Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats
Abstract
:1. Introduction
2. Results
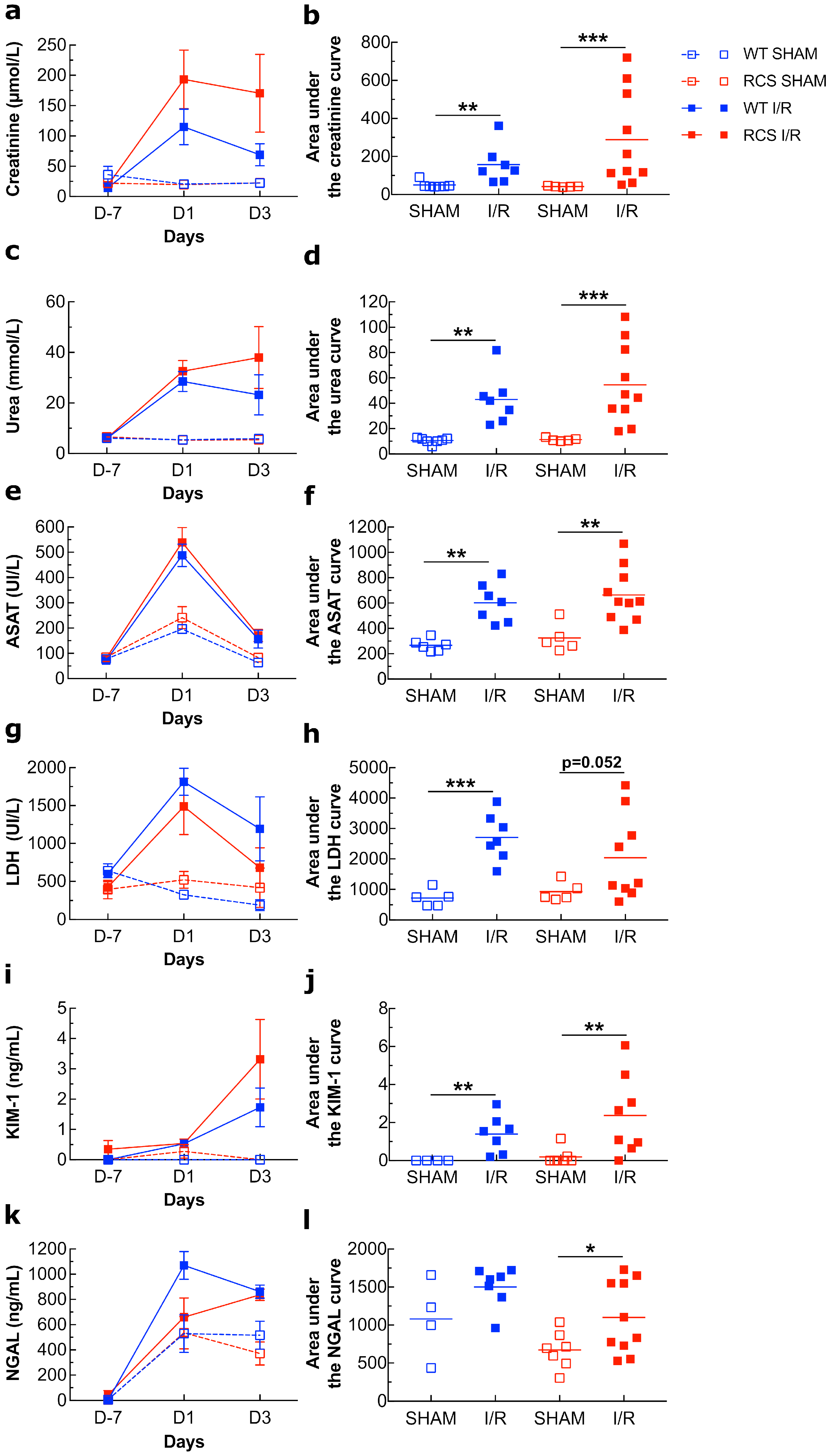
2.1. Renal I/R Induces an Increase in Several Plasma Biomarkers of Injury in Both WT and RCS Rats
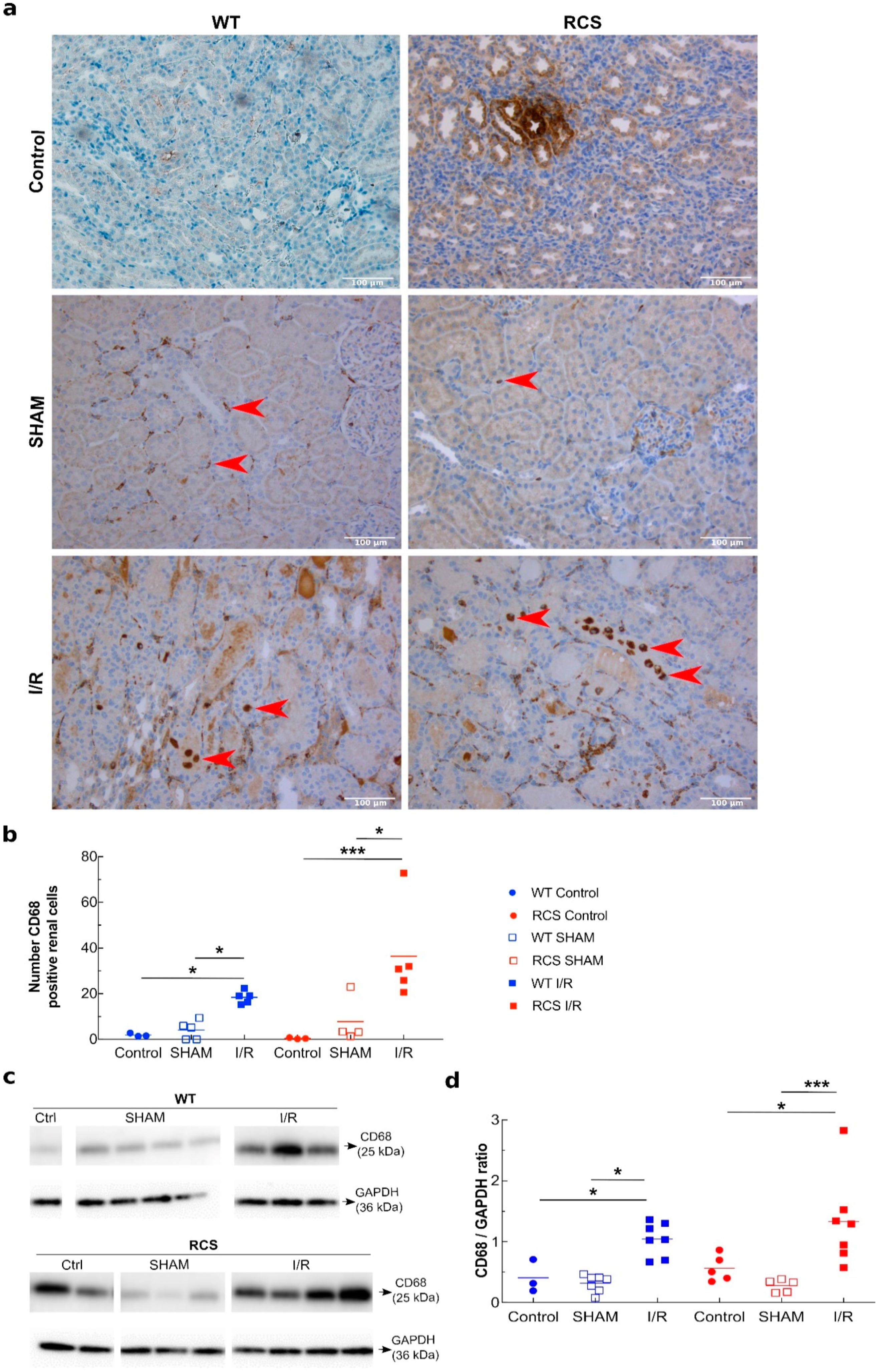
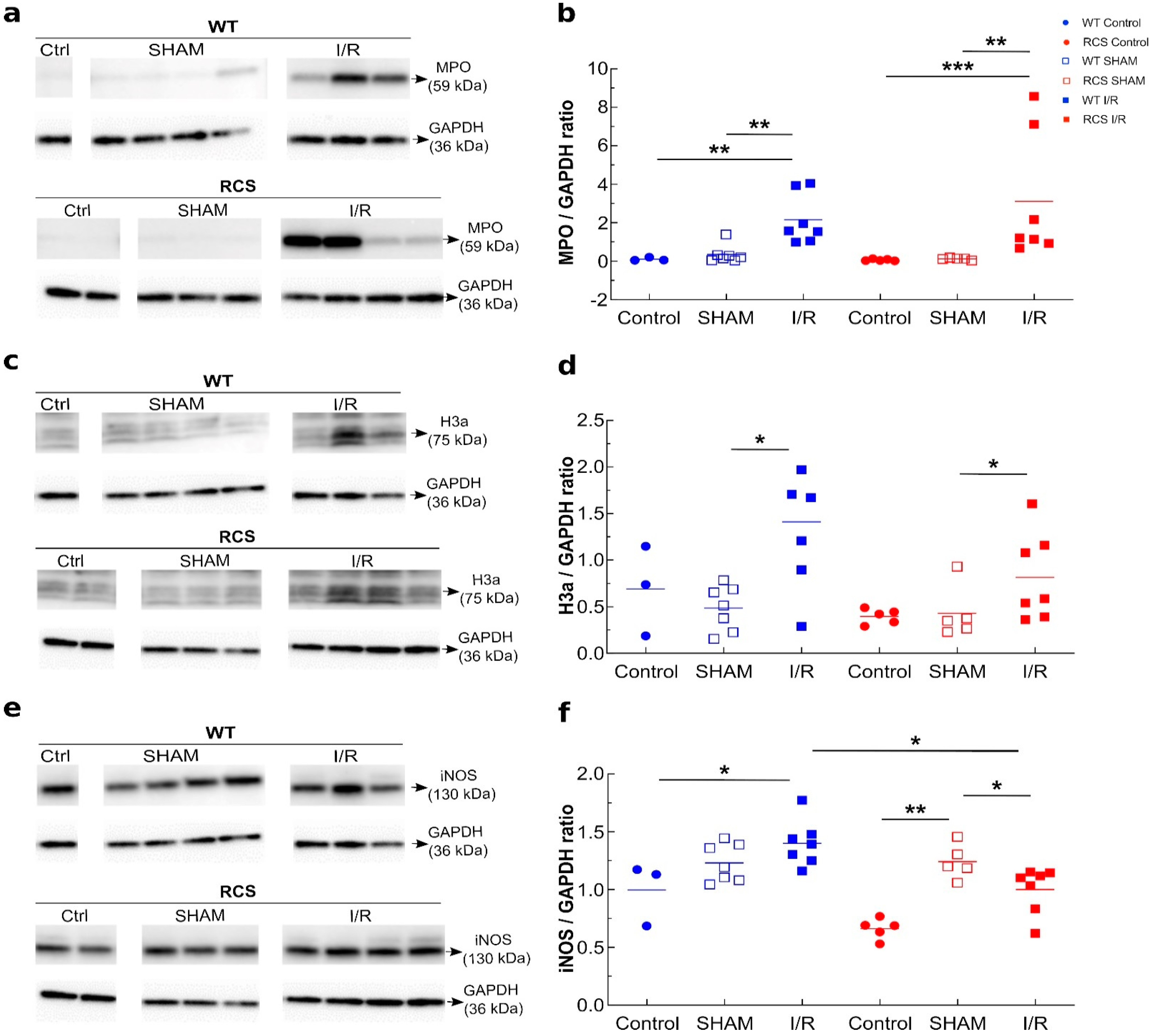
2.2. Renal I/R Sequence Triggers Multiple Inflammatory Responses in Both RCS and WT Rats
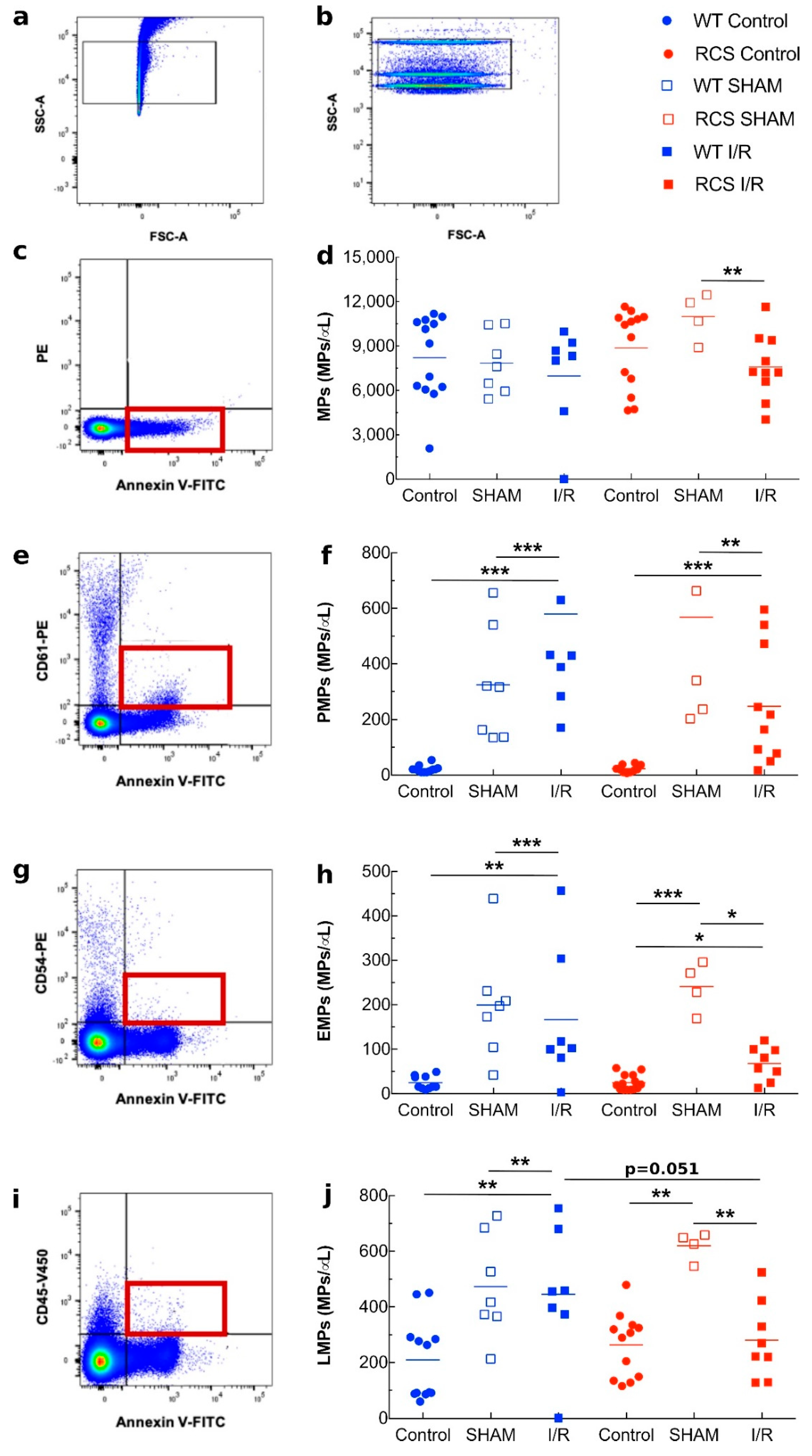
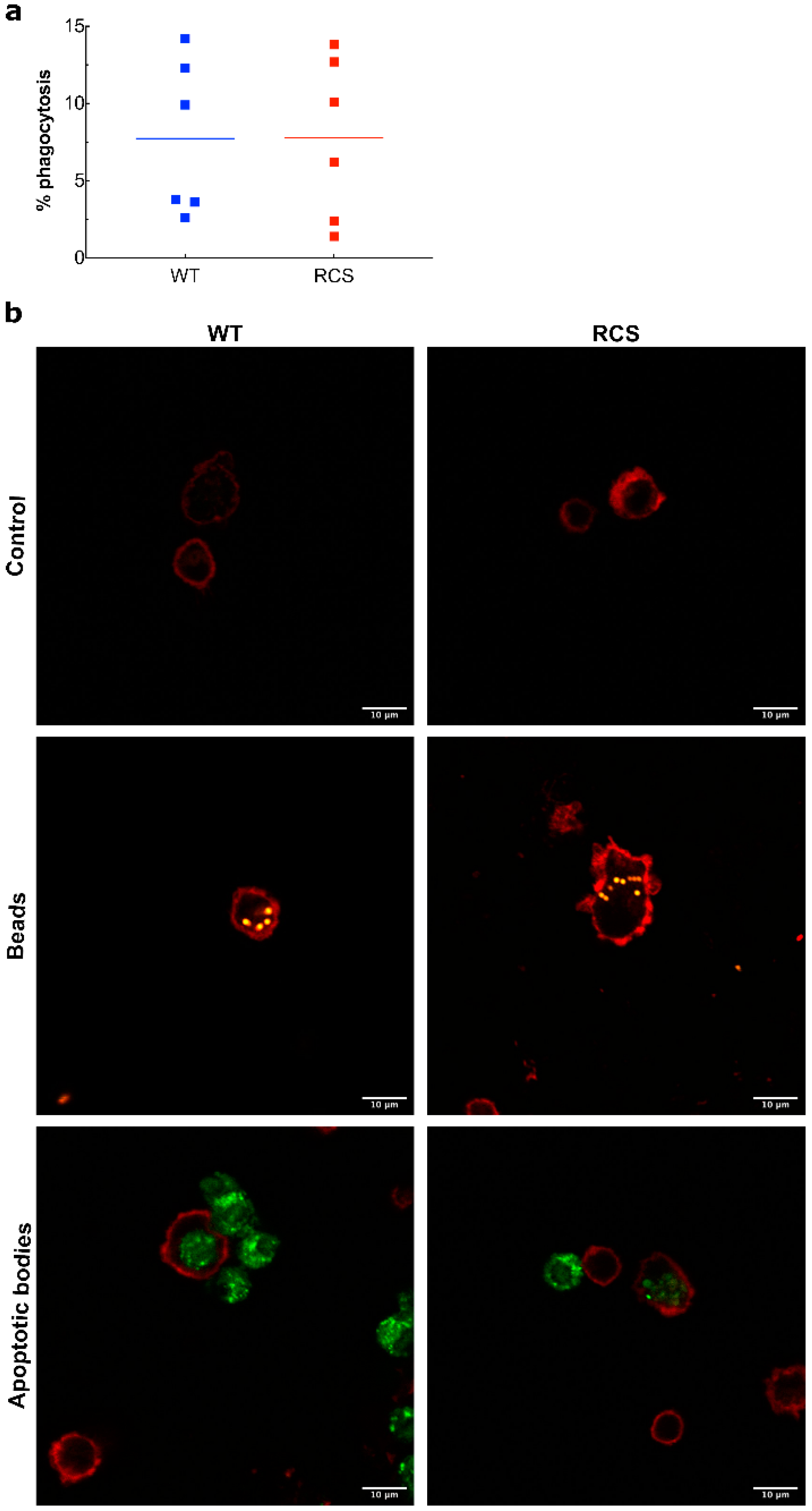
2.3. Effects of Renal I/R on MP Levels and Monocyte Phagocytic Activity in WT and RCS Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ischemia-Reperfusion Bilateral Kidney Injury
4.3. Soluble Biomarker Evaluation
4.4. Western Blot Analyses
4.5. Identification/Quantification MPs
4.6. Histopathology Analyses
4.7. Phagocytosis Test In Vitro
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM-17 | ADAM metallopeptidase domain 17 |
| ASAT | Aspartate aminotransferase |
| BSA | Bovine serum albumin |
| DAMPs | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| EMPs | Endothelial-derived microparticles |
| FACS | Fluorescence-activated single cell sorting |
| H3A | Histone 3A |
| IL | Interleukin |
| iNOS | inducible nitric oxide synthase |
| I/R | Ischemia-reperfusion |
| KIM-1 | Kidney injury molecule -1 |
| LDH | Lactate dehydrogenase |
| LMPs | Leukocyte-derived microparticles |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MPs | Microparticles |
| MPO | Myeloperoxidase |
| NET | Neutrophil extracellular traps |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NO | Nitric oxide |
| PAS | Periodic-acid-Schiff |
| PMPs | Platelet-derived microparticles |
| PPP | Platelet-poor plasma |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| RCS | Royal College of Surgeons |
| TAM | Tyro3, Axl and MerTK family |
| TLR | Toll-like receptors |
| TNFα | Tumor necrosis factor-α |
References
- Chiang-Ting, C.; Tzu-Ching, C.; Ching-Yi, T.; Song-Kuen, S.; Ming-Kuen, L. Adenovirus-Mediated Bcl-2 Gene Transfer Inhibits Renal Ischemia/Reperfusion Induced Tubular Oxidative Stress and Apoptosis. Am. J. Transplant. 2005, 5, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favreau, F.; Giraud, S.; Bon, D.; Chatauret, N.; Thuillier, R.; Hauet, T. Ischemia reperfusion control: The key of kidney graft outcome. Med. Sci. 2013, 29, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated Necrosis in Kidney Ischemia-Reperfusion Injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Wiegele, G.; Brandis, M.; Zimmerhackl, L.B. Apoptosis and Necrosis during Ischaemia in Renal Tubular Cells (LLC-PK1 and MDCK). Nephrol. Dial. Transplant. 1998, 13, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkonda, M.N.; Akbari, S.; Landry, C.; Sun, S.; Xiao, F.; Turner, M.; Holterman, C.E.; Nasrallah, R.; Hébert, R.L.; Kennedy, C.R.J.; et al. Podocyte-Derived Microparticles Promote Proximal Tubule Fibrotic Signaling via P38 MAPK and CD36. J. Extracell. Vesicles 2018, 7, 1432206. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, Z. Neutrophil Extracellular Traps in Ischemic AKI: New Way to Kill. Kidney Int. 2018, 93, 303–305. [Google Scholar] [CrossRef]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; et al. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. J. Am. Soc. Nephrol. 2017, 28, 1753–1768. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Huen, S.C.; Cantley, L.G. Macrophage-Mediated Injury and Repair after Ischemic Kidney Injury. Pediatric Nephrol. 2015, 30, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Huen, S.C.; Cantley, L.G. Macrophages in Renal Injury and Repair. Annu. Rev. Physiol. 2017, 79, 449–469. [Google Scholar] [CrossRef]
- Škrajnar, Š.; Lasnik, M.A.; Zavec, A.B. A Flow Cytometric Method for Determination of the Blood Neutrophil Fraction in Rats. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 152–156. [Google Scholar] [PubMed]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Svenungsson, E.; Pisetsky, D.S. Microparticles as Autoantigens in Systemic Lupus Erythematosus. Eur. J. Clin. Investig. 2018, 48, e13010. [Google Scholar] [CrossRef] [PubMed]
- Vallier, L.; Cointe, S.; Lacroix, R.; Bonifay, A.; Judicone, C.; Dignat-George, F.; Kwaan, H.C. Microparticles and Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 129–134. [Google Scholar] [CrossRef]
- Mohning, M.P.; Thomas, S.M.; Barthel, L.; Mould, K.J.; McCubbrey, A.L.; Frasch, S.C.; Bratton, D.; Henson, P.M.; Janssen, W.J. Phagocytosis of Microparticles by Alveolar Macrophages during Acute Lung Injury Requires MerTK. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 314, L69–L82. [Google Scholar] [CrossRef]
- Freeman, C.M.; Quillin, R.C.; Wilson, G.C.; Nojima, H.; Johnson, B.L.; Sutton, J.M.; Schuster, R.M.; Blanchard, J.; Edwards, M.J.; Caldwell, C.C.; et al. Characterization of Microparticles after Hepatic Ischemia-Reperfusion Injury. PLoS ONE 2014, 9, e97945. [Google Scholar] [CrossRef]
- Shao, W.-H.; Zhen, Y.; Rosenbaum, J.; Eisenberg, R.A.; McGaha, T.L.; Birkenbach, M.; Cohen, P.L. A Protective Role of Mer Receptor Tyrosine Kinase in Nephrotoxic Serum-Induced Nephritis. Clin. Immunol. 2010, 136, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Fraineau, S.; Monvoisin, A.; Clarhaut, J.; Talbot, J.; Simonneau, C.; Kanthou, C.; Kanse, S.M.; Philippe, M.; Benzakour, O. The Vitamin K-Dependent Anticoagulant Factor, Protein S, Inhibits Multiple VEGF-A-Induced Angiogenesis Events in a Mer- and SHP2-Dependent Manner. Blood 2012, 120, 5073–5083. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Perani, D.; Comi, C. TAM Receptor Pathways at the Crossroads of Neuroinflammation and Neurodegeneration. Dis. Markers 2019, 2019, 2387614. [Google Scholar] [CrossRef] [PubMed]
- Ginisty, A.; Gély-Pernot, A.; Abaamrane, L.; Morel, F.; Arnault, P.; Coronas, V.; Benzakour, O. Evidence for a Subventricular Zone Neural Stem Cell Phagocytic Activity Stimulated by the Vitamin K-Dependent Factor Protein S: Phagocytic Activity of Neural Stem Cells. Stem Cells 2015, 33, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Barth, N.D.; Marwick, J.A.; Heeb, M.J.; Gale, A.J.; Rossi, A.G.; Dransfield, I. Augmentation of Human Monocyte Responses to Lipopolysaccharide by the Protein S and Mer/Tyro3 Receptor Tyrosine Kinase Axis. J. Immunol. 2018, 201, 2602–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, D.; Schock, S.C.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and Beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P.L.; Shao, W.-H. Gas6/TAM Receptors in Systemic Lupus Erythematosus. Available online: https://www.hindawi.com/journals/dm/2019/7838195/ (accessed on 30 September 2019).
- Cohen, P.L.; Caricchio, R.; Abraham, V.; Camenisch, T.D.; Jennette, J.C.; Roubey, R.A.S.; Earp, H.S.; Matsushima, G.; Reap, E.A. Delayed Apoptotic Cell Clearance and Lupus-like Autoimmunity in Mice Lacking the c-Mer Membrane Tyrosine Kinase. J. Exp. Med. 2002, 196, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Gore, M.; Zhang, Q.; Camenisch, T.; Boast, S.; Casagranda, F.; Lai, C.; Skinner, M.K.; Klein, R.; Matsushima, G.K.; et al. Tyro-3 Family Receptors Are Essential Regulators of Mammalian Spermatogenesis. Nature 1999, 398, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; LaVail, M.M.; Yasumura, D.; Matthes, M.T.; Yang, H.; Trautmann, N.; Chappelow, A.V.; Feng, W.; Earp, H.S.; Matsushima, G.K.; et al. An RCS-like Retinal Dystrophy Phenotype in Mer Knockout Mice. Investig. Opthalmol. Vis. Sci. 2003, 44, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camenisch, T.D.; Koller, B.H.; Earp, H.S.; Matsushima, G.K. A Novel Receptor Tyrosine Kinase, Mer, Inhibits TNF-Alpha Production and Lipopolysaccharide-Induced Endotoxic Shock. J. Immunol. 1999, 162, 3498–3503. [Google Scholar] [PubMed]
- Nandrot, E.F.; Dufour, E.M. Mertk in Daily Retinal Phagocytosis: A History in the Making. Adv. Exp. Med. Biol. 2010, 664, 133–140. [Google Scholar] [CrossRef]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the Receptor Tyrosine Kinase Gene Mertk in the Retinal Dystrophic RCS Rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [Green Version]
- LaVail, M.M.; Battelle, B.A. Influence of Eye Pigmentation and Light Deprivation on Inherited Retinal Dystrophy in the Rat. Exp. Eye Res. 1975, 21, 167–192. [Google Scholar] [CrossRef]
- Nandrot, E.; Dufour, E.M.; Provost, A.C.; Péquignot, M.O.; Bonnel, S.; Gogat, K.; Marchant, D.; Rouillac, C.; de Condé, B.S.; Bihoreau, M.T.; et al. Homozygous Deletion in the Coding Sequence of the C-Mer Gene in RCS Rats Unravels General Mechanisms of Physiological Cell Adhesion and Apoptosis. Neurobiol. Dis. 2000, 7, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Parinot, C.; Nandrot, E.F. A Comprehensive Review of Mutations in the MERTK Proto-Oncogene. Adv. Exp. Med. Biol. 2016, 854, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, I.; Zagórska, A.; Lew, E.D.; Michail, K.; Lemke, G. Mer Receptor Tyrosine Kinase Mediates Both Tethering and Phagocytosis of Apoptotic Cells. Cell Death Dis. 2015, 6, e1646. [Google Scholar] [CrossRef]
- Zhen, Y.; Finkelman, F.D.; Shao, W.-H. Mechanism of Mer Receptor Tyrosine Kinase Inhibition of Glomerular Endothelial Cell Inflammation. J. Leukoc. Biol. 2018, 103, 709–717. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of Apoptotic Cells in Homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zager, R.A.; Johnson, A.C.M.; Becker, K. Renal Cortical Lactate Dehydrogenase: A Useful, Accurate, Quantitative Marker of In Vivo Tubular Injury and Acute Renal Failure. PLoS ONE 2013, 8, e66776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, A.M.; Bonventre, J.V. Cell Biology and Molecular Mechanisms of Injury in Ischemic Acute Renal Failure. Curr. Opin. Nephrol. Hypertens. 2000, 9, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sung, F.L.; Zhu, T.Y.; Au-Yeung, K.K.W.; Siow, Y.L.; O, K. Enhanced MCP-1 Expression during Ischemia/Reperfusion Injury Is Mediated by Oxidative Stress and NF-KappaB. Kidney Int. 2002, 62, 1160–1170. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of Inflammation in Acute Kidney Injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional Regulation of Macrophage Polarization: Enabling Diversity with Identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, K.K.; Meldrum, D.R.; Meng, X.; Ao, L.; Harken, A.H. TNF-Alpha-Dependent Bilateral Renal Injury Is Induced by Unilateral Renal Ischemia-Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H540–H546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, C.-C.; Song, S.-M.; Wei, S.-Y.; Li, J.-S.; Zhao, S.-L.; Li, B. The Administration of Erythropoietin Attenuates Kidney Injury Induced by Ischemia/Reperfusion with Increased Activation of Wnt/β-Catenin Signaling. J. Formos. Med. Assoc. 2015, 114, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Okusa, M.D. Macrophages, Dendritic Cells, and Kidney Ischemia-Reperfusion Injury. Semin. Nephrol. 2010, 30, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Kwon, K.; Tsitrin, T.; Bekele, S.; Sikorski, P.; Nelson, K.E.; Pieper, R. Characterization of Early-Phase Neutrophil Extracellular Traps in Urinary Tract Infections. PLoS Pathog. 2017, 13, e1006151. [Google Scholar] [CrossRef]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of Circulating Endothelial Microparticles in Patients with Chronic Renal Failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef]
- Teoh, N.C.; Ajamieh, H.; Wong, H.J.; Croft, K.; Mori, T.; Allison, A.C.; Farrell, G.C. Microparticles Mediate Hepatic Ischemia-Reperfusion Injury and Are the Targets of Diannexin (ASP8597). PLoS ONE 2014, 9, e104376. [Google Scholar] [CrossRef] [Green Version]
- Seitz, H.M.; Camenisch, T.D.; Lemke, G.; Earp, H.S.; Matsushima, G.K. Macrophages and Dendritic Cells Use Different Axl/Mertk/Tyro3 Receptors in Clearance of Apoptotic Cells. J. Immunol. 2007, 178, 5635–5642. [Google Scholar] [CrossRef]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Earp, H.S.; Matsushima, G.K. Phagocytosis and Clearance of Apoptotic Cells Is Mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef]
- Myers, K.V.; Amend, S.R.; Pienta, K.J. Targeting Tyro3, Axl and MerTK (TAM Receptors): Implications for Macrophages in the Tumor Microenvironment. Mol. Cancer 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanuth, E.; Breyer, J. Experiences with a Statistical Quality Control System (QCS) for Coagulation Diagnostics Parameters. Folia Haematol. 1988, 115, 539–545. [Google Scholar]
- Grabiec, A.M.; Goenka, A.; Fife, M.E.; Fujimori, T.; Hussell, T. Axl and MerTK Receptor Tyrosine Kinases Maintain Human Macrophage Efferocytic Capacity in the Presence of Viral Triggers. Eur. J. Immunol. 2018, 48, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.-S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Rothlin, C.V.; Ghosh, S.; Zuniga, E.I.; Oldstone, M.B.A.; Lemke, G. TAM Receptors Are Pleiotropic Inhibitors of the Innate Immune Response. Cell 2007, 131, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Sen, P.; Wallet, M.A.; Yi, Z.; Huang, Y.; Henderson, M.; Mathews, C.E.; Earp, H.S.; Matsushima, G.; Baldwin, A.S.; Tisch, R.M. Apoptotic Cells Induce Mer Tyrosine Kinase-Dependent Blockade of NF-KappaB Activation in Dendritic Cells. Blood 2007, 109, 653–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Han, J.-Y.; Byun, J.; Park, H.-J.; Park, E.-M.; Chong, Y.H.; Cho, M.-S.; Kang, J.L. Inhibiting Mer Receptor Tyrosine Kinase Suppresses STAT1, SOCS1/3, and NF-ΚB Activation and Enhances Inflammatory Responses in Lipopolysaccharide-Induced Acute Lung Injury. J. Leuko. Biol. 2012, 91, 921–932. [Google Scholar] [CrossRef]
- Zhen, Y.; Priest, S.O.; Shao, W.-H. Opposing Roles of Tyrosine Kinase Receptors Mer and Axl Determine Clinical Outcomes in Experimental Immune-Mediated Nephritis. J. Immunol. 2016, 197, 2187–2194. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; He, L.; Cao, Z.; Xiang, B.; Liu, L. Effect of Ischemia Preconditioning on Renal Ischemia/Reperfusion Injury in Rats. Int. Braz. J. Urol. 2012, 38, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-F.; Yip, H.-K.; Zhen, Y.-Y.; Lee, C.-C.; Lee, C.-C.; Huang, S.-J.; Huang, C.-C.; Ng, S.-H.; Lin, J.-W. Severe Bilateral Ischemic-Reperfusion Renal Injury: Hyperacute and Acute Changes in Apparent Diffusion Coefficient, T1, and T2 Mapping with Immunohistochemical Correlations. Sci. Rep. 2017, 7, 1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocoglu, H.; Ozturk, H.; Ozturk, H.; Yilmaz, F.; Gulcu, N. Effect of Dexmedetomidine on Ischemia-Reperfusion Injury in Rat Kidney: A Histopathologic Study. Ren. Fail. 2009, 31, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Goujon, J.M.; Hauet, T.; Menet, E.; Levillain, P.; Babin, P.; Carretier, M. Histological Evaluation of Proximal Tubule Cell Injury in Isolated Perfused Pig Kidneys Exposed to Cold Ischemia. J. Surg. Res. 1999, 82, 228–233. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelé, T.; Giraud, S.; Joffrion, S.; Ameteau, V.; Delwail, A.; Goujon, J.-M.; Macchi, L.; Hauet, T.; Dkhissi, F.; Benzakour, O. Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats. Int. J. Mol. Sci. 2021, 22, 12103. https://doi.org/10.3390/ijms222212103
Pelé T, Giraud S, Joffrion S, Ameteau V, Delwail A, Goujon J-M, Macchi L, Hauet T, Dkhissi F, Benzakour O. Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats. International Journal of Molecular Sciences. 2021; 22(22):12103. https://doi.org/10.3390/ijms222212103
Chicago/Turabian StylePelé, Thomas, Sebastien Giraud, Sandrine Joffrion, Virginie Ameteau, Adriana Delwail, Jean-Michel Goujon, Laurent Macchi, Thierry Hauet, Fatima Dkhissi, and Omar Benzakour. 2021. "Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats" International Journal of Molecular Sciences 22, no. 22: 12103. https://doi.org/10.3390/ijms222212103





