. Endocrinol. 2010, 24, 1151–1164. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chiang, J.Y.L. Nuclear receptors in bile acid metabolism. Drug Metab. Rev. 2013, 45, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Rysä, J.; Buler, M.; Savolainen, M.J.; Ruskoaho, H.; Hakkola, J.; Hukkanen, J. Pregnane X receptor agonists impair postprandial glucose tolerance. Clin. Pharmacol. Ther. 2013, 93, 556–563. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gao, J.; Xu, M.; Ren, S.; Stefanovic-Racic, M.; O’Doherty, R.M.; Xie, W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 2013, 62, 1876–1887. [Google Scholar] [CrossRef] [Green Version]
- Andersson-Hall, U.; Gustavsson, C.; Pedersen, A.; Malmodin, D.; Joelsson, L.; Holmäng, A. Higher concentrations of BCAAs and 3-HIB are associated with insulin resistance in the transition from gestational diabetes to type 2 diabetes. J. Diabetes Res. 2018, 2018. [Google Scholar] [CrossRef]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.C.; Delpino-Rius, A.; Samarra, I.; Castellano-Castillo, D.; Muñoz-Garach, A.; Bernal-Lopez, M.R.; Queipo-Ortuño, M.I.; Cardona, F.; Ramos-Molina, B.; Tinahones, F.J. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial. J. Clin. Med. 2019, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Oellgaard, J.; Abitz Winther, S.; Schmidt Hansen, T.; Rossing, P.; Johan von Scholten, B. Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Torrecilla, E.; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Gunsalus, R.P.; Rao, S.S.C.; Zhang, H. Methanogens in human health and disease. Am. J. Gastroenterol. Suppl. 2012, 1, 28. [Google Scholar] [CrossRef]
- Laverdure, R.; Mezouari, A.; Carson, M.A.; Basiliko, N.; Gagnon, J. A role for methanogens and methane in the regulation of GLP-1. Endocrinol. Diabetes Metab. 2018, 1, e00006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, J.; Wang, A.; Su, W.; Rozenbloom, S.R.; Taibi, A.; Comelli, E.M.; Wolever, T.M.S. Age, dietary fiber, breath methane, and fecal short chain fatty acids are interrelated in Archaea-positive humans. J. Nutr. 2013, 143, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Mathur, R.; Goyal, D.; Kim, G.; Barlow, G.M.; Chua, K.S.; Pimentel, M. Methane-producing human subjects have higher serum glucose levels during oral glucose challenge than non-methane producers: A pilot study of the effects of enteric methanogens on glycemic regulation. Res. J. Endocrinol. Metab. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K.; Bull, R.; Rains, J.L.; Bass, P.F.; Levine, S.N.; Reddy, S.; McVie, R.; Bocchini, J.A., Jr. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signal. 2010, 12, 1333–1337. [Google Scholar] [CrossRef]
- Okamoto, M.; Ishizaki, T.; Kimura, T. Protective effect of hydrogen sulfide on pancreatic beta-cells. Nitric Oxide 2015, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Wang, X.-Y.; Bian, J.-S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Zhao, J.; Tang, C.; Jiang, Z.; Geng, B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 2009, 380, 153–159. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: Implications for the pathogenesis and treatment of diabetes mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Jiang, T.; Fang, S.; Zhao, J.; Zhang, H.; Wang, G. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J. Appl. Microbiol. 2016, 121, 1727–1736. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.-R.; Lee, S.-Y.; Lee, N.-K.; Paik, H.-D.; Lim, S.-I. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef]
- Memarrast, F.; Ghafouri-Fard, S.; Kolivand, S.; Nodooshan, S.J.; Neyazi, N.; Sadroddiny, E.; Motevaseli, E. Comparative evaluation of probiotics effects on plasma glucose, lipid, and insulin levels in streptozotocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2017, 33, e2912. [Google Scholar] [CrossRef] [PubMed]
- Manaer, T.; Yu, L.; Nabi, X.-H.; Dilidaxi, D.; Liu, L.; Sailike, J. The beneficial effects of the composite probiotics from camel milk on glucose and lipid metabolism, liver and renal function and gut microbiota in db/db mice. BMC Complement. Med. Ther. 2021, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, Y.; Zhang, Y.; Zhou, J.; Zhou, F.; Zhao, Q.; Xu, G.; Hua, Z. Protective role of nano-selenium-enriched Bifidobacterium longum in delaying the onset of streptozotocin-induced diabetes. R. Soc. Open Sci. 2021, 5, 181156. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhu, H.; Gao, F.; Qian, Z.; Mao, W.; Yin, Y.; Tan, J.; Chen, D. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020, 11, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Ben Othman, M.; Sakamoto, K. Effect of inactivated Bifidobacterium longum intake on obese diabetes model mice (TSOD). Food Res. Int. 2020, 129, 108792. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Zeng, Z.; Qin, Y.; Shen, Q.; Li, P. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J. Funct. Foods 2020, 67, 103843. [Google Scholar] [CrossRef]
- Sakai, T.; Taki, T.; Nakamoto, A.; Shuto, E.; Tsutsumi, R.; Toshimitsu, T.; Makino, S.; Ikegami, S. Lactobacillus plantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2013, 59, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017, 8, 3155–3164. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, R.K.; Malhotra, S.; Pothuraju, R.; Shandilya, U.K. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef. Microbes 2017, 8, 243–255. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, 1900457. [Google Scholar] [CrossRef] [PubMed]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. ScienceDirect Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef]
- Hsieh, P.-S.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Tseng, H.-Y.; Kao, H.-F.; Wang, J.-Y. Lactobacillus salivarius AP-32 and Lactobacillus reuteri GL-104 decrease glycemic levels and attenuate diabetes-mediated liver and kidney injury in db/db mice. BMJ Open Diabetes Res. Care 2020, 8, e001028. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Lee, D.; Park, G.-S.; Ko, S.-H.; Park, J.; Lee, Y.-K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Eslami, R.D.; Tanomand, A. Effect of Lactobacillus delbrueckii Subsp. lactis PTCC1057 on Serum Glucose, Fetuin-A, and Sestrin 3 Levels in Streptozotocin-Induced Diabetic Mice. Probiotics Antimicrob. Proteins 2021, 13, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, F.; Zhao, P.; Zhang, R.; Zeng, Q. Effect of heat-killed Streptococcus thermophilus on type 2 diabetes rats. PeerJ 2019, 7, e7117. [Google Scholar] [CrossRef] [Green Version]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinai, H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Asghari Jafar-Abadi, M.; Faraji, I.; Hassanalilou, T.; Mesgari Abbasi, M.; Vaghef-Mehrabany, E.; Alizadeh Sani, M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran. Biomed. J. 2019, 23, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: A double-blind randomized clinical trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. 2018, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress, and inflammation and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Ren, J.; Huang, L.; Pang, B.; Liu, X.; Liu, X.; Li, B.; Shan, Y. Antidiabetic Effects of Lactobacillus casei Fermented Yogurt through Reshaping Gut Microbiota Structure in Type 2 Diabetic Rats. J. Agric. Food Chem. 2018, 66, 12696–12705. [Google Scholar] [CrossRef]
- Milshteyn, A.; Colosimo, D.A.; Brady, S.F. Accessing Bioactive Natural Products from the Human Microbiome. Cell Host Microbe 2018, 23, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.J.; Esterhazy, D.; Kim, S.-H.; Lemetre, C.; Aguilar, R.R.; Gordon, E.A.; Pickard, A.J.; Cross, J.R.; Emiliano, A.B.; Han, S.M.; et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.-J.; Chang, F.-Y.; Wyche, T.P.; Backus, K.M.; Acker, T.M.; Funabashi, M.; Taketani, M.; Donia, M.S.; Nayfach, S.; Pollard, K.S.; et al. Discovery of Reactive Microbiota-Derived Metabolites that Inhibit Host Proteases. Cell 2017, 168, 517–526.e18. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Nwe, P.-K.; Yang, Y.; Rosen, C.E.; Bielecka, A.A.; Kuchroo, M.; Cline, G.W.; Kruse, A.C.; Ring, A.M.; Crawford, J.M. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell 2019, 177, 1217–1231. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef]
- Ahmad, A.M.R.; Ahmed, W.; Iqbal, S.; Javed, M.; Rashid, S.; ul Haq, I. Prebiotics and iron bioavailability? Unveiling the hidden association-A review. Trends Food Sci. Technol. 2021, 110, 584–590. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017, 26, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, P.F.; Frissen, M.N.; De Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
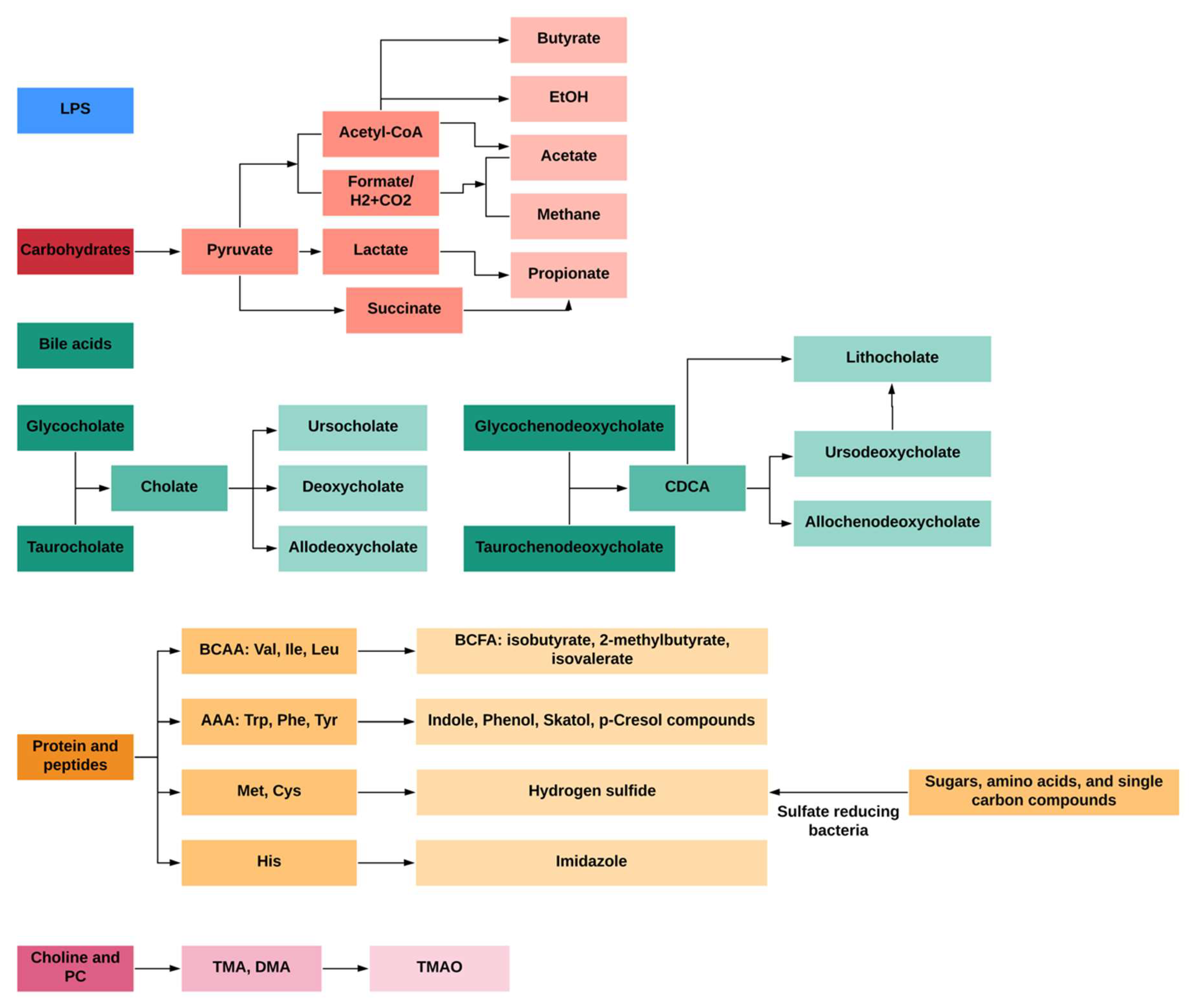
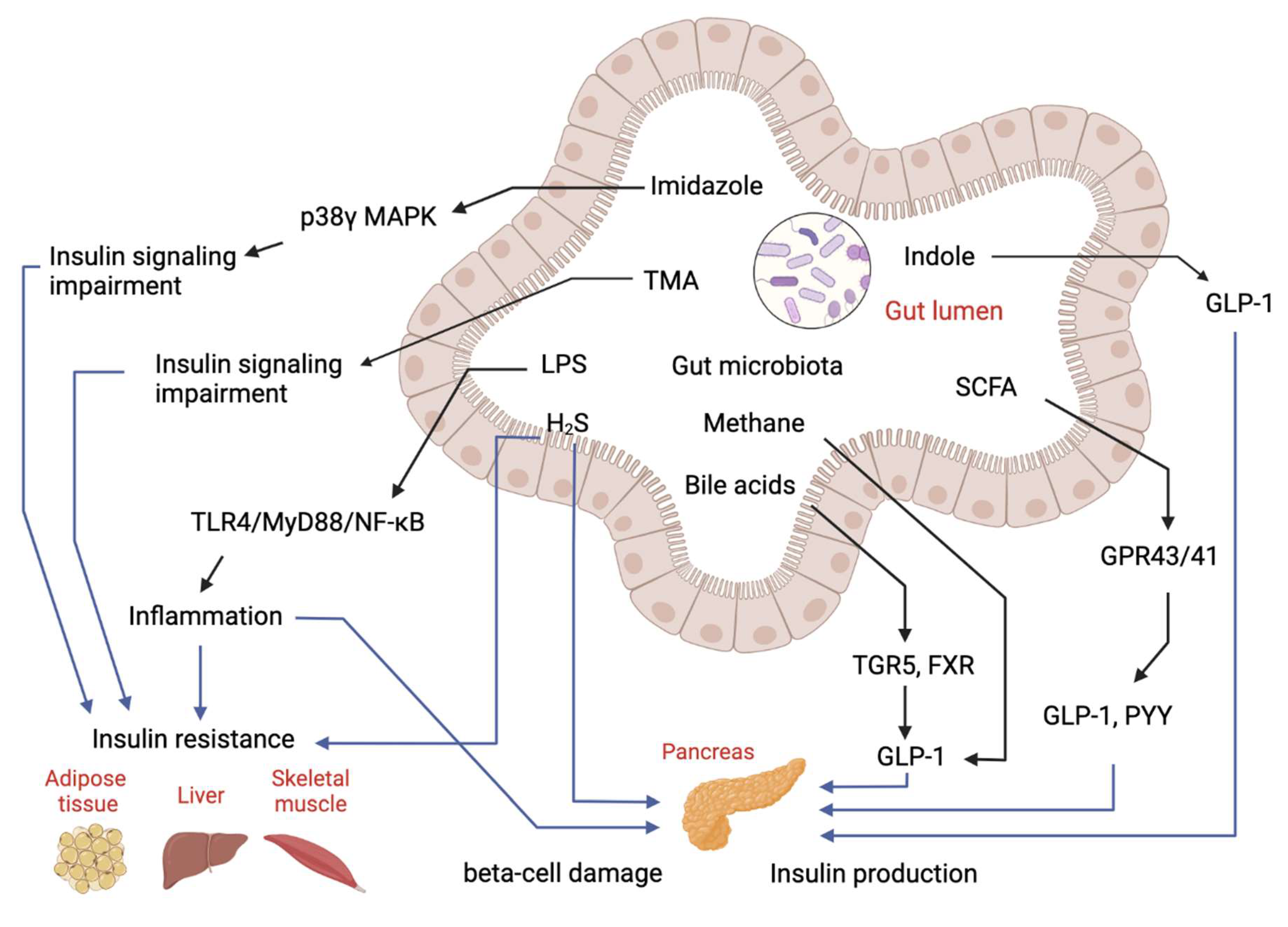
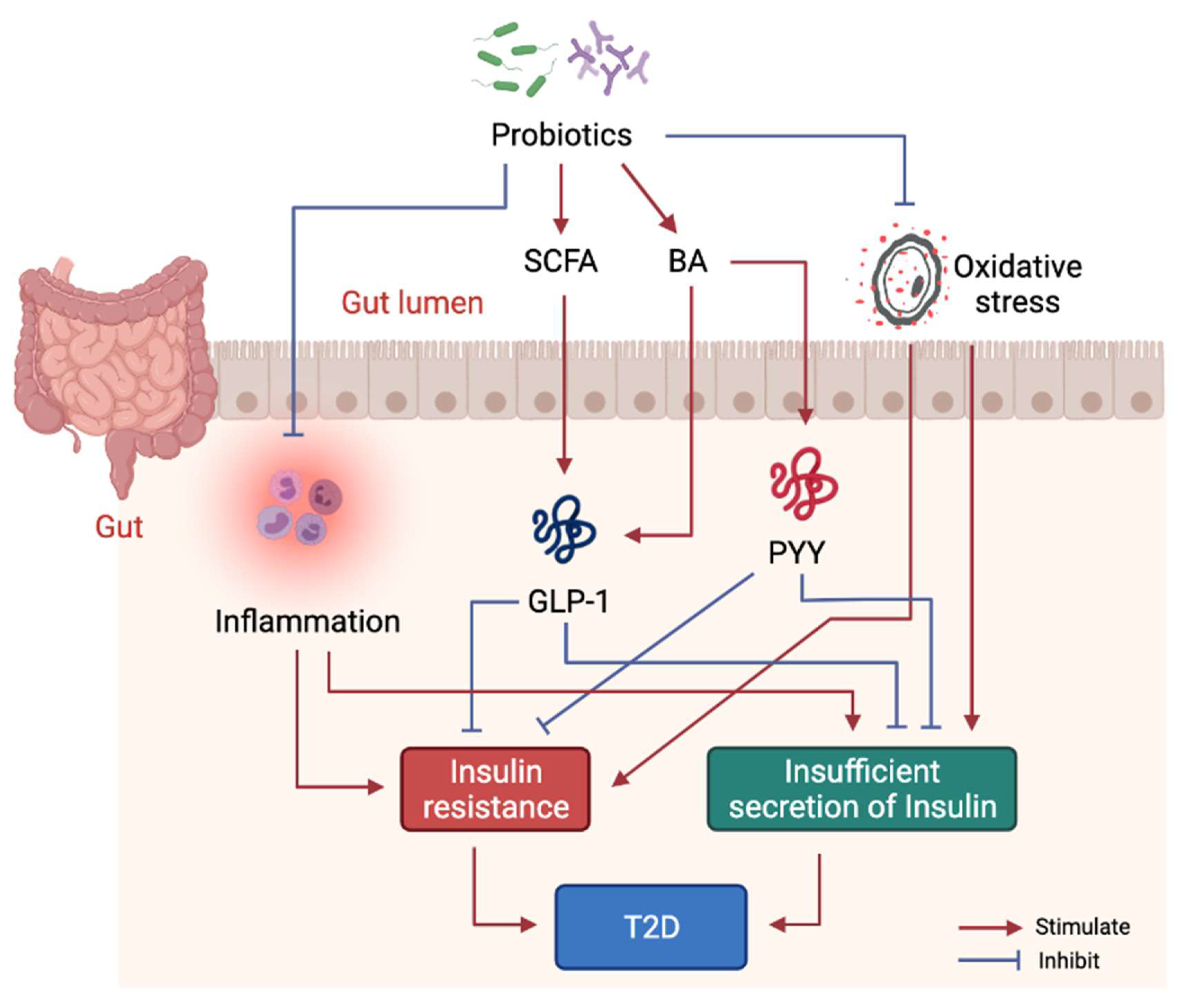
| Category | Metabolite | Serum/Plasma | Urine | Feces/Colon | References |
|---|---|---|---|---|---|
| LPS | LPS | 0.39 ± 0.06 EU/mL | – | 0.27 ± 0.04 EU/mL in feces | [10] |
| SCFAs | Acetate | 5–200 µM | 82.89 ± 60.0 μM | 35.86 ± 16.8 µmol/g in feces | [11] |
| SCFAs | Butyrate | <12 µM | 2.98 ± 1.88 μM | 6.35 ± 3.13 µmol g in feces | |
| SCFAs | Propionate | <13 µM | 108.2 ± 78.1 μM | 11.40 ± 4.74 µmol g in feces | |
| SCFAs | Succinate | 5–200 µM | 10 ± 0.2 μM | 3.1 + 0.9 mmol/kg in the proximal colon; 2.1 ± 1.0 mmol/kg in the sigmoid colon | [7] |
| BCFA | Total | – | – | 18.87 mmol/kg | [7] |
| BCFA | isobutyrate | 2.6–4.7 µM | – | 0.04–0.24 mg/g | [12] |
| BCFA | 2-methylbutyrate | – | – | 4079.7 nmol/g wet feces | |
| BCFA | isovalerate | 11.2–44.4 µM | – | 0.05–0.37 mg/g | |
| Amino acids | Total amines (agmatine, cadaverine, histamine, phenylethylamine, putrescine, spermidine, spermine, tryptamine and tyramine) | – | – | 22.32 mmol/kg | [7] |
| Amino acids | Ammonia | 22–55 µM | – | 160.93 mmol/kg | [7] |
| Amino acids | Phenolic acids | – | – | 2.39 mmol/kg (total phenols) | [13] |
| Amino acids | Indole | – | – | 2.6 mM | [14] |
| Amino acids | p–cresol | 5.556 ± 9.259 µM | 52.6 (38.8–71.0) umol/mmol creatinine | 2.12 mmol/kg | [15] |
| Bile acids | Deoxycholic acid | 0.57 ± 0.35 µM | – | 1920.10 +/− 1390.50 nmol/g dry feces | [16] |
| Bile acids | Lithocholic acid | 0.0103 µM | – | 1016.60 +/− 647.31 nmol/g dry feces | |
| Bile acids | Ursodesoxycholic acid | 0.1975 µM | – | 27.05 +/− 61.13 nmol/g dry feces | [16] |
| Choline | TMA | 26.55 (7.07) µM | 0.24–2.33 µmol/mmol creatinine | – | [17] |
| Choline | TMAO | 38.81 ± 20.37 µM | 20–125 µmol/mmol creatinine | 18417.506 (9541.599–27,293.412) nmol/g wet feces | [18] |
| Gas | Methane | – | – | – | |
| Gas | Carbon dioxide | – | – | – | |
| Gas | Hydrogen sulfide | 37.6 (27.4–41.3) µM | – | – | [19] |
| Probiotic Species/Strains | Disease Model | Main Results | References |
|---|---|---|---|
| Lactobacillus plantarum CCFM0236 | HFD+STZ | Blood glucose ↓, leptin level ↓, insulin resistance ↓ | [72] |
| Lactobacillus plantarum Ln4 | HFD | Insulin resistance ↓, insulin response ↑ | [73] |
| Lactobacillus fermentum MTCC 5689, Lactobacillus plantarum MTCC 5690 | HFD | Glucose ↓, HbA1c↓, plasma insulin ↓, HOMA-IR ↓ | [74] |
| Lactobacillus paracasei TD062 | HFD+STZ | FBG↓, Glucose tolerance ↓ | [75] |
| Lactobacillus reuteri, Lactobacillus crispatus and Bacillus subtiliso | STZ | Plasma glucose ↓, HbA1c ↓, plasma insulin ↑ | [76] |
| Lactobacillus kefiranofaciens, Lactobacillus plantarum, Lactobacillus helveticus, Lactococcus lactis and Issatchenkia orientalis | db/db | FBG ↓, OGTT ↓, HbAlc ↓ IRI ↓, plasma TC ↓, TG ↓, LDL-C ↓, | [77] |
| Nano-selenium-enriched Bifidobacterium longum | STZ | Blood glucose ↓, renal function damage ↓ | [78] |
| Bifidobacterium longum DD98 and selenium-enriched B. longum DD98 | HFD+STZ | FBG and HbA1c ↓ | [79] |
| Inactivated Bifidobacterium longum BR-108 | TSOD mouse | Blood glucose ↓ | [80] |
| Bifidobacterium animalis 01 | HFD+STZ | OGTT and HOMA-IR ↓, pro-inflammatory cytokines ↓ | [81] |
| Lactobacillus plantarum OLL2712 | HFD | Blood glucose ↓, IL-1beta ↓ | [82] |
| Lactobacillus casei CCFM419 | HFD+STZ | FBG ↓, glucose intolerance↓, insulin resistance ↓, TNF-alpha and IL-6 ↓, GLP-1 ↑ | [83] |
| Lactobacillus rhamnosus NCDC 17 | HFD+STZ | FBG ↓, plasma insulin ↓, HbA1c ↓, free fatty acids ↓, TG ↓ and TC ↓, | [84] |
| Lactobacillus paracasei NL41 | HFD+STZ | Insulin resistance↓, HbA1c ↓, glucagon ↓ and leptin ↓, oxidative stress status ↓ | [85] |
| Lactobacillus rhamnosus, Lactobacillus acidophilus and Bifidobacterium bifidum | HFD | Plasma glucose ↓, intestinal permeability ↓, LPS translocation ↓, systemic low-grade inflammation ↓ | [86] |
| Clostridium butyricum CGMCC0313.1 | Db/db mice and HFD+STZ | FBG ↓, HbA1c ↓, GLP-1 ↑ and inflammatory responses ↓ | [87] |
| Lactobacillus salivarius AP-32 and L. reuteri GL-104 | db/db mice | FBG ↓, TG ↓, TC ↓ | [88] |
| Lactobacillus plantarum HAC01 | HFD+STZ | FBG ↓, HbA1c ↓ and insulin-positive β-cell mass ↑ | [89] |
| Lactobacillus delbrueckii subsp. lactis PTCC1057 | STZ | FBG ↓, fetuin-A ↓ and sestrin ↑ | [90] |
| Streptococcus thermophilus | Zucker diabetic fatty (ZDF) | FBG ↓, glucose intolerance ↓, TC ↓, LPS ↓, IL-6 ↓, TNF-α ↓ and IL-10 ↑ | [91] |
| Lactobacillus plantarum, L. bulgaricus, L casei, L. acidophilus, Bifidobacterium infantis, B. longum, B. breve | HFD+STZ | Plasma glucose ↓, GLP-1 ↑ and total antioxidant capacity ↑ | [92] |
| Probiotic Species/Strains | Period | Sample Size | Main Results | References |
|---|---|---|---|---|
| Lactobacillus reuteri DSM 17938 | 12 weeks | 46 Placebo (n = 15) L. reuteri low (n = 15) L. reuteri high (n = 14) | Insulin sensitivity index (ISI) ↑, HbA1c not affected | [93] |
| Lactobacillus case 431® | 8 weeks | 40 Probiotic (n = 20) and placebo (n = 20) | FBG ↓, insulin ↓ and insulin resistance ↓ | [94] |
| Lactobacillus acidophilus, Bifidobacterium lactis, B.bifidum, B. longum | 24 weeks | 85 (27 in probiotic, 30 in synbiotic and 28 in placebo groups) | FPG ↓, HbA1c ↓and HOMA-IR ↓ | [95] |
| Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58 | 12 weeks | 78 placebo (n = 39) and probiotics (n = 39). | HOMA-IR ↓ | [96] |
| Symbiter, containing 14 alive probiotic strains of Lactobacillus, Lactococcus, Bifidobacterium, Propionibacterium and Acetobacter genera. | 8 weeks | 53 probiotic (n = 31) and placebo (n = 22) | HOMA-IR ↓, HbA1c ↓, TNF-α ↓ and IL-1β ↓ | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.-X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. https://doi.org/10.3390/ijms222312846
Zhai L, Wu J, Lam YY, Kwan HY, Bian Z-X, Wong HLX. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. International Journal of Molecular Sciences. 2021; 22(23):12846. https://doi.org/10.3390/ijms222312846
Chicago/Turabian StyleZhai, Lixiang, Jiayan Wu, Yan Y. Lam, Hiu Yee Kwan, Zhao-Xiang Bian, and Hoi Leong Xavier Wong. 2021. "Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes" International Journal of Molecular Sciences 22, no. 23: 12846. https://doi.org/10.3390/ijms222312846
APA StyleZhai, L., Wu, J., Lam, Y. Y., Kwan, H. Y., Bian, Z.-X., & Wong, H. L. X. (2021). Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. International Journal of Molecular Sciences, 22(23), 12846. https://doi.org/10.3390/ijms222312846







