The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms
Abstract
1. Introduction
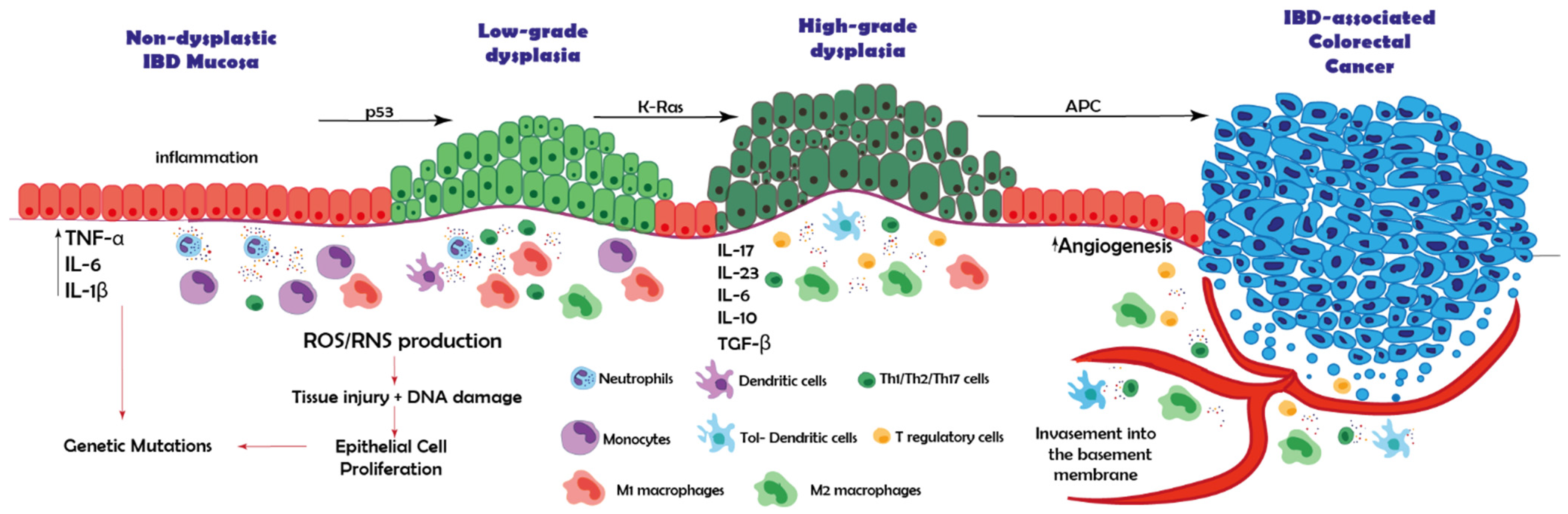
2. IBD-Associated Cancer: From Inflamed Tissue to Carcinoma
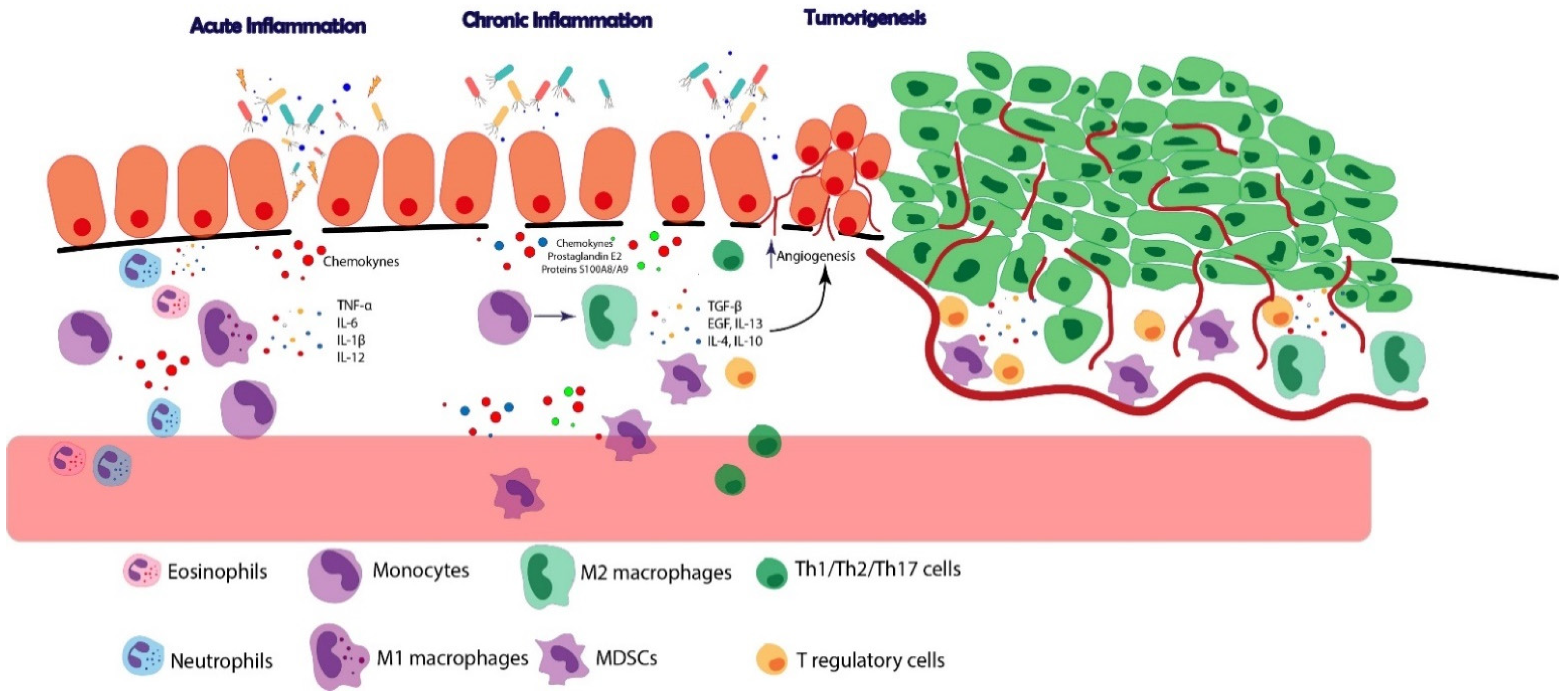
3. The Role of the Immune System in Cancers Developing on the Background of Chronic Inflammation
4. The Role of the Immune System in IBD-Associated Cancer
4.1. Immune Signaling Pathways in IBD: Contribution to Cancer Onset
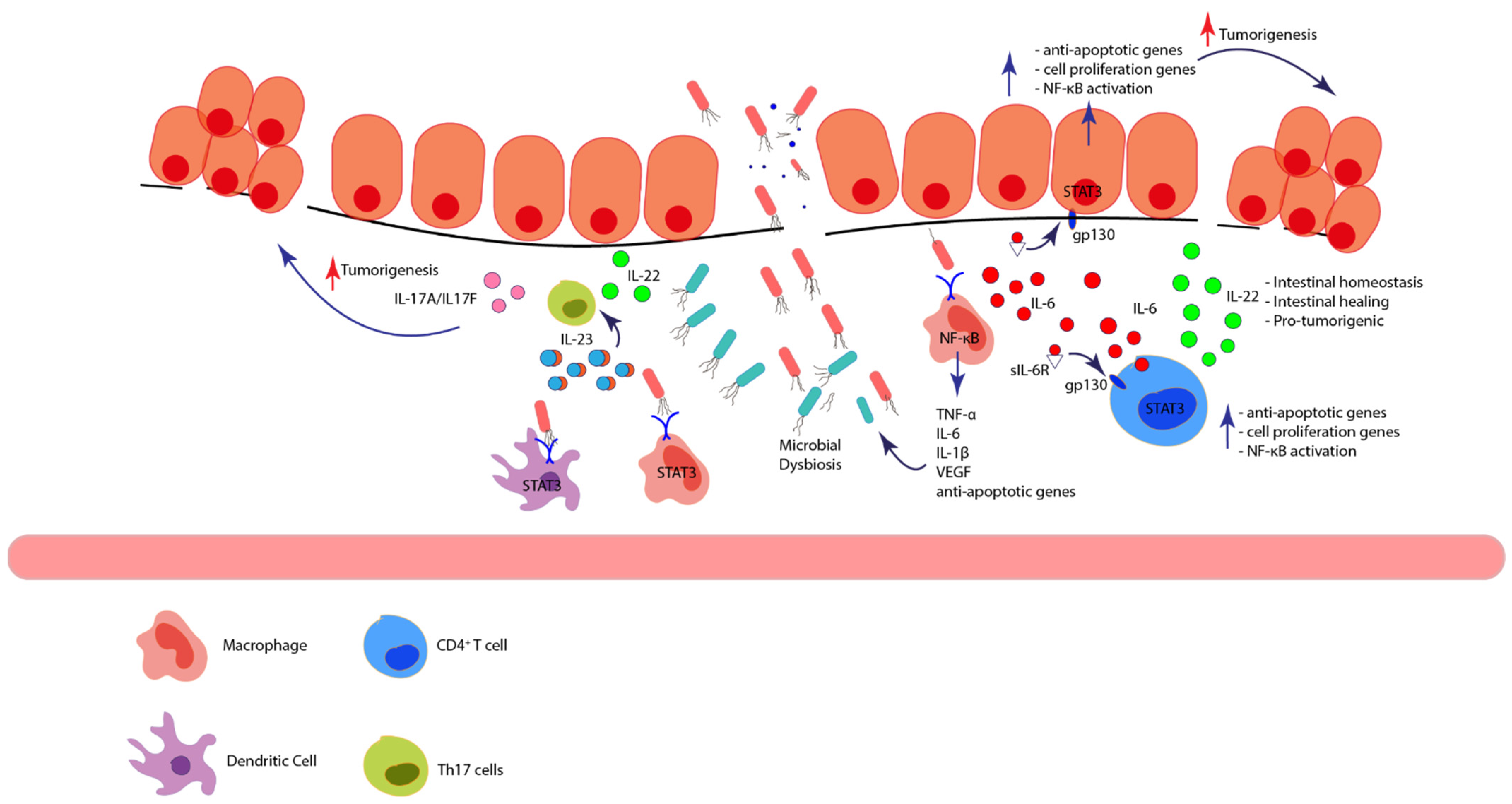
4.1.1. NF-κB/TNF-α
4.1.2. IL-6/STAT3 and IL-22/STAT3
4.1.3. NFAT
4.1.4. IL23/STAT3/Th17 Signaling Pathway
4.2. Immune Cells in IBD and Its Contribution to IBD-Associated Cancer
4.2.1. Macrophages
4.2.2. Neutrophils and Eosinophils
4.2.3. Dendritic Cells
4.2.4. T Cells
4.2.5. Innate Lymphoid Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ASA | 5-Aminosalicylic acid |
| AOM | azoxymethane |
| APC | antigen presenting cell |
| CAC | colitis-associated cancer |
| CCL | C-C motif ligand |
| CCR | C-C chemokine receptor |
| CD | crohn’s disease |
| CRC | colorectal cancer |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein |
| CXCL | C-X-C motif ligand |
| CXCR | C-X-C chemokine receptor |
| DC | dendritic cell |
| DSS | dextran sodium sulphate |
| HGD | high-grade dysplasia |
| IBD | inflammatory bowel disease |
| IECs | intestinal epithelial cells |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| ILC | innate lymphoid cell |
| ILC3 | type 3 innate lymphoid cell |
| iNOS | inducible nitric oxide synthase |
| LGD | low-grade dysplasia |
| MDSC | myeloid-derived suppressor cell |
| NK | natural killer |
| PD-1 | programmed cell death protein 1 |
| pDC | plasmacytoid dendritic cell |
| PD-L1 | programmed death ligand 1 |
| PSC | primary sclerosing cholangitis |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor alpha |
| Th | helper T cell |
| Treg | regulatory T cell |
| UC | ulcerative colitis |
References
- Romano, M.; De Francesco, F.; Zarantonello, L.; Ruffolo, C.; Ferraro, G.A.; Zanus, G.; Giordano, A.; Bassi, N.; Cillo, U. From Inflammation to Cancer in Inflammatory Bowel Disease: Molecular Perspectives. Anticancer Res. 2016, 36, 1447–1460. [Google Scholar]
- Saraggi, D.; Fassan, M.; Mescoli, C.; Scarpa, M.; Valeri, N.; Michielan, A.; D’Incá, R.; Rugge, M. The molecular landscape of colitis-associated carcinogenesis. Dig. Liver Dis. 2017, 49, 326–330. [Google Scholar] [CrossRef]
- Okayasu, I. Development of ulcerative colitis and its associated colorectal neoplasia as a model of the organ-specific chronic inflammation-carcinoma sequence. Pathol. Int. 2012, 62, 368–380. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Fantini, M.C.; Guadagni, I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021, 53, 558–565. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Van Der Sloot, K.W.J.; Le, L.P.; Batten, J.M.; Han, J.Y.; Fan, K.C.; Siegel, C.A.; Srivastava, A.; Park, Y.; Chen, J.-H.; et al. Colorectal cancer in Crohn’s colitis is comparable to sporadic colorectal cancer. Int. J. Colorectal. Dis. 2016, 31, 973–982. [Google Scholar] [CrossRef]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef]
- De Campos Silva, E.F.; Baima, J.P.; de Barros, J.R.; Tanni, S.E.; Schreck, T.; Saad-Hossne, R.; Sassaki, L.Y. Risk factors for ulcerative colitis-associated colorectal cancer: A retrospective cohort study. Medicine 2020, 99, e21686. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Yvellez, O.V.; Rai, V.; Sossenheimer, P.H.; Hart, J.; Turner, J.R.; Weber, C.; El Jurdi, K.; Rubin, D.T. Cumulative Histologic Inflammation Predicts Colorectal Neoplasia in Ulcerative Colitis: A Validation Study. Inflamm. Bowel Dis. 2021, 27, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D.; Din, S. Inflammatory bowel disease-associated colorectal cancer: Translational risks from mechanisms to medicines. J. Crohns Colitis 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Low, D.; Mino-Kenudson, M.; Mizoguchi, E. Recent advancement in understanding colitis-associated tumorigenesis. Inflamm. Bowel Dis. 2014, 20, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Sandborn, W.J.; Gupta, S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev. Res. 2016, 9, 887–894. [Google Scholar] [CrossRef]
- Du, L.; Kim, J.J.; Shen, J.; Chen, B.; Dai, N. KRAS and TP53 mutations in inflammatory bowel disease-associated colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 22175–22186. [Google Scholar] [CrossRef] [PubMed]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar]
- Osawa, E.; Nakajima, A.; Fujisawa, T.; Kawamura, Y.I.; Toyama-Sorimachi, N.; Nakagama, H.; Dohi, T. Predominant T helper type 2-inflammatory responses promote murine colon cancers. Int. J. Cancer 2006, 118, 2232–2236. [Google Scholar] [CrossRef]
- Jauch, D.; Martin, M.; Schiechl, G.; Kesselring, R.; Schlitt, H.J.; Geissler, E.K.; Fichtner-Feigl, S. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut 2011, 60, 1678–1686. [Google Scholar] [CrossRef]
- Punkenburg, E.; Vogler, T.; Büttner, M.; Amann, K.; Waldner, M.; Atreya, R.; Abendroth, B.; Mudter, J.; Merkel, S.; Gallmeier, E.; et al. Batf-dependent Th17 cells critically regulate IL-23 driven colitis-associated colon cancer. Gut 2016, 65, 1139–1150. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Mitsuyama, K.; Yamamoto, M.; Tsuruta, O.; Sata, M.; Scheller, J.; Rose-John, S.; Kado, S.I.; Takada, T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 2010, 184, 1543–1551. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Fedorak, R.N.; Hanson, J.; Wong, C.K. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can. J. Gastroenterol. 2007, 21, 443–446. [Google Scholar] [CrossRef]
- Munkholm, P. Review article: The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 18, 1–5. [Google Scholar] [CrossRef]
- Selinger, C.P.; Andrews, J.M.; Titman, A.; Norton, I.; Jones, D.B.; McDonald, C.; Barr, G.; Selby, W.; Leong, R. Sydney IBD Cohort Study Group. Long-term follow-up reveals low incidence of colorectal cancer, but frequent need for resection, among Australian patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2014, 12, 644–650. [Google Scholar] [CrossRef]
- Gatenby, G.; Glyn, T.; Pearson, J.; Gearry, R.; Eglinton, T. The long-term incidence of dysplasia and colorectal cancer in a Crohn’s colitis population-based cohort. Colorectal. Dis. 2021, 23, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Matkowskyj, K.A.; Chen, Z.E.; Rao, M.S.; Yang, G.Y. Dysplastic lesions in inflammatory bowel disease: Molecular pathogenesis to morphology. Arch. Pathol. Lab. Med. 2013, 137, 338–350. [Google Scholar] [CrossRef]
- Riddell, R.H.; Goldman, H.; Ransohoff, D.F.; Appelman, H.D.; Fenoglio, C.M.; Haggitt, R.C.; Hren, C.; Correa, P.; Hamilton, S.R.; Morson, B.C.; et al. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical applications. Hum. Pathol. 1983, 14, 931–968. [Google Scholar] [CrossRef]
- Harpaz, N.; Ward, S.C.; Mescoli, C.; Itzkowitz, S.H.; Polydorides, A.D. Precancerous lesions in inflammatory bowel disease. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 257–267. [Google Scholar] [CrossRef]
- Gui, X.; Iacucci, M.; Ghosh, S.; Ferraz, J.G.P.; Lee, S. Revisiting the distinct histomorphologic features of inflammatory bowel disease-associated neoplastic precursor lesions in the SCENIC and post-DALM Era. Hum. Pathol. 2020, 100, 24–37. [Google Scholar] [CrossRef]
- Chiu, K.; Riddell, R.H.; Schaeffer, D.F. DALM, rest in peace: A pathologist’s perspective on dysplasia in inflammatory bowel disease in the post-DALM era. Mod. Pathol. 2018, 31, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Hagland, H.R.; Berg, M.; Jolma, I.W.; Carlsen, A.; Søreide, K. Molecular pathways and cellular metabolism in colorectal cancer. Dig. Surg. 2013, 30, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Itzkowitz, S.H. Cancer in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 378–389. [Google Scholar] [CrossRef]
- Wijnands, A.M.; de Jong, M.E.; Lutgens, M.; Hoentjen, F.; Elias, S.G.; Oldenburg, B.; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology 2021, 160, 1584–1598. [Google Scholar] [CrossRef]
- Löwenberg, M.; Van Der Vlugt, M. Risk of Progression of Low-Grade Dysplasia to Advanced Neoplasia in Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 1483–1484. [Google Scholar] [CrossRef] [PubMed]
- Bezzio, C.; Festa, S.; Saibeni, S.; Papi, C. Chemoprevention of colorectal cancer in ulcerative colitis: Digging deep in current evidence. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 339–347. [Google Scholar] [CrossRef]
- Lopez, A.; Pouillon, L.; Beaugerie, L.; Danese, S.; Peyrin-Biroulet, L. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef]
- Nagahori, M.; Kochi, S.; Hanai, H.; Yamamoto, T.; Nakamura, S.; Omuro, S.; Watanabe, M.; Hibi, T. Real life results in using 5-ASA for maintaining mild to moderate UC patients in Japan, a multi-center study, OPTIMUM Study. BMC Gastroenterol. 2017, 17, 47. [Google Scholar] [CrossRef]
- Van de Meeberg, M.M.; Schultheiss, J.P.D.; Oldenburg, B.; Fidder, H.H.; Huitema, A.D.R. Does the 5-Aminosalicylate Concentration Correlate with the Efficacy of Oral 5-Aminosalicylate and Predict Response in Patients with Inflammatory Bowel Disease? A Systematic Review. Digestion 2020, 101, 245–261. [Google Scholar] [CrossRef]
- Koelink, P.J.; Robanus-Maandag, E.C.; Devilee, P.; Hommes, D.W.; Lamers, C.B.; Verspaget, H.W. 5-Aminosalicylic acid inhibits colitis-associated but not sporadic colorectal neoplasia in a novel conditional Apc mouse model. Carcinogenesis 2009, 30, 1217–1224. [Google Scholar] [CrossRef][Green Version]
- Bos, C.L.; Diks, S.H.; Hardwick, J.C.; Walburg, K.V.; Peppelenbosch, M.P.; Richel, D.J. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/beta-catenin pathway activity. Carcinogenesis 2006, 27, 2371–2382. [Google Scholar] [CrossRef]
- Campregher, C.; Honeder, C.; Chung, H.; Carethers, J.M.; Gasche, C. Mesalazine reduces mutations in transforming growth factor beta receptor II and activin type II receptor by improvement of replication fidelity in mononucleotide repeats. Clin. Cancer Res. 2010, 16, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Gulamhusein, A.; Bernstein, C.N. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: A meta-analysis of non-referral populations. Am. J. Gastroenterol. 2012, 107, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Li, J.Y.; Yu, T.; Chen, G.C.; Yuan, Y.H.; Chen, Q.K. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: An updated meta-analysis. PLoS ONE 2014, 9, e94208. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Roy, A.; Lawlor, G.; Korelitz, B.; Lichtiger, S. Thiopurines and inflammatory bowel disease: Current evidence and a historical perspective. World J. Gastroenterol. 2016, 22, 10103–10117. [Google Scholar] [CrossRef]
- Zhu, Z.; Mei, Z.; Guo, Y.; Wang, G.; Wu, T.; Cui, X.; Huang, Z.; Zhu, Y.; Wen, D.; Song, J.; et al. Reduced Risk of Inflammatory Bowel Disease-associated Colorectal Neoplasia with Use of Thiopurines: A Systematic Review and Meta-analysis. J. Crohns Colitis 2018, 12, 546–558. [Google Scholar] [CrossRef]
- Carrat, F.; Seksik, P.; Colombel, J.F.; Peyrin-Biroulet, L.; Beaugerie, L. The effects of aminosalicylates or thiopurines on the risk of colorectal cancer in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 533–541. [Google Scholar] [CrossRef]
- Lu, M.J.; Qiu, X.Y.; Mao, X.Q.; Li, X.T.; Zhang, H.H.H.J. Systematic review with meta-analysis: Thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 47, 318–331. [Google Scholar] [CrossRef]
- Fleit, H.B. Chronic Inflammation. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 300–314. [Google Scholar]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Irish J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Ohata, H.; Kitauchi, S.; Yoshimura, N.; Mugitani, K.; Iwane, M.; Nakamura, H.; Yoshikawa, A.; Yanaoka, K.; Arii, K.; Tamai, H.; et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 2004, 109, 138–143. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, P.Y.; Aboul-Soud, M.A.M. From inflammation to gastric cancer: Role of Helicobacter pylori. Oncol. Lett. 2017, 13, 543–548. [Google Scholar] [CrossRef]
- Nair, S.; Pillai, M.R. Human papillomavirus and disease mechanisms: Relevance to oral and cervical cancers. Oral Dis. 2005, 11, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 258–261. [Google Scholar]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef]
- Teng, W.; Liu, Y.C.; Jeng, W.J.; Su, C.W. Tertiary Prevention of HCC in Chronic Hepatitis B or C Infected Patients. Cancers 2021, 13, 1729. [Google Scholar] [CrossRef]
- Palmela, C.; Peerani, F.; Castaneda, D.; Torres, J.; Itzkowitz, S.H. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal. Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum Dis. 2011, 70 (Suppl. 1), i104–i108. [Google Scholar] [CrossRef]
- Li, R.; Wen, A.; Lin, J. Pro-Inflammatory Cytokines in the Formation of the Pre-Metastatic Niche. Cancers 2020, 12, 3752. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J.S. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Németh, J.; Stein, I.; Haag, D.; Riehl, A.; Longerich, T.; Horwitz, E.; Breuhahn, K.; Gebhardt, C.; Schirmacher, P.; Hahn, M.; et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology 2009, 50, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Montrose, D.C.; Nakanishi, M.; Murphy, R.C.; Zarini, S.; McAleer, J.P.; Vella, A.T.; Rosenberg, D.W. The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat. 2015, 116–117, 26–36. [Google Scholar] [CrossRef]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Narumiya, S. Prostaglandin E(2)-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflamm. Regen. 2017, 37, 4. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Bollrath, J.; Phesse, T.J.; von Burstin, V.A.; Putoczki, T.; Bennecke, M.; Bateman, T.; Nebelsiek, T.; Lundgren-May, T.; Canli, Ö.; Schwitalla, S.; et al. gp130-Mediated Stat3 Activation in Enterocytes Regulates Cell Survival and Cell-Cycle Progression during Colitis-Associated Tumorigenesis. Cancer Cell 2009, 15, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer: The Role of Inflammation and Chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef]
- Burns, J.M.; Summers, B.C.; Wang, Y.; Melikian, A.; Berahovich, R.; Miao, Z.; Penfold, M.E.T.; Sunshine, M.J.; Littman, D.R.; Kuo, C.J.; et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2006, 203, 2201–2213. [Google Scholar] [CrossRef]
- Salcedo, R.; Ponce, M.L.; Young, H.A.; Wasserman, K.; Ward, J.M.; Kleinman, H.K.; Oppenheim, J.J.; Murphy, W.J. Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000, 96, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Louie, M.C.; Desai, S.J.; Yang, J.; Chen, H.W.; Evans, C.P.; Kung, H.J. Interleukin-8 confers androgen-independent growth and migration of LNCaP: Differential effects of tyrosine kinases Src and FAK. Oncogene 2004, 23, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Ueha, S.; Shand, F.; Matsushima, K. Cellular and Molecular Mechanisms of Chronic Inflammation-Associated Organ Fibrosis. Front. Immunol. 2012, 3, 71. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Hao, N.B.; Lü, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, L.; Peek, R.M.; Hao, X.; Polk, D.B.; Li, H.; Yan, F. Activation of Epidermal Growth Factor Receptor in Macrophages Mediates Feedback Inhibition of M2 Polarization and Gastrointestinal Tumor Cell Growth. J. Biol. Chem. 2016, 291, 20462–20472. [Google Scholar] [CrossRef]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Kanterman, J.; Sade-Feldman, M.; Baniyash, M. New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol. 2012, 22, 307–318. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef]
- Lorusso, G.; Rüegg, C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem. Cell Biol. 2008, 130, 1091–1103. [Google Scholar] [CrossRef]
- Rao, S.; Gharib, K.; Han, A. Chapter Five—Cancer Immunosurveillance by T Cells. In International Review of Cell and Molecular Biology; Galluzzi, L., Rudqvist, N.-P., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 149–173. [Google Scholar]
- Belkaid, Y.; Oldenhove, G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity 2008, 29, 362–371. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where We Are and What Comes Next? Front. Immunol. 2017, 8, 1578. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Zou, L.; Zhu, G.; Mottram, P.; Xu, H.; Chen, L.; Zou, W. Cutting edge: Induction of B7-H4 on APCs through IL-10: Novel suppressive mode for regulatory T cells. J. Immunol. 2006, 177, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005, 6, 338–344. [Google Scholar] [CrossRef]
- Beswick, E.J.; Johnson, J.R.; Saada, J.I.; Humen, M.; House, J.; Dann, S.; Qiu, S.; Brasier, A.R.; Powell, D.W.; Reyes, V.E.; et al. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J. Immunol. 2014, 193, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Y.; Qu, Q.; Zhu, J.; Liu, Z.; Ning, W.; Zeng, H.; Zhang, N.; Du, W.; Chen, C.; et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int. J. Clin. Oncol. 2017, 22, 1026–1033. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Petrelli, A.; Mijnheer, G.; van Konijnenburg, D.H.; Van Der Wal, M.M.; Giovannone, B.; Mocholi, E.; Vazirpanah, N.; Broen, J.C.; Hijnen, D.; Oldenburg, B.; et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Investig. 2018, 128, 4669–4681. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal. Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Schottelius, A.J.; Baldwin, A.S., Jr. A role for transcription factor NF-kappa B in intestinal inflammation. Int. J. Colorectal. Dis. 1999, 14, 18–28. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Merlin, D. NF-κB pathway in colitis-associated cancers. Transl. Gastrointest. Cancer 2013, 2, 21–29. [Google Scholar]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Wilson, J.E.; Schneider, M.; Lich, J.D.; Roberts, R.A.; Arthur, J.C.; Woodford, R.M.T.; Davis, B.K.; Uronis, J.M.; Herfarth, H.H.; et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 2012, 36, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chatterjee, I.; Gujral, T.; Alakkam, A.; Coffing, H.; Anbazhagan, A.N.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; et al. Activation of Nuclear Factor-κB by Tumor Necrosis Factor in Intestinal Epithelial Cells and Mouse Intestinal Epithelia Reduces Expression of the Chloride Transporter SLC26A3. Gastroenterology 2017, 153, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X.; Wang, C.L.; Wang, L.; Sun, C.; Zhang, D.B.; Liu, J.L.; Liang, Y.N.; Tang, D.X.; Tang, Z.S. Tryptanthrin Protects Mice against Dextran Sulfate Sodium-Induced Colitis through Inhibition of TNF-α/NF-κB and IL-6/STAT3 Pathways. Molecules 2018, 23, 1062. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.; Jiang, R.; Zhang, M.; Yin, B.; Wang, J.; Li, Z.; Li, B.; Zhou, X. Suppression of Transmembrane Tumor Necrosis Factor Alpha Processing by a Specific Antibody Protects Against Colitis-Associated Cancer. Front. Immunol. 2021, 12, 687874. [Google Scholar] [CrossRef] [PubMed]
- Zidi, I.; Mestiri, S.; Bartegi, A.; Amor, N.B. TNF-alpha and its inhibitors in cancer. Med. Oncol. 2010, 27, 185–198. [Google Scholar] [CrossRef]
- Caspersen, S.; Elkjaer, M.; Riis, L.; Pedersen, N.; Mortensen, C.; Jess, T.; Sarto, P.; Hansen, T.S.; Wewer, V.; Bendtsen, F.; et al. Infliximab for inflammatory bowel disease in Denmark 1999-2005: Clinical outcome and follow-up evaluation of malignancy and mortality. Clin. Gastroenterol. Hepatol. 2008, 6, 1212–1217. [Google Scholar] [CrossRef]
- Schnitzler, F.; Fidder, H.; Ferrante, M.; Noman, M.; Arijs, I.; Van Assche, G.; Hoffman, I.; Van Steen, K.; Vermeire, S.; Rutgeerts, P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: Results from a single-centre cohort. Gut 2009, 58, 492–500. [Google Scholar] [CrossRef]
- Neurath, M.F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014, 7, 6–19. [Google Scholar] [CrossRef]
- Li, Y.; de Haar, C.; Chen, M.; Deuring, J.; Gerrits, M.M.; Smits, R.; Xia, B.; Kuipers, E.J.; van der Woude, C.J. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 2010, 59, 227–235. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Backert, I.; Koralov, S.; Wirtz, S.; Kitowski, V.; Billmeier, U.; Martini, E.; Hofmann, K.; Hildner, K.; Wittkopf, N.; Brecht, K.; et al. STAT3 activation in Th17 and Th22 cells controls IL-22-mediated epithelial host defense during infectious colitis. J. Immunol. 2014, 193, 3779–3791. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Putoczki, T.L.; Ernst, M. STAT3-Activating Cytokines: A Therapeutic Opportunity for Inflammatory Bowel Disease? J. Interferon Cytokine Res. 2015, 35, 340–350. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Yu, L.Z.; Wang, H.Y.; Yang, S.P.; Yuan, Z.P.; Xu, F.Y.; Sun, C.; Shi, R.H. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J. Gastroenterol. 2013, 19, 2638–2649. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, H.; Deng, L.; Hou, J.; Shi, R.; Yao, M.; Gao, Y.; Yao, A.; Wang, X.; Yu, L.; et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013, 13, 59. [Google Scholar] [CrossRef]
- Pan, M.G.; Xiong, Y.; Chen, F. NFAT gene family in inflammation and cancer. Curr. Mol. Med. 2013, 13, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, J.; Krummey, S.; Lu, W.; Cai, H.; Lenardo, M.J. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 2011, 12, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, B.; Lehr, H.A.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; Stevens, S.; Schmidt, J.; Galle, P.R.; Rose-John, S.; Neurath, M.F. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J. Exp. Med. 2008, 205, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, K.; Daniel, C.; Lehr, H.A.; Nikolaev, A.; Gerlach, T.; Atreya, R.; Rose-John, S.; Neurath, M.F.; Weigmann, B. Transcription factor NFATc2 controls the emergence of colon cancer associated with IL-6-dependent colitis. Cancer Res. 2012, 72, 4340–4350. [Google Scholar] [CrossRef]
- Lin, Y.; Koumba, M.H.; Qu, S.; Wang, D.; Lin, L. Blocking NFATc3 ameliorates azoxymethane/dextran sulfate sodium induced colitis-associated colorectal cancer in mice via the inhibition of inflammatory responses and epithelial-mesenchymal transition. Cell. Signal. 2020, 74, 109707. [Google Scholar] [CrossRef]
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 Pathway in Crohn’s Disease. Front. Immunol. 2021, 12, 622934. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Qi, H.; Yang, H.; Xu, G.; Ren, J.; Hua, W.; Shi, Y.; Torsvik, M.; Florholmen, J.; Cui, G. Therapeutic efficacy of IL-17A antibody injection in preventing the development of colitis associated carcinogenesis in mice. Immunobiology 2015, 220, 54–59. [Google Scholar] [CrossRef]
- Eken, A.; Oukka, M. Interleukin 23 in IBD Pathogenesis. In New Insights into Inflammatory Bowel Disease; Samuel Huber, IntechOpen: London, UK, 2016; pp. 93–123. [Google Scholar]
- Hyun, Y.S.; Han, D.S.; Lee, A.R.; Eun, C.S.; Youn, J.; Kim, H.Y. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis 2012, 33, 931–936. [Google Scholar] [CrossRef]
- Fiorino, G.; Allocca, M.; Correale, C.; Roda, G.; Furfaro, F.; Loy, L.; Zilli, A.; Peyrin-Biroulet, L.; Danese, S. Positioning ustekinumab in moderate-to-severe ulcerative colitis: New kid on the block. Expert Opin. Biol. Ther. 2020, 20, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Argollo, M.C.; Allocca, M.; Furfaro, F.; Peyrin-Biroulet, L.; Danese, S. Interleukin-23 Blockers: Born to be First-line Biologic Agents in Inflammatory Bowel Disease? Curr. Pharm. Des. 2019, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Taniguchi, M.; Street, S.E. The anti-tumor activity of IL-12: Mechanisms of innate immunity that are model and dose dependent. J. Immunol. 2000, 165, 2665–2670. [Google Scholar] [CrossRef]
- Teng, M.W.; Vesely, M.D.; Duret, H.; McLaughlin, N.; Towne, J.E.; Schreiber, R.D.; Smyth, M.J. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012, 72, 3987–3996. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Roblin, X.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Mowat, A.M. To respond or not to respond—A personal perspective of intestinal tolerance. Nat. Rev. Immunol. 2018, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative contex.xt-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Thiesen, S.; Janciauskiene, S.; Uronen-Hansson, H.; Agace, W.; Högerkorp, C.-M.; Spee, P.; Håkansson, K.; Grip, O. CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J. Leukoc. Biol. 2014, 95, 531–541. [Google Scholar] [CrossRef]
- Bernardo, D.; Marin, A.C.; Fernández-Tomé, S.; Montalban-Arques, A.; Carrasco, A.; Tristán, E.; Ortega-Moreno, L.; Mora-Gutiérrez, I.; Díaz-Guerra, A.; Caminero-Fernández, R.; et al. Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018, 11, 1114–1126. [Google Scholar] [CrossRef]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.; Sanchez-Elsner, T. Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Seyedizade, S.S.; Afshari, K.; Bayat, S.; Rahmani, F.; Momtaz, S.; Rezaei, N.; Abdolghaffari, A.H. Current Status of M1 and M2 Macrophages Pathway as Drug Targets for Inflammatory Bowel Disease. Arch. Immunol. Ther. Exp. 2020, 68, 10. [Google Scholar] [CrossRef]
- Shin, S.H.; Kadayakkara, D.K.; Bulte, J.W. In Vivo (19)F MR Imaging Cell Tracking of Inflammatory Macrophages and Site-specific Development of Colitis-associated Dysplasia. Radiology 2017, 282, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Keshavarzian, A.; Gounaris, E.; Melson, J.E.; Cheon, E.C.; Blatner, N.R.; Chen, Z.E.; Tsai, F.-N.; Lee, G.; Ryu, S.H.; et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin. Cancer Res. 2013, 19, 2342–2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.E.I.; Li, X.; Zheng, D.; Zhang, D.; Peng, X.; Zhang, X.; Ai, F.; Wang, X.; Ma, J.; Xiong, W.; et al. Dynamic changes and functions of macrophages and M1/M2 subpopulations during ulcerative colitis-associated carcinogenesis in an AOM/DSS mouse model. Mol. Med. Rep. 2015, 11, 2397–2406. [Google Scholar] [CrossRef]
- Kvorjak, M.; Ahmed, Y.; Miller, M.L.; Sriram, R.; Coronnello, C.; Hashash, J.G.; Hartman, D.J.; Telmer, C.A.; Miskov-Zivanov, N.; Finn, O.J.; et al. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2020, 8, 167–178. [Google Scholar] [CrossRef]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef]
- Moreira, T.G.; Horta, L.S.; Gomes-Santos, A.C.; Oliveira, R.P.; Queiroz, N.M.G.P.; Mangani, D.; Daniel, B.; Vieira, A.T.; Liu, S.; Rodrigues, A.M.; et al. CLA-supplemented diet accelerates experimental colorectal cancer by inducing TGF-β-producing macrophages and T cells. Mucosal Immunol. 2019, 12, 188–199. [Google Scholar] [CrossRef]
- Wang, L.; Yu, S.; Chan, E.R.; Chen, K.-Y.; Liu, C.; Che, D.; Awadallah, A.; Myers, J.; Askew, D.; Huang, A.Y.; et al. Notch-Regulated Dendritic Cells Restrain Inflammation-Associated Colorectal Carcinogenesis. Cancer Immunol. Res. 2021, 9, 348–361. [Google Scholar] [CrossRef]
- Hong, E.-H.; Cho, J.; Ahn, J.-H.; Kwon, B.-E.; Kweon, M.-N.; Seo, S.-U.; Yoon, B.-I.; Chang, S.-Y.; Ko, H.-J. Plasmacytoid dendritic cells regulate colitis-associated tumorigenesis by controlling myeloid-derived suppressor cell infiltration. Cancer Lett. 2020, 493, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Amy, I.Y.; Zhao, L.; Eaton, K.A.; Ho, S.; Chen, J.; Poe, S.; Becker, J.; Gonzalez, A.; McKinstry, D.; Hasso, M.; et al. Gut Microbiota Modulate CD8 T Cell Responses to Influence Colitis-Associated Tumorigenesis. Cell Rep. 2020, 31, 107471. [Google Scholar]
- Yassin, M.; Sadowska, Z.; Djurhuus, D.; Nielsen, B.; Tougaard, P.; Olsen, J.; Pedersen, A.E. Upregulation of PD-1 follows tumour development in the AOM/DSS model of inflammation-induced colorectal cancer in mice. Immunology 2019, 158, 35–46. [Google Scholar] [CrossRef]
- Michael-Robinson, J.; Pandeya, N.; Walsh, M.D.; Biemer-Huttmann, A.-E.; Eri, R.; Buttenshaw, R.; Lincoln, D.; Clouston, A.; Jass, J.; Radford-Smith, G. Characterization of tumour-infiltrating lymphocytes and apoptosis in colitis-associated neoplasia: Comparison with sporadic colorectal cancer. J. Pathol. 2006, 208, 381–387. [Google Scholar] [CrossRef]
- Pastille, E.; Bardini, K.; Fleissner, D.; Adamczyk, A.; Frede, A.; Wadwa, M.; Von Smolinski, D.; Kasper, S.; Sparwasser, T.; Gruber, A.D.; et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014, 74, 4258–4269. [Google Scholar] [CrossRef] [PubMed]
- Olguín, J.E.; Medina-Andrade, I.; Molina, E.; Vázquez, A.; Pacheco-Fernández, T.; Saavedra, R.; Perez-Plasencia, C.; Chirino, Y.I.; Vaca-Paniagua, F.; Arias-Romero, L.E.; et al. Early and Partial Reduction in CD4(+)Foxp3(+) Regulatory T Cells during Colitis-Associated Colon Cancer Induces CD4(+) and CD8(+) T Cell Activation Inhibiting Tumorigenesis. J. Cancer 2018, 9, 239–249. [Google Scholar] [CrossRef]
- Zaiatz Bittencourt, V.; Jones, F.; Tosetto, M.; Doherty, G.A.; Ryan, E.J. Dysregulation of Metabolic Pathways in Circulating Natural Killer Cells Isolated from Inflammatory Bowel Disease Patients. J. Crohns Colitis. 2021, 15, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Chapuy, L.; Bsat, M.; Rubio, M.; Bernard, G.; Arslanian, E.; Orlicka, K.; Weber, A.; Panzini, B.; Dorais, J.; et al. Recruitment of activated neutrophils correlates with disease severity in adult Crohn’s disease. Clin. Exp. Immunol. 2019, 195, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Alhmoud, T.; Gremida, A.; Steele, D.C.; Fallahi, I.; Tuqan, W.; Nandy, N.; Ismail, M.; Altamimi, B.A.; Xiong, M.-J.; Kerwin, A.; et al. Outcomes of inflammatory bowel disease in patients with eosinophil-predominant colonic inflammation. BMJ Open Gastroenterol. 2020, 7, e000373. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, L.; Liu, Y.; Wang, Y.; Wang, Q.N.; Wang, H.L.; Shi, G.; Li, J.S.; Yang, Q.M.; Chen, S.; et al. Intestinal epithelium-derived BATF3 promotes colitis-associated colon cancer through facilitating CXCL5-mediated neutrophils recruitment. Mucosal Immunol. 2021, 14, 187–198. [Google Scholar] [CrossRef]
- Wéra, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Schins, R.P.; Polat, D.; Becker, A.; Borm, P.J. Mechanisms of neutrophil-induced DNA damage in respiratory tract epithelial cells. Mol. Cell Biochem. 2002, 234–235, 143–151. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, K.; Song, Y.; Yang, W.; Shu, W.; Yu, T.; Yu, L.; Lin, M.; Weigang, S.; Chen, C.; et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis 2018, 39, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K. Neutrophils Restrict Tumor-Associated Microbiota to Reduce Growth and Invasion of Colon Tumors in Mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef]
- Lampinen, M.; Backman, M.; Winqvist, O.; Rorsman, F.; Rönnblom, A.; Sangfelt, P.; Carlson, M. Different regulation of eosinophil activity in Crohn’s disease compared with ulcerative colitis. J. Leukoc. Biol. 2008, 84, 1392–1399. [Google Scholar] [CrossRef]
- Leoncini, G.; Villanacci, V.; Marin, M.G.; Crisafulli, V.; Cadei, M.; Antonelli, E.; Leoci, C.; Bassotti, G. Colonic hypereosinophilia in ulcerative colitis may help to predict the failure of steroid therapy. Tech. Coloproctol. 2018, 22, 941–946. [Google Scholar] [CrossRef]
- Sastre, B.; Rodrigo-Muñoz, J.M.; Garcia-Sanchez, D.A.; Cañas, J.A.; Del Pozo, V. Eosinophils: Old Players in a New Game. J. Investig. Allergol. Clin. Immunol. 2018, 28, 289–304. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: A meta-analysis. BMC Cancer 2020, 20, 454. [Google Scholar] [CrossRef]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Bokemeyer, C.; Langner, C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod. Pathol. 2015, 28, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Prizment, A.E.; Vierkant, R.; Smyrk, T.C.; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Kienzl, M.; Hasenoehrl, C.; Valadez-Cosmes, P.; Maitz, K.; Sarsembayeva, A.; Sturm, E.; Heinemann, A.; Kargl, J.; Schicho, R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology 2020, 9, 1776059. [Google Scholar] [CrossRef]
- Jaensson, E.; Uronen-Hansson, H.; Pabst, O.; Eksteen, B.; Tian, J.; Coombes, J.L.; Berg, P.L.; Davidsson, T.; Powrie, F.; Johansson-Lindbom, B.; et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008, 205, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Thomas, S.; Przesdzing, I.; Metzke, D.; Bielecki, C.; Lehmann, S.M.; Lehnardt, S.; Dörffel, Y.; Sturm, A.; Scheffold, A.; et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin. Exp. Immunol. 2009, 157, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; Vogelpoel, L.T.; van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Matsuno, H.; Kayama, H.; Nishimura, J.; Sekido, Y.; Osawa, H.; Barman, S.; Ogino, T.; Takahashi, H.; Haraguchi, N.; Hata, T.; et al. CD103+ Dendritic Cell Function Is Altered in the Colons of Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Pool, L. Intestinal Classical Dendritic in T Cell Induced Colitis and Colitis Associated Colorectal Cancer; DTU Nanotech, Technical University of Denmark: Lyngby, Denmark, 2018. [Google Scholar]
- Garrett, W.S.; Punit, S.; Gallini, C.A.; Michaud, M.; Zhang, D.; Sigrist, K.S.; Lord, G.M.; Glickman, J.N.; Glimcher, L.H. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 2009, 16, 208–219. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef]
- Groth, C.; Arpinati, L.; Shaul, M.; Winkler, N.; Diester, K.; Gengenbacher, N.; Weber, R.; Arkhypov, I.; Lasser, S.; Petrova, V.; et al. Blocking Migration of Polymorphonuclear Myeloid-Derived Suppressor Cells Inhibits Mouse Melanoma Progression. Cancers 2021, 13, 726. [Google Scholar] [CrossRef]
- James, S.P.; Kwan, W.C.; Sneller, M.C. T cells in inductive and effector compartments of the intestinal mucosal immune system of nonhuman primates differ in lymphokine mRNA expression, lymphokine utilization, and regulatory function. J. Immunol. 1990, 144, 1251–1256. [Google Scholar] [PubMed]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, F.; Cheroutre, H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev. Clin. Immunol. 2010, 6, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef]
- Scarpa, M.; Brun, P.; Scarpa, M.; Morgan, S.; Porzionato, A.; Kotsafti, A.; Bortolami, M.; Buda, A.; D’Incà, R.; Macchi, V.; et al. CD80-CD28 signaling controls the progression of inflammatory colorectal carcinogenesis. Oncotarget 2015, 6, 20058–20069. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yamada, H.; Shibata, K.; Muta, H.; Wajjwalku, W.; Podack, E.R.; Yoshikai, Y. A novel role of CD30L/CD30 signaling by T-T cell interaction in Th1 response against mycobacterial infection. J. Immunol. 2008, 181, 6316–6327. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, X.; Wang, X.; Wang, B.; Meng, X.; Yoshikai, Y.; Wang, Y.; Sun, X. CD30L/CD30 signaling regulates the formation of the tumor immune microenvironment and inhibits intestinal tumor development of colitis-associated colon cancer in mice. Int. Immunopharmacol. 2020, 84, 106531. [Google Scholar] [CrossRef]
- Catakovic, K.; Klieser, E.; Neureiter, D.; Geisberger, R. T cell exhaustion: From pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017, 15, 1. [Google Scholar] [CrossRef]
- Ozawa, N.; Yokobori, T.; Osone, K.; Katayama, C.; Suga, K.; Komine, C.; Shibasaki, Y.; Shiraishi, T.; Okada, T.; Kato, R.; et al. PD-L1 upregulation is associated with activation of the DNA double-strand break repair pathway in patients with colitic cancer. Sci. Rep. 2021, 11, 13077. [Google Scholar] [CrossRef]
- Kryczek, I.; Wu, K.; Zhao, E.; Wei, S.; Vatan, L.; Szeliga, W.; Huang, E.; Greenson, J.; Chang, A.; Rolinski, J.M.; et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J. Immunol. 2011, 186, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Boussiotis, V.A. The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clin. Immunol. 2013, 148, 246–253. [Google Scholar] [CrossRef]
- Ueno, A.; Jijon, H.; Chan, R.; Ford, K.; Hirota, C.; Kaplan, G.G.; Beck, P.L.; Iacucci, M.; Gasia, M.F.; Barkema, H.W.; et al. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Di Giovangiulio, M.; Stolfi, C.; Franzè, E.; Fehling, H.-J.; Carsetti, R.; Giorda, E.; Colantoni, A.; Ortenzi, A.; Rugge, M.; et al. RORγt-Expressing Tregs Drive the Growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in Dendritic Cells. Cancer Immunol. Res. 2018, 6, 1082–1092. [Google Scholar] [CrossRef]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, S.; Grootjans, J.; Tschurtschenthaler, M.; Krupka, N.; Matute, J.D.; Flak, M.B.; Martinez-Naves, E.; Del Moral, M.G.; Glickman, J.N.; Ohira, M.; et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J. Exp. Med. 2017, 214, 2985–2997. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Cui, Y.; Wang, X.; Guo, C.; Huang, Z.; Kan, Q.; Liu, Z.; Liu, Y. Il-21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1133–1144. [Google Scholar] [CrossRef]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Bahri, R.; Pateras, I.S.; D’Orlando, O.; Goyeneche-Patino, D.A.; Campbell, M.; Polansky, J.K.; Sandig, H.; Papaioannou, M.; Evangelou, K.; Foukas, P.G.; et al. IL-15 suppresses colitis-associated colon carcinogenesis by inducing antitumor immunity. Oncoimmunology 2015, 4, e1002721. [Google Scholar] [CrossRef] [PubMed]
- Kuen, D.S.; Kim, B.S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Smyth, M.J.; Teng, M.W.L. Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer. Cold Spring Harb Perspect Biol. 2018, 10, a028530. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
| Immune Cell | Mechanism |
|---|---|
| Macrophages |
|
| |
| Dendritic Cells (DCs) |
|
| |
| Myeloid-derived suppressor cells (MDSCs) |
|
| T cells |
|
| |
| |
| Natural Killer (NK) cells |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigerio, S.; Lartey, D.A.; D’Haens, G.R.; Grootjans, J. The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms. Int. J. Mol. Sci. 2021, 22, 12739. https://doi.org/10.3390/ijms222312739
Frigerio S, Lartey DA, D’Haens GR, Grootjans J. The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms. International Journal of Molecular Sciences. 2021; 22(23):12739. https://doi.org/10.3390/ijms222312739
Chicago/Turabian StyleFrigerio, Sofía, Dalia A. Lartey, Geert R. D’Haens, and Joep Grootjans. 2021. "The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms" International Journal of Molecular Sciences 22, no. 23: 12739. https://doi.org/10.3390/ijms222312739
APA StyleFrigerio, S., Lartey, D. A., D’Haens, G. R., & Grootjans, J. (2021). The Role of the Immune System in IBD-Associated Colorectal Cancer: From Pro to Anti-Tumorigenic Mechanisms. International Journal of Molecular Sciences, 22(23), 12739. https://doi.org/10.3390/ijms222312739





