Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of Gelatin from Seabream, Seabass and Trout Skin
2.2. Characterization of Gelatin
2.2.1. Amino acid Profiling
2.2.2. Molecular Weight Distributions
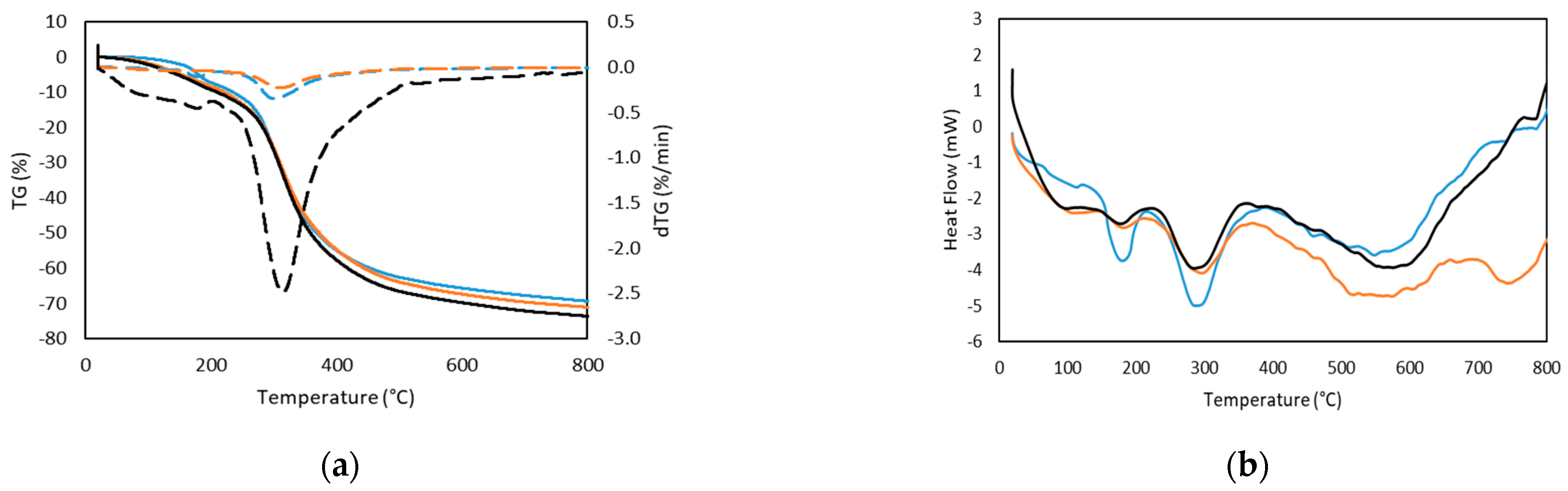
2.2.3. Thermal Stability
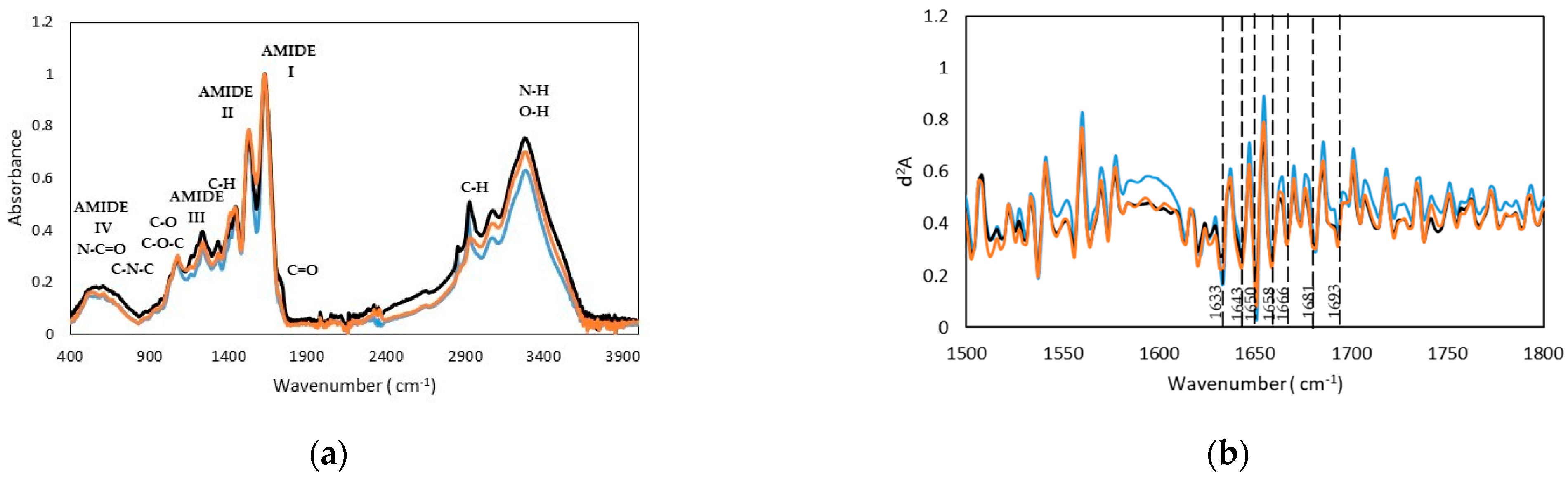
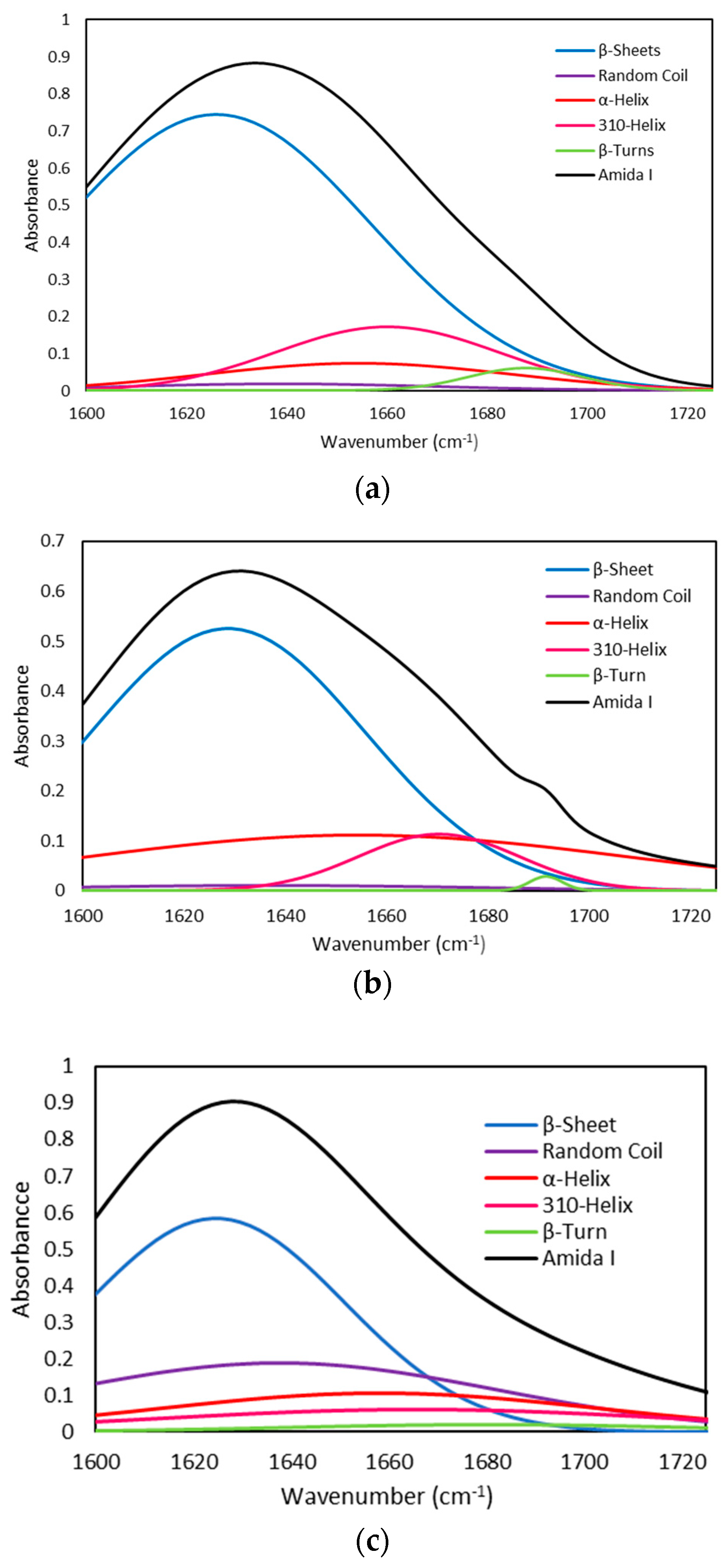
2.2.4. Fourier Transform Infrared (FTIR) Spectra
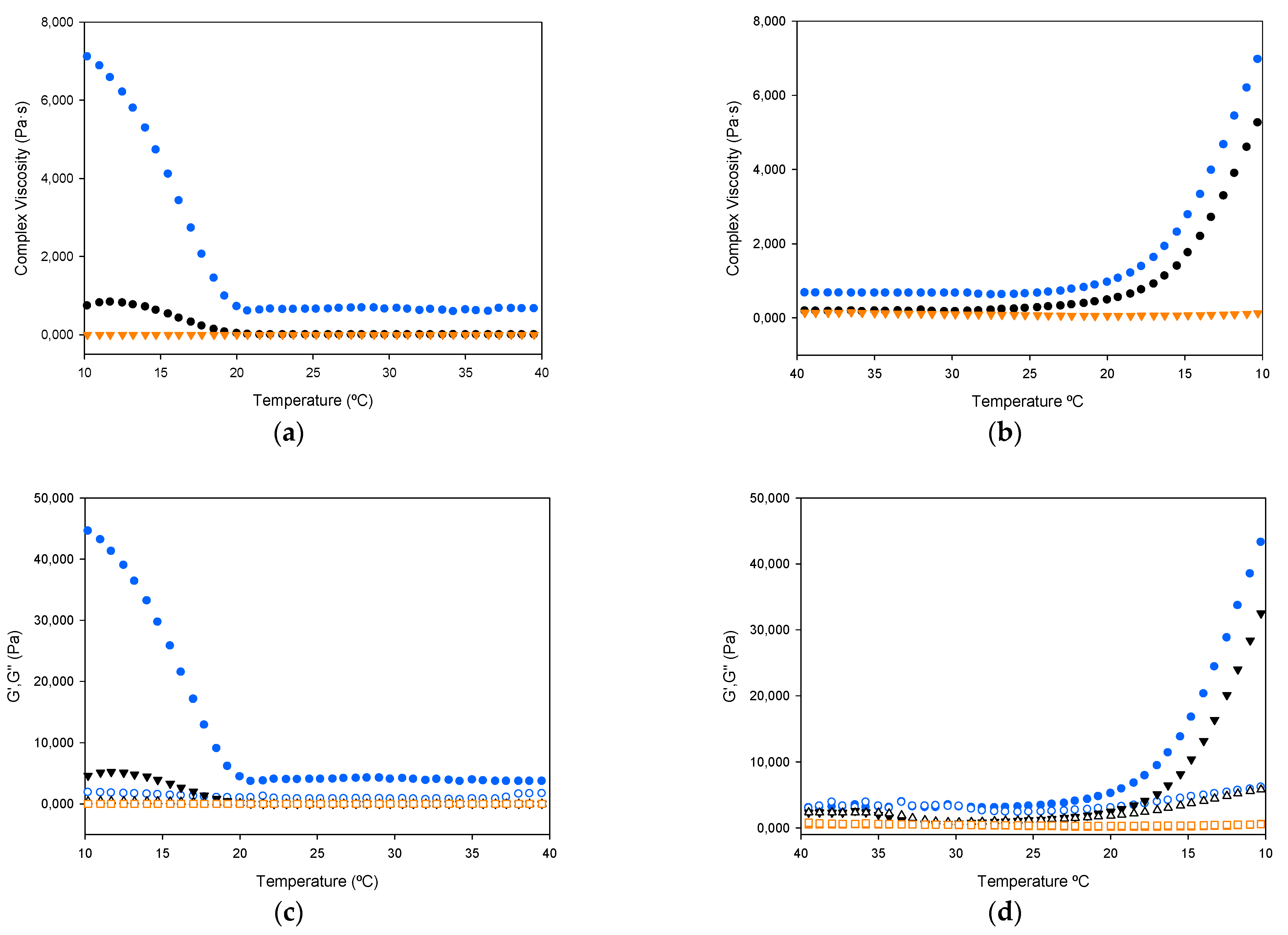
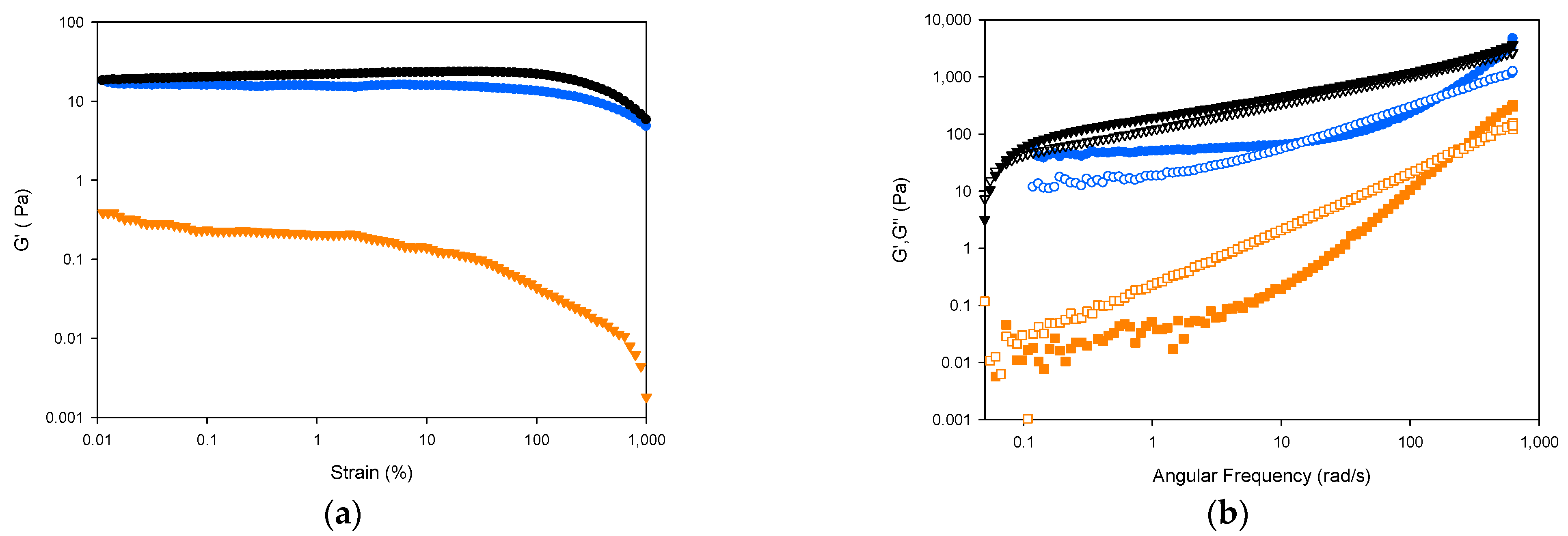
2.2.5. Rheology
3. Conclusions
4. Materials and Methods
4.1. Skin By-Products from Aquaculture Fish
4.2. Extraction of Aquaculture Gelatin
4.3. Yield and Amino acid Profile
4.4. Molecular Weight
4.5. Gel Strength
4.6. Fourier Transform Infrared (FTIR) Spectroscopy
4.7. Thermal Stability
4.8. Rheology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO GLOBEFISH Highlights April 2019 ISSUE, with January–December 2018 Statistics—A Quarterly Update on World Seafood Markets; Globefish Highlights no. 2-2019 CC BY-NC-SA 3.0 IGO 2019; FAO: Rome, Italy, 2019.
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Fraguas, J.; Mirón, J.; Valcárcel, J.; Perez-Martin, R.; Antelo, L.T. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. J. Clean. Prod. 2020, 246, 119027. [Google Scholar] [CrossRef]
- Valcarcel, J.; Fraguas, J.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Vázquez, J.A. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Mar. Drugs 2021, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Johansen, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Valcarcel, J. Characterization of Gelatin and Hydrolysates from Valorization of Farmed Salmon Skin By-Products. Polymers (Basel) 2021, 13, 2828. [Google Scholar] [CrossRef] [PubMed]
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- García-Santiago, X.; Franco-Uría, A.; Antelo, L.T.; Vázquez, J.A.; Pérez-Martín, R.; Moreira, M.T.; Feijoo, G. Eco-efficiency of a marine biorefinery for valorization of cartilaginous fish biomass. J. Ind. Ecol. 2020. [Google Scholar] [CrossRef]
- Hodar, A.R.; Vasava, R.J.; Mahavadiya, D.R.; Joshi, N.H. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Alfaro, A.D.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejska, I.; Skierka, E.; Sadowska, M.; Kołodziejski, W.; Niecikowska, C. Effect of extracting time and temperature on yield of gelatin from different fish offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S.; O’Brien, N.M.; Kishimura, H. Characteristics and functional properties of gelatin from seabass skin as influenced by defatting. Int. J. Food Sci. Technol. 2016, 51, 1204–1211. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Molecular characteristics and properties of gelatin from skin of seabass with different sizes. Int. J. Biol. Macromol. 2015, 73, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TInrAT, S.; SIlA-ASnA, M. Optimization of gelatin extraction and physico-chemical properties of fish skin and bone gelatin: Its application to Panna Cotta formulas. Curr. Res. Nutr. Food Sci. J. 2017, 5, 263–273. [Google Scholar] [CrossRef]
- Kalkan, H.; Dinçer, M.T. Comparison the effects of using different organic acids on gelatin extracted from cultured sea bream (Sparus aurata) scales Farklı organik asitlerin kullanımının kültüre edilmiş çipura (Sparus aurata) pullarından elde edilen jelatin üzerine etkilerinin ka. Ege J. Fish. Aquat. Sci. 2018, 35, 311–317. [Google Scholar]
- Tabarestani, H.S.; Maghsoudlou, Y.; Motamedzadegan, A.; Mahoonak, A.S. Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresour. Technol. 2010, 101, 6207–6214. [Google Scholar] [CrossRef] [PubMed]
- Tabarestani, H.S.; Maghsoudlou, Y.; Motamedzadegan, A.; Mahoonak, A.R.S. Effect of Pretreatment Conditions on Physicochemical Properties of Rainbow Trout Skin Gelatin. J. Aquat. Food Prod. Technol. 2014, 23, 14–24. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Kim, D.; Min, S.C. Trout Skin Gelatin-Based Edible Film Development. J. Food Sci. 2012, 77, E240–E246. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Turnay, J.; Fernández-Dı́az, M.D.; Ulmo, N.; Lizarbe, M.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Kamer, D.D.A.; Palabiyik, I.; Işık, N.O.; Akyuz, F.; Demirci, A.S.; Gumus, T. Effect of confectionery solutes on the rheological properties of fish (Oncorhynchus mykiss) gelatin. LWT 2018, 101, 499–505. [Google Scholar] [CrossRef]
- Correia, D.M.; Padrão, J.; Rodrigues, L.; Dourado, F.; Lanceros-Mendez, S.; Sencadas, V. Thermal and hydrolytic degradation of electrospun fish gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.E.O.; Sousa, J.R.; Claudino, R.L.; Lino, S.C.O.; Vale, D.A.D.; Silva, A.L.C.; Morais, J.P.S.; Filho, M.D.S.M.D.S.; De Souza, B.W.S. Thermal and Chemical Properties of Gelatin from Tilapia (Oreochromis niloticus) Scale. J. Aquat. Food Prod. Technol. 2018, 27, 1120–1133. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Protein structure by Fourier transform infrared spectroscopy: Second derivative spectra. Biochem. Biophys. Res. Commun. 1983, 115, 391–397. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.; Kolotova, D.S.; Voron’Ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Bandekar, J.; Krimm, S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of -turns. Proc. Natl. Acad. Sci. USA 1979, 76, 774–777. [Google Scholar] [CrossRef] [Green Version]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Litvinov, R.; Faizullin, D.A.; Zuev, Y.; Weisel, J.W. The α-Helix to β-Sheet Transition in Stretched and Compressed Hydrated Fibrin Clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokol, V.; Pottathara, Y.B.; Mihelčič, M.; Perše, L.S. Rheological properties of gelatine hydrogels affected by flow- and horizontally-induced cooling rates during 3D cryo-printing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126356. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT 2014, 55, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Process. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Gordon, M.B.; Kloxin, C.J.; Wagner, N.J. The rheology and microstructure of an aging thermoreversible colloidal gel. J. Rheol. 2017, 61, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Atma, Y. Synthesis and Application of Fish Gelatin for Hydrogels/Composite Hydrogels: A Review. Peptides 2021, 22, 23. [Google Scholar]
- Shi, X.-D.; Huang, J.-J.; Wu, J.-L.; Cai, X.-X.; Tian, Y.-Q.; Rao, P.-F.; Huang, J.-L.; Wang, S.-Y. Fabrication, interaction mechanism, functional properties, and applications of fish gelatin-polysaccharide composites: A review. Food Hydrocoll. 2022, 122, 107106. [Google Scholar] [CrossRef]
- Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC (Association of Official Analytical Chemistry). Methods Analysis; AOAC: Arlington, VA, USA, 1997. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulphonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar]
- Blanco, M.; Sanz, N.; Valcarcel, J.; Pérez-Martín, R.I.; Sotelo, C.G. Does Subunit Composition Influence the Intermolecular Crosslinking of Fish Collagen? A Study with Hake and Blue Shark Skin Collagens. Polymers 2020, 12, 1734. [Google Scholar] [CrossRef]
- Wainewright, F.W. Physical tests for gelatin and gelatin products. In The Science and Technology of Gelatin; Academic Press: New York, NY, USA, 1977; pp. 507–531. [Google Scholar]
- Fernández-Dı́az, M.D.; Montero, P.; Gómez-Guillén, M.C. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by the coenhancers magnesium sulphate, glycerol and transglutaminase. Food Chem. 2001, 74, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Hermida-Merino, C.; Pérez-Rodríguez, M.; Pereiro, A.B.; Piñeiro, M.M.; Pastoriza-Gallego, M.J. Tailoring Nanofluid Thermophysical Profile through Graphene Nanoplatelets Surface Functionalization. ACS Omega 2018, 3, 744–752. [Google Scholar] [CrossRef]

| Species | Yield (%, w of Gelatin/w of Skin) | Gel Strength (Bloom, g) |
|---|---|---|
| Seabream | 6.60 ± 0.46 a | 229.5 ± 12.7 a |
| Seabass | 6.83 ± 0.39 a | 181.0 ± 7.8 b |
| Trout | 1.56 ± 0.24 b | 95.0 ± 2.0 c |
| AA | Seabream | Seabass | Trout |
|---|---|---|---|
| Asp | 5.51 ± 0.15 | 5.98 ± 0.05 | 6.31 ± 0.05 |
| Thr | 2.94 ± 0.18 | 3.02 ± 0.02 | 2.75 ± 0.02 |
| Ser | 4.32 ± 0.08 | 4.58 ± 0.20 | 4.94 ± 0.09 |
| Glu | 9.46 ± 0.03 | 10.15 ± 0.12 | 10.03 ± 0.10 |
| Gly | 23.02 ± 0.38 a | 21.42 ± 0.29 b | 24.16 ± 0.26 c |
| Ala | 10.18 ± 0.02 | 10.59 ± 0.12 | 9.14 ± 0.07 |
| Cys | 0.37 ± 0.02 | 0.27 ± 0.02 | 0.41 ± 0.08 |
| Val | 2.08 ± 0.15 | 1.97 ± 0.06 | 1.81 ± 0.05 |
| Met | 2.41 ± 0.27 | 2.05 ± 0.01 | 3.09 ± 0.09 |
| Ile | 1.01 ± 0.04 | 0.99 ± 0.03 | 1.41 ± 0.02 |
| Leu | 2.52 ± 0.05 | 2.49 ± 0.07 | 2.59 ± 0.08 |
| Tyr | 0.73 ± 0.09 | 0.70 ± 0.09 | 0.70 ± 0.05 |
| Phe | 2.24 ± 0.20 | 2.41 ± 0.05 | 2.17 ± 0.04 |
| His | 0.78 ± 0.14 | 0.80 ± 0.05 | 1.04 ± 0.27 |
| Lys | 3.70 ± 0.01 | 3.93 ± 0.21 | 3.52 ± 0.13 |
| Arg | 8.33 ± 0.00 | 8.75 ± 0.18 | 7.76 ± 0.07 |
| OHPro | 9.29 ± 0.37 a | 8.34 ± 0.37 b | 8.28 ± 0.54 c |
| Pro | 11.11 ± 0.19 a | 11.56 ± 0.01 b | 9.90 ± 0.60 c |
| Pr (%) | 92.4 ± 4.4 a | 89.9 ± 4.4 ab | 84.6 ± 3.1 b |
| TE/TA (%) | 26.1 ± 0.1 a | 26.4 ± 0.6 a | 26.1 ± 0.1 a |
| Peak Number | Rt (min) | Mw (kDa) | PDI | Peak Area (%) | |
|---|---|---|---|---|---|
| Seabream | 1-high Mw | 35.2–44.02 | - | - | 17.5 ± 6.0 |
| 2 | 45.2 ± 0.1 | 164 ± 7 | 1.016 | 26.1 ± 1.8 | |
| 3 | 48.0 ± 0.1 | 112 ± 10 | 1.003 | 16.1 ± 2.4 | |
| 4-low Mw | 49.1–60.2 | <100 | - | 32.8 ± 2.0 | |
| 55.6 ± 0.1 | 43 ± 7 | 1.002 | 7.5 ± 1.4 | ||
| Seabass | 1-high Mw | 34.2–44.1 | - | - | 20.5 ± 4.2 |
| 2 | 45.2 ± 0.0 | 176 ± 2 | 1.016 | 27.0 ± 0.2 | |
| 3 | 47.9 ± 0.0 | 112 ± 2 | 1.004 | 17.1 ± 1.4 | |
| 4-low Mw | 49.1–64.22 | <100 | - | 27.3 ± 3.0 | |
| 55.7.0 ± 0.0 | 32 ± 4 | 1.001 | 8.1 ± 1.2 | ||
| Trout | 1-high Mw | 35.8–42.3 | - | - | 3.2 ± 0.8 |
| 2 | 43.8 ± 0.1 | 342 ± 49 | 1.012 | 4.9 ± 0.3 | |
| 3 | 44.8 ± 0.1 | 207 ± 12 | 1.023 | 20.3 ± 3.7 | |
| 4 | 48.1 ± 0.0 | 117 ± 5 | 1.006 | 30.2 ± 3.8 | |
| 5-low Mw | 49.1–62.5 | <100 | - | 41.4 ± 8.6 |
| Steps | T Onset (°C) | Tmax (°C) | Weight loss (%) | |
|---|---|---|---|---|
| Seabass | 1 | 158.86 | 257.48 | −8.07 |
| 2 | 180.03 | 301.34 | −61.46 | |
| Seabream | 1 | 36.44 | 88.20 | −9.46 |
| 2 | 255.67 | 315.22 | −64.62 | |
| Trout | 1 | 44.53 | 254.58 | −8.53 |
| 2 | 112.90 | 312.46 | −62.70 |
| Wavenumber (cm−1) | Assignment | ||
|---|---|---|---|
| Seabass | Seabream | Trout | |
| 3284 | 3277 | 3272 | N-H/O-H |
| 2850–3000 | 2850–3000 | 2850–3000 | Aliphatic Tension CH |
| 1333, 1444 | 1334, 1445 | 1338, 1454 | Aliphatic Flexion CH |
| 1632, 1525, 1236 | 1631, 1530, 1235 | 1628, 1529, 1236 | Amide I,II,III |
| 973 | 974 | - | CNC |
| 521, 601, 706 | 560, 603, 702 | 532, 607, 704 | Amide IV,V,VI |
| 1162–1077 | 1172, 1079, 1034 | 1161, 1078, 1032 | Bond Tension C-O, C-O-C |
| 600 | 600 | 600 | Water |
| - | - | 1720, 1737 | Lipid C=O |
| Gelatin Type | Peak center (cm−1) | Area % | Height | FWHM | Assignment |
|---|---|---|---|---|---|
| Seabass | 1629 | 57.35 | 0.74 | 72.42 | β-Sheet |
| 1639 | 1.40 | 0.02 | 73.00 | Random Coil | |
| 1654 | 5.69 | 0.07 | 72.2 | α-Helix | |
| 1660 | 9.23 | 0.17 | 52.35 | 310-Helix | |
| 1687 | 1.77 | 0.06 | 26.62 | β-turn | |
| Seabream | 1628 | 35.60 | 0.53 | 63.60 | β-Sheet |
| 1636 | 1.13 | 0.01 | 105.80 | Random Coil | |
| 1654 | 14.99 | 0.11 | 127.28 | α-Helix | |
| 1669 | 4.52 | 0.11 | 37.19 | 310-Helix | |
| 1689 | 0.23 | 0.03 | 7.54 | β-turn | |
| Trout | 1624 | 38.51 | 0.58 | 62.06 | β-Sheet |
| 1637 | 20.63 | 0.18 | 105.49 | Random Coil | |
| 1658 | 10.67 | 0.09 | 106.6 | α-Helix | |
| 1666 | 11.69 | 0.09 | 124.8 | 310-Helix | |
| 1681 | 2.15 | 0.02 | 96.2 | β-turn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valcarcel, J.; Hermida-Merino, C.; Piñeiro, M.M.; Hermida-Merino, D.; Vázquez, J.A. Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture. Int. J. Mol. Sci. 2021, 22, 12104. https://doi.org/10.3390/ijms222212104
Valcarcel J, Hermida-Merino C, Piñeiro MM, Hermida-Merino D, Vázquez JA. Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture. International Journal of Molecular Sciences. 2021; 22(22):12104. https://doi.org/10.3390/ijms222212104
Chicago/Turabian StyleValcarcel, Jesus, Carolina Hermida-Merino, Manuel M. Piñeiro, Daniel Hermida-Merino, and José Antonio Vázquez. 2021. "Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture" International Journal of Molecular Sciences 22, no. 22: 12104. https://doi.org/10.3390/ijms222212104
APA StyleValcarcel, J., Hermida-Merino, C., Piñeiro, M. M., Hermida-Merino, D., & Vázquez, J. A. (2021). Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture. International Journal of Molecular Sciences, 22(22), 12104. https://doi.org/10.3390/ijms222212104










