Abstract
Functions of selenium are diverse as antioxidant, anti-inflammation, increased immunity, reduced cancer incidence, blocking tumor invasion and metastasis, and further clinical application as treatment with radiation and chemotherapy. These functions of selenium are mostly related to oxidation and reduction mechanisms of selenium metabolites. Hydrogen selenide from selenite, and methylselenol (MSeH) from Se-methylselenocyteine (MSeC) and methylseleninicacid (MSeA) are the most reactive metabolites produced reactive oxygen species (ROS); furthermore, these metabolites may involve in oxidizing sulfhydryl groups, including glutathione. Selenite also reacted with glutathione and produces hydrogen selenide via selenodiglutathione (SeDG), which induces cytotoxicity as cell apoptosis, ROS production, DNA damage, and adenosine-methionine methylation in the cellular nucleus. However, a more pronounced effect was shown in the subsequent treatment of sodium selenite with chemotherapy and radiation therapy. High doses of sodium selenite were effective to increase radiation therapy and chemotherapy, and further to reduce radiation side effects and drug resistance. In our study, advanced cancer patients can tolerate until 5000 μg of sodium selenite in combination with radiation and chemotherapy since the half-life of sodium selenite may be relatively short, and, further, selenium may accumulates more in cancer cells than that of normal cells, which may be toxic to the cancer cells. Further clinical studies of high amount sodium selenite are required to treat advanced cancer patients.
1. Introduction
Selenium (Se) is an essential trace element for animals and human, and it has several important functions, such as cancer, antioxidant, increasing immunity, and antiviral activity. Se compounds have emerged as nutritional supplements with the most consistent anticancer effects among a number of micronutrients treated in animal experiments and further clinical trials in human [1]. Se deficiency is related to the occurrence of chronic disease, including certain tumor in human [2,3,4]. Cancer is a leading cause of death for people in the world. In 2020, more than 19 million new incidences occurred, and nearly 10 million cancer death were account for, estimated by WHO. Most of cancer death is mainly related to tumor metastasis.
A large body of evidence indicates that Se is physiologically important to prevent cancer incidence and block tumor metastasis. Se was first mentioned as possibly protective against cancer risk almost 60 years ago [1]. Epidemiological studies have been shown that a population with low Se intake and low plasma Se levels have an increased incidence of cancer, including cancer of breast, lung, stomach, bladder, ovaries, pancreas, thyroid, esophagus, head and neck, cerebellum, and melanoma [5,6,7,8,9,10,11,12]. Most of these studies have found Se status to be inversely associated with cancer risk. We would like to emphasize here that higher amount of sodium selenite (super nutritional levels) could block cancer incidence, tumor invasion, and metastasis, and, further, high-level of sodium selenite can be used to adjuvant therapy of radiation and anticancer drugs in patients with advanced cancer [13,14]. Our clinical treatment to advanced cancer or terminal cancer patients are tolerable to 5 mg or more doses of sodium selenite. In the clinical trials, a high dose of Se returns to almost a normal level in serum less than 260 μg/mL 24 h after injection. We postulate that relatively short half-life of Se and more absorption of sodium selenite in cancer cells would be more become toxic to cancer cells and therefore pose less toxicity to normal cells. It seems to be sodium selenite an excellent drug to treat cancer patients. However, further studies are required to define the most therapeutic doses of sodium selenite in each cancer patients in situation of anticancer drugs, radiation, drug resistance, stages of cancer, and, further, side effects of the treatment.
As mentioned, in organic Se compounds, such as MSeC, its metabolite, methylselenol, can generate radicals to induce apoptosis and blocking tumor invasion. Sodium selenite is also oxidants as accepting electrons without involving oxygen atoms. A simplified mechanism is disulfide isomerase (PDI), which can convert to inactive disulfide form according to following formula, as shown in the prevention of corona virus infections, which was hypothesized by Kieliszek and Lipinski (PDI-(SH)2 + Se4+ -> PDI-SS-PDI + Se2+) [15]. The hypothesis can apply to sodium selenite and MSeC, which have been implicated as an important factor in aging, antiviral activities, and aging related diseases, especially cancer, and, further, subsequent treatment of high doses of sodium selenite with radiation and chemotherapy has been performed to advanced cancer patients at several Institutions.
2. Metabolism of Selenium Compounds
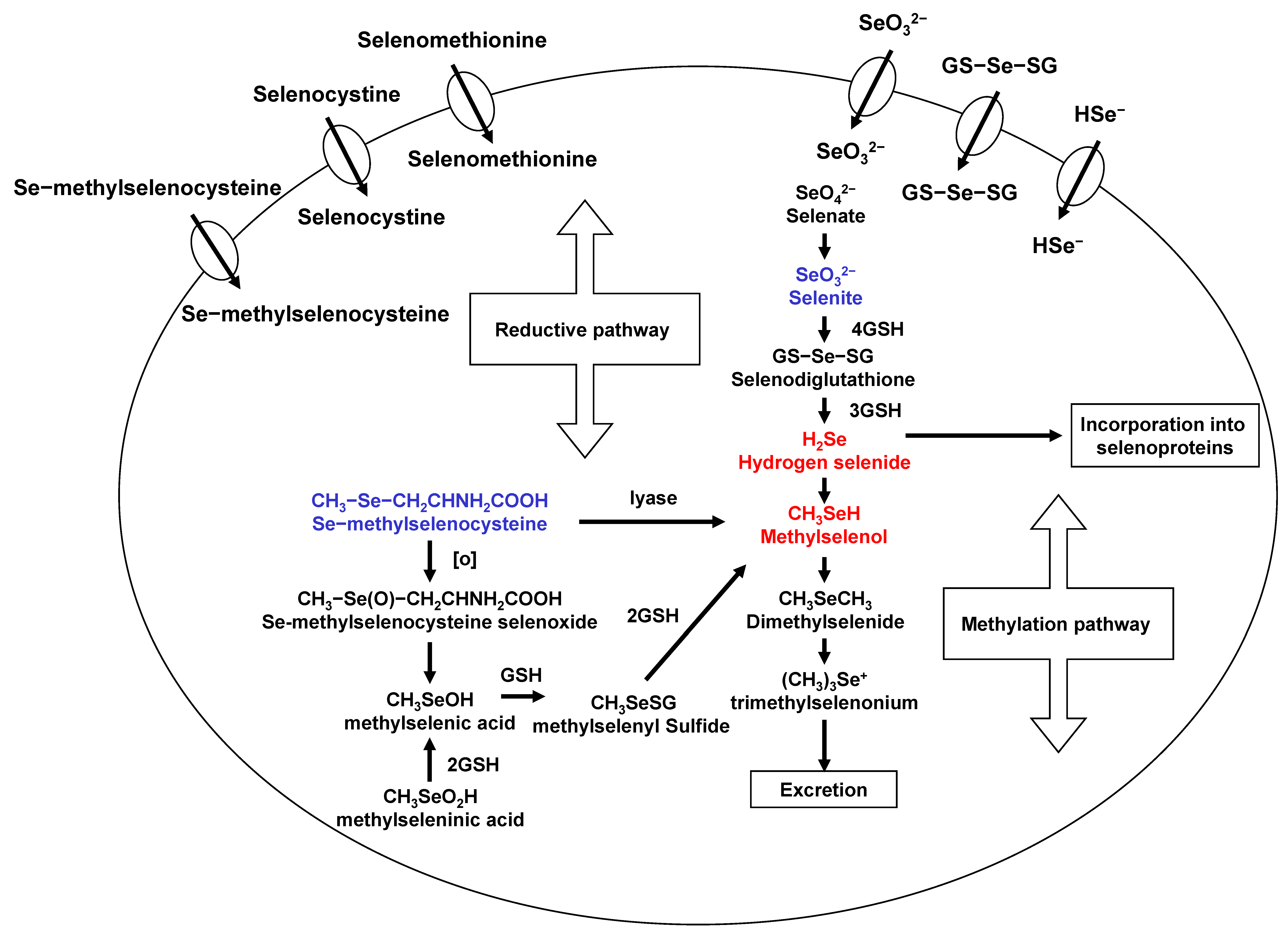
Se exists in both organic and inorgarnic forms in natural diets. Organic Se is presented in the form of selenometathionine (SeM), selenocyteine, and MSeC, whereas inorganic forms occurs either as selenite or selenate. Among organic forms, SeM is the predominant forms of Se in Se-enriched yeast [16]. Se-methylselenocysteine (MSeC) is most abundant in garlic, broccoli, walnut, and some other plant products. Selenite, an inorganic form, has been used for laboratory and clinical trials, even though it has some toxicity. The various degree antitumor activity of different chemical forms of Se may be related to their metabolism in vivo. A flowchart describing the events in Se metabolism is presented in Figure 1. Se is important due to its localization within the active site of some selenoproteins with several functions and, further, the maintenance of redox balance in cells. Most Se compounds are readily absorbed from the diet and are mainly metabolized in the liver. Hydrogen selenide from selenite and MSeH from MSeC are important metabolites related to antitumor activities. Selenomethionine gets incorporated into the general body proteins as methionine. Therefore, it is less effective compared to MSeC and selenite. Metabolism of selenite is tightly regulated and is reduced to selenide. This selenide and SeDG serve as a precursor for the synthesis of selenoproteins, such as GPx and TrxR. Moreover, Se can be methylated mono-, di-, and tri-methyl forms of Se. This methylation is reversible in vivo. However, MSeC is directly converted to MSeH by β-lyase. In our studies, MSeC is the most effective Se compound to induce apoptosis, as well as block tumor invasion and metastasis, in cancer cells [17,18,19]. More studies on MSeC in vivo are required to measure pharmacokinetic of this compound and to determine half-life and maximum tolerable doses of MSeC. This compound is commercially available now. Even further, it would be interesting to see clinical application of this compound in cancer patients.
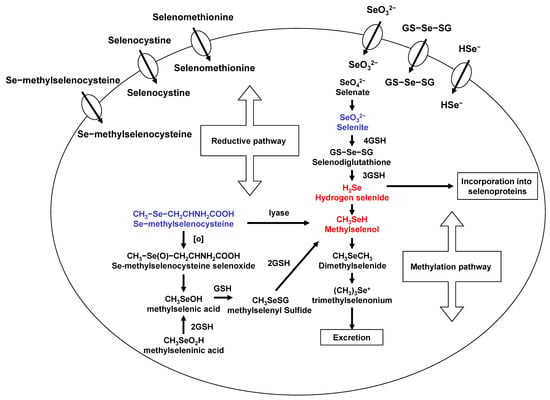
Figure 1.
Selenium metabolism.
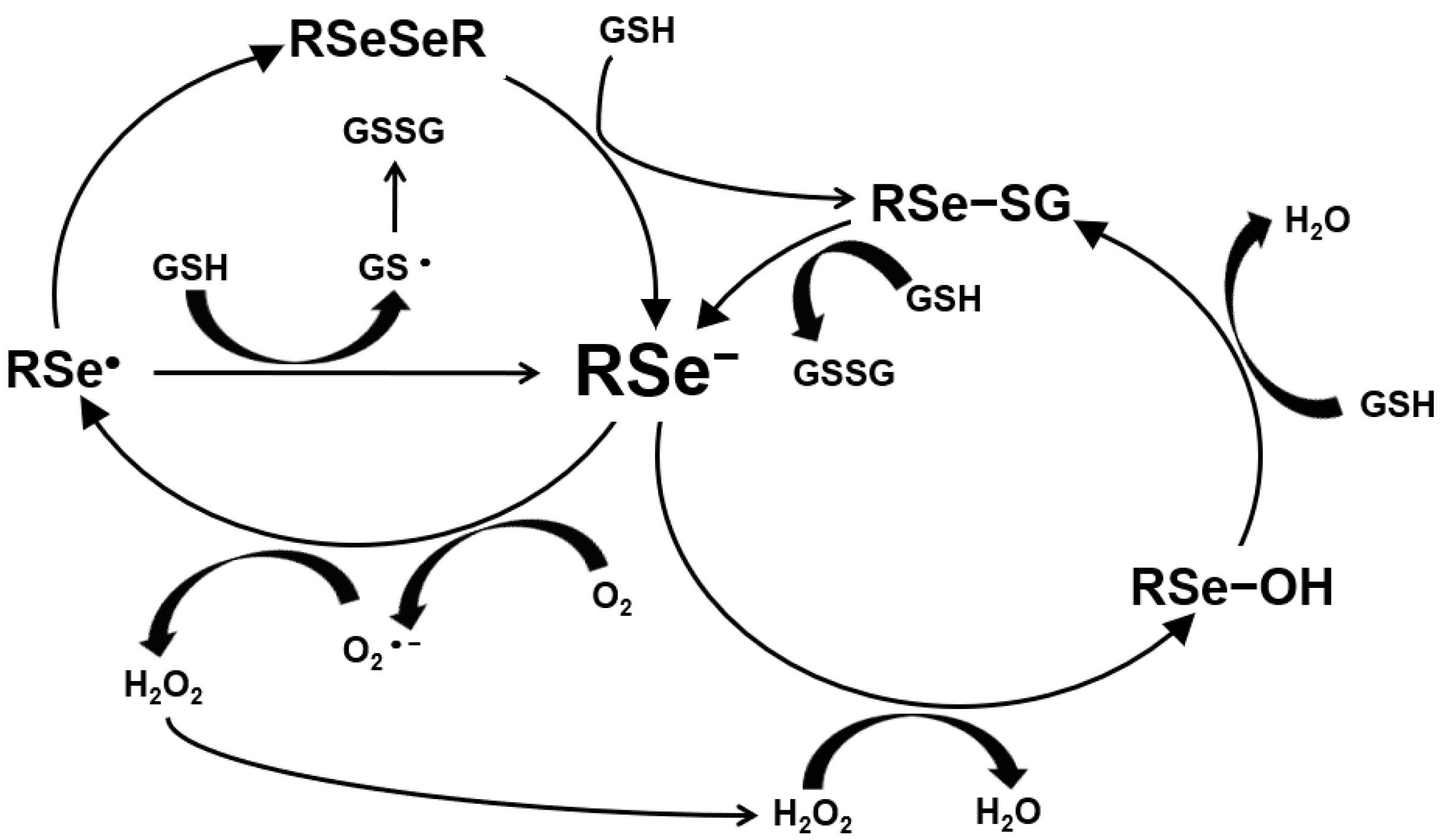
The ROS production by Se is shown in Figure 2. Hydrogen selenide from selenite and MSeH from MSeC react with GSH or sulfhydryl groups and produce O2− and, further, H2O2. Selenide produces reactive oxygen species (ROS), such as superoxide anion and H2O2, which induce DNA single-strand breaks, the cell cycle arrest by blocking S/G2 phase and, further, cell death by apoptosis [20,21,22]. Selenide induced apoptosis through this mechanism as the genotoxic and pro-apoptotic effects by the production of superoxide anion on leukemia. Mammary or prostate cancer cells have been shown to be blocked by a superoxide dismutase or its mimics [23] but not by a hydroxyl radical scavenger [24]. Further, catalase added to the cell culture medium blocked the induction of cell death by selenite [25]. Selenite is metabolized to hydrogen selenide and induces DNA single-strand breaks and, subsequently, cell death by a combination of acute lysis (necrosis) and apoptosis in several cell lines [24,26,27,28]. Selenite-induced cell death is independent of these death proteases [17,29,30,31].
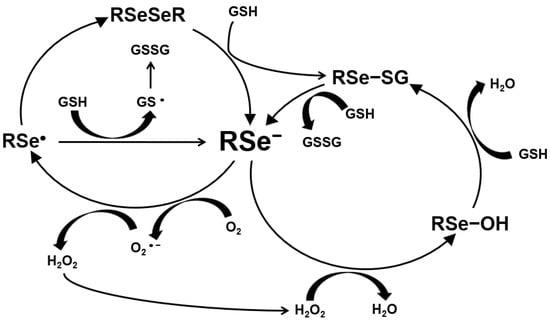
Figure 2.
Production of ROS by selenium.
On the other hand, the anti-carcinogenic effects of Se mediated byCH3SeH or its derivatives have been shown in the papers by References [32,33,34,35,36,37,38]. They have shown that the CH3SeH-precusor MSeCN and MSeC can inhibit mammary cell growth, arresting cells in G1 phase and inducing apoptosis [21,22,30,39,40,41,42]. This effect is related to caspase-dependent apoptosis [31]. Methylselenol induced apoptosis by ROS production, subsequently altered mitochondrial membrane potential, and, further, induced caspases’ activity. The production of ROS triggers cytochrome c release into cytoplasm, which can activate either caspase-9 or -8 and, further, caspase-3, and, subsequently, induce apoptosis. In addition, MSeH induced apoptosis through the direct oxidation of vicinal sulfhydryl groups within the catalytic domains of cellular enzymes (e.g., protein kinase C) and generation of superoxide anion and other ROS by MSeH [43]. The difference in the cytotoxicity between selenide from selenite and MSeH from MSeC was well discussed in a recent review paper of Reference [44]. Selenide is very reactive and may redox cycle with oxygen, form elemental Se, and undergo methylation to monomethylselenol, dimethylselenide or trimethylselenium or incorperated into selenoproteins as selenocysteine. The methylation procedure is reversible, and there are methylation and demethylation reactions by methytransferases and demethyltransferases, respectively [45]. The most predominant excretory urinary metabolites are seleno-methyl-N-acetylgalatosamine, seleno-methyl-N-acetylglucosamine, and trimethylselenium [46,47].
3. Effects of Selenium on Cancer Cells Depended on Its Concentration and Species
The viability of human breast cancer cells was inhibited in vitro in a dose-dependent manner. By Se supplementation, however, a normal cell line was relatively resistant to Se concentration [48,49]. It has been demonstrated that neoplastic breast tissues contain a high amount of Se compared to its surrounding non-neoplastic tissues [50]. Similar results have been reported in colorectal cancer and gastric cancer patients by the same group. In conjunction, several investigations clearly indicated increased expression of the selenoprotein, TrxR in the neoplastic tissues compared to the normal tissues [51]. Neoplastic transformation is often associated with increased cell proliferation during initiation and promotion of tumor development. These observations, together, imply the importance of Se in the established neoplastic tissue. On the other hand, a high dose of Se compounds inhibits neoplastic growth by production ROS [52]. Redox active Se compounds are evolving as promising chemotherapeutic agents through tumor selectivity and multi-target response, which are of great benefit in preventing development of drug resistance. Generation of ROS is implicated in Se-mediated cytotoxic effects on cancer cells. Recent findings have indicated that activation of diverse intracellular signaling leads to cell death in single cell line upon treatment with Se compounds, including selenite and SeDG. Both selenite and SeDG exhibited similar toxicity but different mechanisms. Morphologically and molecular SeDG induced apoptosis-like cell death. The above results imply that the diverse cytotoxic effects and variable potential redox active Se compounds on the survival of the cells and, thereby, substantiate the potential of chemical species-specific usage of Se in the treatment of cancers.
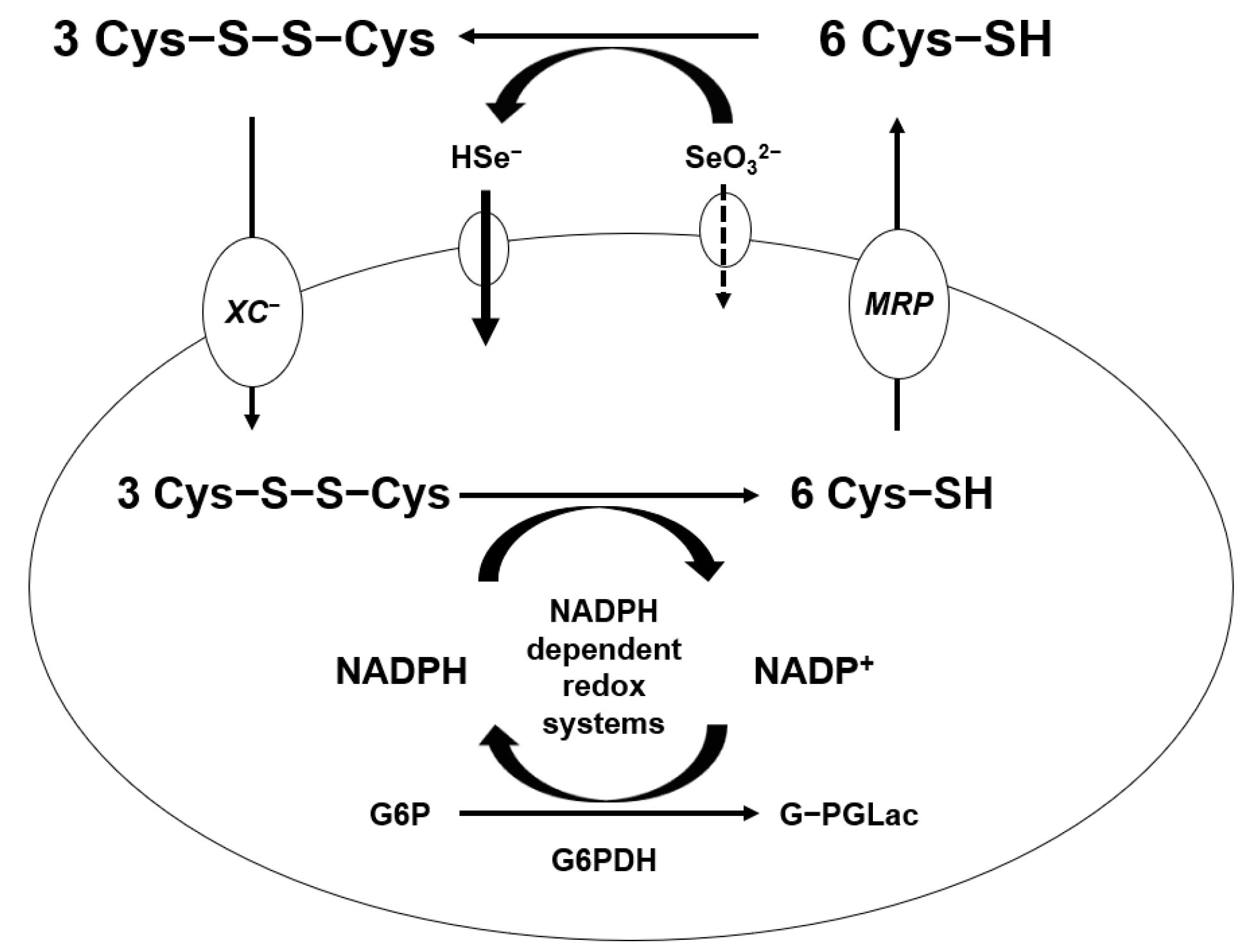
Generation of ROS from the reaction of Se compounds with thiols was shown in mammary tumor cells [53]. It was suggested that a free radical, the superoxide anion (O2−), and H2O2 are produced from the reaction of selenite and cysteine as glutathione. The capacity of cells to form Se metabolites that more easily oxidize glutathione and other thiols in the tumor cells produce ROS and peroxides. A significant difference in thiol concentration between cancer cells and normal cells has been observed [54]. Treatment with high amount of sodium selenite selectively eliminates cancer cells by generating more free radicals than that of normal cells without a systemic Se toxicity [55]. The mechanism of selenite penetrates in the cell is shown in Figure 3 in this paper. Selenite coverts hydrogen selenide by cysteine oxidation in extracellular compartment. Both hydrogen selenide and cystine get into the intracellular compartment by the χc− cystine/glutamate antiport, which reduces intracellularly to cysteine by NADPH-dependent redox systems. A significant fraction of intracellular cysteine is resected back to the extracellular compartment by multidrug resistant protein transport systems. More hydrogen selenide can be absorbed in the cancer cells, which is the most reactive Se compound [56]. Metastatic tumor or drug-resistance tumor cells absorb more hydrogen selenide, which can be more toxic to tumor cells by the glutathione oxidation-reduction system. Glutathione is the most stable form of cysteine in the cells and, further, can be synthesized more under oxidative stress, such as cancer cells.
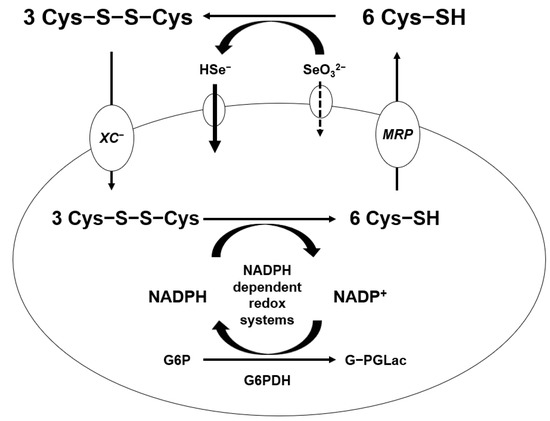
Figure 3.
Absorption mechanism of high dose of sodium selenite.
4. Selenium on Immune Functions and Anti-Inflammatory Activity
Similar to other cell types, immune cells respond to amount of dietary Se by increasing expression of many selenoproteins, although not all selenoproteins are equivalently affected. GPx1 and GPx4 in human lymphocytes were increased by supplementation with sodium selenite (50 μg or 100 μg/day) [57], similar to mice studies [58]. Se activates immune functions via the activation of IL-2 receptor [59]. IL-2 activated by T helper cells increases the activity of immune system and differentiates immune cells. The antioxidant functions of Se by the action of selenoproteins can scavenger ROS and protect cells, and Se activates the IL-2 receptor, which can activate the immune cells and regulate the immune functions.
Se has both antioxidant and anti-inflammatory functions. These functions may be explained by the roles of selenoproteins, such as GPx, TrxR, selenoprotein P, and selenoprotein W [35,60,61,62]. The roles of these enzymes have been extensively reviewed in relation to their potential functions in physiological phenomena as antioxidant defense, redox regulation of cytokine, differentiation, apoptosis, and tumorigenesis [63]. Glutathione-peroxidase and other selenoproteins can reduce hydrogen peroxide and phospholipid peroxides, thereby blocking the propagation of free radicals and reactive oxygen species, and they can also reduce hydrogen peroxides intermediate in the cyclooxygenase and lipoxygenase pathways diminishing the production of inflammatory prostaglandin and leukotrienes [64]. Any condition associated with increased oxidative stress or inflammation might be expected to be influenced by Se levels, which may be the case in rheumatoid arthritis, pancreatitis, and asthma. Supplementation with 200 μg Se in a group of rheumatoid arthritis patients for three months significantly reduced pain and joint involvement [65]. Administration of 600 μg Se along with other antioxidants to patients with chronic and recurrent pancreatitis significantly reduced pain and frequency attacks [66]. A protective relationship was found between dietary Se intake and asthma in a large occupation-based case control study [67].
Se deficiency could increase cancer risk, which might be expected on the basis of the known functions of selenoproteins in antioxidant protection and redox regulation and, thus, in the metabolic defense against carcinogenic free radicals. Mutagenic oxidative stress is generally thought to be a major factor in the initiation of human carcinogenesis, as the electron-rich DNA bases are susceptible to electrophilic attack by reactive oxygen species (ROS), including superoxide radical, hydrogen peroxide, hydroxyl radicals, and electrophilic metabolite of xenobiotics and other reactive intermediated metabolites. These ROS can cause genetic damage and the reproduction of mutant oncogenes and tumor suppressor genes, as well as epigenetic changes that affect expression. Se protection against such changes has been shown in the results from the induction of skin tumors by other ultraviolet irradiation [68,69] or the levels of phorbol esters [70], which was inversely related to skin GPx activity in animal models, and protection by selenite against (2-oxopropyl) amine-induced intrahepatic cholangio carcinomas in Syrian golden hamsters, which was correlated with the restoration of hepatic GPx activity [71].
Se plays an important role in defense against acute illness, such as sepsis syndrome. Se concentration was decreased during an intensive care unit (ICU) stay in all groups, as well as severity of sepsis syndrome, such as systemic inflammatory response and multiorgan failure. Plasma Se was inversely related to admission acute physiology [71], and treatment of high-dose sodium selenite reduced the mortality rate in patients with severe sepsis and, especially, in septic shock [72]; high doses of Se also reduced ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation [73]. Mechanisms of the beneficial effects of Se treatment on sepsis were postulated by blocking lipopolysaccharide production, as well as inhibition of myocardial cytochrome c oxidase activity and mechanically ventilated septic patients. Recently, intravenous administration of high doses Se did not improve outcome results from severe sepsis patients guided by procalcitonin therapy algorithm [74]. There may be a few debates against using high-doses of Se to sepsis. However, there was strong evidence that Se might enhance the activities of important selenoenzymes involved in the maintenance of redox-homeostasis, as well as immune and endothelial functions.
5. Blocking Tumor Invasion and Metastasis
Metastasis is one of the major causes of cancer mortality. Earlier studies have shown that dietary supplementation of Se reduces lung metastasis of melanoma cells, and Se-enriched yeast inhibits the spread of Lewis lung carcinoma cells in mice [73,74,75]. These findings collectively suggest that a nutritional adjuvant containing Se may be beneficial in reducing metastasis.
Matrix metalloproteinases (MMPs) play a major role in promoting angiogenesis and tumor metastasis [76]. MMP-2 and MMP-9 are crucial enzymes in the process of tumor metastasis derived from ECM degradation [76]. Several experiments by our group demonstrate that selenite inhibits tumor invasion by blocking MMP-2 and -9 expression [28], and MSeA inhibits tumor invasion induced by PMA via blocking MMP-2 activation [77]. Another experiment indicates that MSeH reduces metastasis of melanoma cells as a nutritional adjuvant due to suppression of integrin expression and the inhibition of MMPs [78].
Another experiment has shown that MSeA, in contrast to selenite, specifically induced human macro-vascular endothelial (HUVEC) G1 cell cycle arrest [42] and apoptotic death by caspase-mediated execution [31], inhibited endothelial MMP-2 expression [79,80], and inhibited cancer epithelial expression of VEGF [80]. Regarding its anti-proliferative action, it has been reported that MSeA can inhibit HUVEC cell cycle G1 to S progression stimulated by angiogenic factors [42], but the mechanism remains to be elucidated.
Finally, Se blocks tumor invasion and metastasis by inhibition of MMPs. These MMPs are involved in ECM degradation, basement membrane invasion, intravasation, extravasation, and, further, angiogenesis. Furthermore, animal and human intervention studies are required to establish whether Se compounds can be effectively used for chemoprevention and blocking tumor metastasis.
6. Clinical Application of High Doses of Sodium Selenite with Radiation Therapy and Chemotherapies for Advanced Cancer Patients
Selenite by itself is efficient in targeting certain cancers. However, a more pronounced effect has been shown in the subsequent chemotherapy and radiation therapy. The metastatic cancer patients were orally administrated by selenite (5.5 to 49.5 mg) as single dose 2 h before each radiation therapy. It was shown that sodium selenite was effective to increase radiation therapy, reduce radiation side effects, such as pain, stabilize the cancer status, and, further, to reduce PSA in prostate cancer patients [81]. It was measured that the half-life of sodium selenite was 18.5 h, and no adverse effects were shown at the level of selenite until 33 mg dose. The results from a phase 1 study of sodium selenite were safe and efficient to treat patients with metastatic cancer in combination with palliative radiation therapy until a 33 mg dose. Similar results were shown in other study [82]. In this study, 34 patients with different chemo-resistant tumors received intravenously sodium selenite daily for five consecutive days, either two weeks or four weeks. Plasma half-life of Se was 18.25 h, and the maximum tolerated dose was 10.2 mg/m2 in cancer patients with chemotherapy. The most common adverse events were fatigue, nausea, and cramps in fingers and legs. Dose limiting toxicities were acute of short duration and reversible. Biomarkers for organ functions indicated no major systemic toxicity. It was concluded that sodium selenite is safe and tolerable when administrated under their current protocol. It would be interesting to see that the result of ongoing prolonged infusion study with sodium selenite is more effective treatment for advanced cancer patients. Another phase 1 trial of sodium selenite plus chemotherapy was shown in gynecological cancer patients [83]. Sodium selenite was administrated to the patients from 50 μg to 500 μg doses. Grade 3/4 toxicities included neutropenia, pain, infection, neurologic, and pulmonary adverse effects. The maximum tolerated dose was not reached. Se had no effect on carboplatin pharmacokinetics in the treatment of chemo-naive women with gynecologic cancers. Correlative studies showed post-treatment downregulation of RAD51AP1, a protein involved DNA repair, in both cancer cell lines and patient tumors. The addition of Se to carboplatin/paclitaxel treatment was safe and well tolerated, and it did not alter carboplatin pharmacokinetics. A 5000 μg dose of sodium selenite is suggested as the dose of evaluated in a phase II clinical trial in their ongoing studies. In our study, several terminal cancer patients could tolerate more than 5000 μg of sodium selenite with chemotherapies and radiotherapy. Serum Se concentrations were monitored every time at 24 h after injection of high doses of sodium selenite and was reduced to less than 260 μg/mL, even more than 5000 μg of sodium selenite treatment at Sangkyungwon Integrate Medical Cancer Hospital (SIMCH) (unpublished data; Table 1) in South Korea. Three hundred and ten patients were supplemented by high dose of sodium selenite with advanced drug-resistant metastatic cancers, such as breast, gynecological, gastrointestinal tract, liver cancer, pancreatic, gall bladder, renal, bladder cancers, and lymphoma, from July 2019 to August 2021. The results and procedures of this clinical trial are shown here (unpublished data). During and after a high dose of the selenite treatment, no irreversible adverse events were found in most cancer patients, but a few reversible mild gastroenteric symptoms were found. After the injection of the high dose of sodium selenite, Se levels were less than 260 μg/mL at 24 h, which may not be toxic. We present two advanced gynecologic cancer patients successfully treated with high doses of sodium selenite in which continual treatment reduced the size of tumors and improved other symptoms, as shown in Figure 4.

Table 1.
Sodium selenite as integrative medical application to cancer patients at SIMCH.
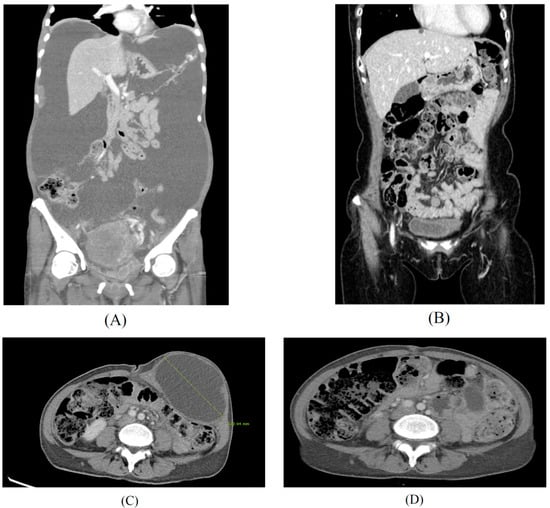
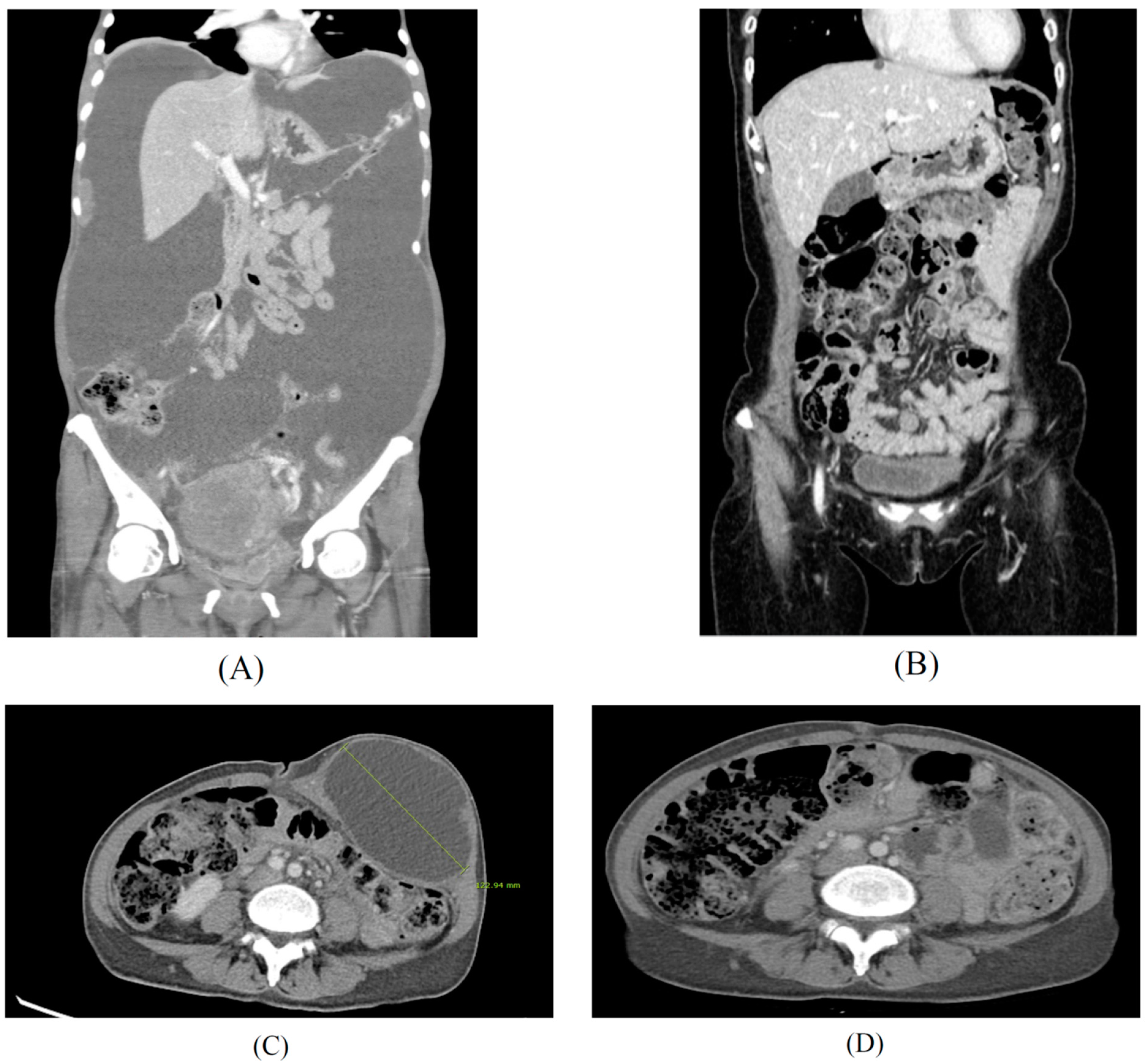
Figure 4.
High doses of sodium selenite in treatment of advanced metastasis cancer patients. Case 1: Findings of abdomino-pelvic CT before (A) and after (B) the multidisciplinary treatment for a 51-year-old patient diagnosed with advanced stage ovarian cancer. Case 2: Findings of abdomino-pelvic CT before (C) and after (D) the integrative treatment for a 48-year-old patient diagnosed with recurrent ovarian cancer after primary intensive pelvic surgery with adjuvant chemotherapy, since 2017. Peritoneal carcinomatosis with massive ascites (A) and no evidence of disease (B) after 18 months of chemotherapy, interval debulking surgery, and PARP inhibitor maintenance therapy with high dose sodium selenite treatment according to regimen of Table 1 (SIMCH). Metastatic para-aortic lymph nodes with 12 cm-sized port site metastatic mass at left lower abdominal wall was noted (C), and no evidence of disease at left lower abdomen and stable disease of metastatic lymph nodes (D) after 16 months of metastatectomy of abdominal wall and maintenance immune checkpoint inhibitor (pembrolizumab) with high dose sodium selenite treatment according to regimen of Table 1 (SIMCH).
It has been postulated that a short half-life of sodium selenite and more accumulation of the Se in the cancer cells may be more toxic in cancer cells than that in normal cells. As the patients were treated with multiple lines of chemotherapy, they eventually showed drug resistance. The patients were treated with several drugs and showed drug resistance. High doses of sodium selenite were applied to these chemo-resistant patients, and some of them were terminal patients. The detailed segment modification of dosage and duration were performed upon individual cases for 3 months, 6 months, and 12 months and reevaluated, including quality of life. The results suggest that high doses of sodium selenite as conventional treatment with chemotherapy and radiation can be considered a prominent method. An integrated medical treatment of sodium selenite with chemotherapy, radiation, immunotherapy, surgery, and resistance to chemotherapy may be applied to advanced cancer patients. In the future, it would be interesting to find that each cancer patient can apply modification doses and duration in treatment with or without combination of standard procedure, such as surgery, irradiation, chemotherapy, and, further, immunotherapy.
In conclusion, high dose Se treatment can be a possible therapy in some cases for advanced cancer patients. We discuss the pertinent literature in a mine review style and also describe, through our recent clinical understanding, that Se may be tolerable agent without any significant severe adverse events as an integrative medical treatment combination with chemotherapy, radiotherapy, and radical surgery in the advanced stage cancer of patients in chemo-resistant status or to prevent developing it. In the beginning, the individual takes a modification in therapy of dose of duration of treatment with or without combination of standard treatment, such as surgery, irradiation, chemotherapy, and also immunotherapy. In the future, randomized controlled prospective clinical trials in terms of high dose inorganic Se treatment will be necessary to be carried out at various institutions internationally.
Author Contributions
Conceptualization and methodology, S.J.K. and A.S.C.; investigation, S.J.K., M.C.C. and J.M.P.; data curation, M.C.C. and J.M.P.; writing and original draft preparation, S.J.K. and A.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Ip, C. Lessons from basic research in selenium and cancer prevention. J. Nutr. 1998, 128, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, G.F., Jr. Status of selenium in prostate cancer prevention. Br. J. Cancer 2004, 91, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.L.; Sheu, J.Y.; Lin, T.H. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin. Biochem. 1999, 32, 131–136. [Google Scholar] [CrossRef]
- Glattre, E.; Thomassen, Y.; Thoresen, S.O.; Haldorsen, T.; Lund-Larsen, P.G.; Theodorsen, L.; Aaseth, J. Prediagnostic serum selenium in a case-control study of thyroid cancer. Int. J. Epidemiol. 1989, 18, 45–49. [Google Scholar] [CrossRef]
- Jaskiewicz, K.; Marasas, W.F.; Rossouw, J.E.; Van Niekerk, F.E.; Heine Tech, E.W. Selenium and other mineral elements in populations at risk for esophageal cancer. Cancer 1988, 62, 2635–2639. [Google Scholar] [CrossRef]
- Westin, T.; Ahlbom, E.; Johansson, E.; Sandstrom, B.; Karlberg, I.; Edstrom, S. Circulating levels of selenium and zinc in relation to nutritional status in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 1989, 115, 1079–1082. [Google Scholar] [CrossRef]
- Philipov, P.; Tzatchev, K. Selenium concentrations in serum of patients with cerebral and extracerebral tumors. Zent. Neurochir. 1988, 49, 344–347. [Google Scholar]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges (Review). Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Ha, P.; Gu, L. Determination of the selenomethionine content in grain and human blood. Wei Sheng Yan Jiu 1997, 26, 113–116. [Google Scholar] [PubMed]
- Kim, T.; Jung, U.; Cho, D.Y.; Chung, A.S. Se-methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis 2001, 22, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.; Zheng, X.; Yoon, S.O.; Chung, A.S. Se-methylselenocysteine induces apoptosis mediated by reactive oxygen species in HL-60 cells. Free Radic. Biol. Med. 2001, 31, 479–489. [Google Scholar] [CrossRef]
- Kim, A.; Jung, J.Y.; Son, M.; Lee, S.H.; Lim, J.S.; Chung, A.S. Long exposure of non-cytotoxic concentrations of methylselenol suppresses the invasive potential of B16F10 melanoma. Oncol. Rep. 2008, 20, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J. Apoptosis and angiogenesis in cancer prevention by selenium. Adv. Exp. Med. Biol. 2001, 492, 131–145. [Google Scholar] [CrossRef]
- Lu, J.; Pei, H.; Ip, C.; Lisk, D.J.; Ganther, H.; Thompson, H.J. Effect on an aqueous extract of selenium-enriched garlic on in vitro markers and in vivo efficacy in cancer prevention. Carcinogenesis 1996, 17, 1903–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeck, M.; Lu, J.; Strange, R.; Ip, C.; Ganther, H.E.; Thompson, H.J. Differential induction of growth arrest inducible genes by selenium compounds. Biochem. Pharm. 1997, 53, 921–926. [Google Scholar] [CrossRef]
- Zhong, W.; Oberley, T.D. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001, 61, 7071–7078. [Google Scholar] [PubMed]
- Lu, J.; Kaeck, M.; Jiang, C.; Wilson, A.C.; Thompson, H.J. Selenite induction of DNA strand breaks and apoptosis in mouse leukemic L1210 cells. Biochem. Pharm. 1994, 47, 1531–1535. [Google Scholar] [CrossRef]
- Zhu, Z.; Kimura, M.; Itokawa, Y.; Aoki, T.; Takahashi, J.A.; Nakatsu, S.; Oda, Y.; Kikuchi, H. Apoptosis induced by selenium in human glioma cell lines. Biol. Trace Elem. Res. 1996, 54, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.S.; Davis, R.L.; Walsh, L.P.; Pence, B.C. Induction of differentiation and apoptosis by sodium selenite in human colonic carcinoma cells (HT29). Cancer Lett. 1997, 117, 35–40. [Google Scholar] [CrossRef]
- Shen, H.M.; Yang, C.F.; Ong, C.N. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int. J. Cancer 1999, 81, 820–828. [Google Scholar] [CrossRef]
- Yoon, S.O.; Kim, M.M.; Chung, A.S. Inhibitory effect of selenite on invasion of HT1080 tumor cells. J. Biol. Chem. 2001, 276, 20085–20092. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1059–1066. [Google Scholar] [PubMed]
- Wang, Z.; Jiang, C.; Ganther, H.; Lu, J. Antimitogenic and proapoptotic activities of methylseleninic acid in vascular endothelial cells and associated effects on PI3K-AKT, ERK, JNK and p38 MAPK signaling. Cancer Res. 2001, 61, 7171–7178. [Google Scholar] [PubMed]
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001, 61, 3062–3070. [Google Scholar]
- Ip, C.; Ganther, H.E. Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis 1992, 13, 1167–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Jiang, C. Selenium and cancer chemoprevention: Hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxid. Redox Signal. 2005, 7, 1715–1727. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A. The Main Cytotoxic Effects of Methylseleninic Acid on Various Cancer Cells. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, M.; Ali, W.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennicke, C.; Rahn, J.; Bukur, J.; Hochgrafe, F.; Wessjohann, L.A.; Lichtenfels, R.; Seliger, B. Modulation of MHC class I surface expression in B16F10 melanoma cells by methylseleninic acid. Oncoimmunology 2017, 6, e1259049. [Google Scholar] [CrossRef] [Green Version]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of Selenium Enrichment and Measurement in Brassicaceous Vegetables, and Their Application to Human Health. Front. Plant. Sci. 2017, 8, 1365. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Jiang, C.; Kaeck, M.; Ganther, H.; Vadhanavikit, S.; Ip, C.; Thompson, H. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem. Pharm. 1995, 50, 213–219. [Google Scholar] [CrossRef]
- Ip, C.; Vadhanavikit, S.; Ganther, H. Cancer chemoprevention by aliphatic selenocyanates: Effect of chain length on inhibition of mammary tumors and DMBA adducts. Carcinogenesis 1995, 16, 35–38. [Google Scholar] [PubMed]
- Ip, C.; Zhu, Z.; Thompson, H.J.; Lisk, D.; Ganther, H.E. Chemoprevention of mammary cancer with Se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Res. 1999, 19, 2875–2880. [Google Scholar]
- Wang, Z.; Jiang, C.; Lu, J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol. Carcinog. 2002, 34, 113–120. [Google Scholar] [CrossRef]
- Drake, E.N. Cancer chemoprevention: Selenium as a prooxidant, not an antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wallenberg, M.; Misra, S.; Bjornstedt, M. Selenium cytotoxicity in cancer. Basic Clin. Pharm. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra, S.; Tisato, F.; Marzano, C.; Rigobello, M.P.; Kumar, S.; Bjornstedt, M. Methylselenol formed by spontaneous methylation of selenide is a superior selenium substrate to the thioredoxin and glutaredoxin systems. PLoS ONE 2012, 7, e50727. [Google Scholar] [CrossRef] [Green Version]
- Francesconi, K.A.; Pannier, F. Selenium metabolites in urine: A critical overview of past work and current status. Clin. Chem. 2004, 50, 2240–2253. [Google Scholar] [CrossRef] [Green Version]
- Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Identification of a novel selenium metabolite, Se-methyl-N-acetylselenohexosamine, in rat urine by high-performance liquid chromatography--inductively coupled plasma mass spectrometry and--electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 767, 301–312. [Google Scholar] [CrossRef]
- Watrach, A.M.; Milner, J.A.; Watrach, M.A.; Poirier, K.A. Inhibition of human breast cancer cells by selenium. Cancer Lett. 1984, 25, 41–47. [Google Scholar] [CrossRef]
- Woo, J.; Kim, J.B.; Cho, T.; Yoo, E.H.; Moon, B.I.; Kwon, H.; Lim, W. Selenium inhibits growth of trastuzumab-resistant human breast cancer cells via downregulation of Akt and beclin-1. PLoS ONE 2021, 16, e0257298. [Google Scholar] [CrossRef]
- Charalabopoulos, K.; Kotsalos, A.; Batistatou, A.; Charalabopoulos, A.; Vezyraki, P.; Peschos, D.; Kalfakakou, V.; Evangelou, A. Selenium in serum and neoplastic tissue in breast cancer: Correlation with CEA. Br. J. Cancer 2006, 95, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Selenius, M.; Rundlof, A.K.; Olm, E.; Fernandes, A.P.; Bjornstedt, M. Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid. Redox Signal. 2010, 12, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Bjornstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharm. 1993, 45, 429–437. [Google Scholar]
- Dirican, N.; Dirican, A.; Sen, O.; Aynali, A.; Atalay, S.; Bircan, H.A.; Ozturk, O.; Erdogan, S.; Cakir, M.; Akkaya, A. Thiol/disulfide homeostasis: A prognostic biomarker for patients with advanced non-small cell lung cancer? Redox Rep. 2016, 21, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, B. Sodium Selenite as an Anticancer Agent. Anticancer Agents Med. Chem. 2017, 17, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlof, A.K.; Larsen, E.H.; Danielsson, O.; Bjornstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x(c)- cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [CrossRef] [Green Version]
- Broome, C.S.; McArdle, F.; Kyle, J.A.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef]
- Sunde, R.A.; Li, J.L.; Taylor, R.M. Insights for Setting of Nutrient Requirements, Gleaned by Comparison of Selenium Status Biomarkers in Turkeys and Chickens versus Rats, Mice, and Lambs. Adv. Nutr. 2016, 7, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Kiremidjian-Schumacher, L.; Roy, M.; Glickman, R.; Schneider, K.; Rothstein, S.; Cooper, J.; Hochster, H.; Kim, M.; Newman, R. Selenium and immunocompetence in patients with head and neck cancer. Biol. Trace Elem. Res. 2000, 73, 97–111. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.C.; Lei, X.G.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Boylan, L.M.; Larsen, H.S. Advances in understanding selenium’s role in the immune system. Ann. N. Y. Acad. Sci. 1990, 587, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Neve, J.; Duchateau, J.; Famaey, J.P. Adjuvant treatment of recent onset rheumatoid arthritis by selenium supplementation: Preliminary observations. Br. J. Rheumatol. 1992, 31, 281–282. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R. Chronic pancreatitis at Manchester, UK. Focus on antioxidant therapy. Digestion 1998, 59 (Suppl. S4), 36–48. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Sterne, J.A.; Tompson, R.L.; Songhurst, C.E.; Margetts, B.M.; Burney, P.G. Dietary antioxidants and asthma in adults. Eur. Respir. J. 1999, 14 (Suppl. S30), 141s. [Google Scholar] [CrossRef]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell. Longev. 2018, 2018, 5895439. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef] [PubMed]
- Perchellet, J.P.; Abney, N.L.; Thomas, R.M.; Guislain, Y.L.; Perchellet, E.M. Effects of combined treatments with selenium, glutathione, and vitamin E on glutathione peroxidase activity, ornithine decarboxylase induction, and complete and multistage carcinogenesis in mouse skin. Cancer Res. 1987, 47, 477–485. [Google Scholar] [PubMed]
- Kise, Y.; Yamamura, M.; Kogata, M.; Nakagawa, M.; Uetsuji, S.; Takada, H.; Hioki, K.; Yamamoto, M. Inhibition by selenium of intrahepatic cholangiocarcinoma induction in Syrian golden hamsters by N′-nitrosobis(2-oxopropyl)amine. Nutr. Cancer 1991, 16, 153–164. [Google Scholar] [CrossRef]
- Angstwurm, M.W.; Engelmann, L.; Zimmermann, T.; Lehmann, C.; Spes, C.H.; Abel, P.; Strauss, R.; Meier-Hellmann, A.; Insel, R.; Radke, J.; et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit. Care Med. 2007, 35, 118–126. [Google Scholar] [CrossRef]
- Yan, L.; Yee, J.A.; McGuire, M.H.; Graef, G.L. Effect of dietary supplementation of selenite on pulmonary metastasis of melanoma cells in mice. Nutr. Cancer 1997, 28, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yee, J.A.; Li, D.; McGuire, M.H.; Graef, G.L. Dietary supplementation of selenomethionine reduces metastasis of melanoma cells in mice. Anticancer Res. 1999, 19, 1337–1342. [Google Scholar] [PubMed]
- Liu, Y.H.; Tian, H.S.; Wang, D.X. Inhibitory effect of selenium yeast on the metastasis of Lewis lung carcinoma in C57BL mice. Studies with reference of histochemistry and ultrastructure. Chin. Med. J. 1987, 100, 549–554. [Google Scholar] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, A.; Oh, J.H.; Chung, A.S. Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation mediated by MT1-MMP expression and further tumor invasion through suppression of NF-kappaB activation. Carcinogenesis 2007, 28, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Oh, J.H.; Park, J.M.; Chung, A.S. Methylselenol generated from selenomethionine by methioninase downregulates integrin expression and induces caspase-mediated apoptosis of B16F10 melanoma cells. J. Cell Physiol. 2007, 212, 386–400. [Google Scholar] [CrossRef]
- Marciel, M.P.; Hoffmann, P.R. Selenoproteins and Metastasis. Adv. Cancer Res. 2017, 136, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Pei, Z.; Chen, S.; Yang, X.; Chen, Y.; Lin, D.; Ma, R.Z. Methylseleninic acid restricts tumor growth in nude mice model of metastatic breast cancer probably via inhibiting angiopoietin-2. BMC Cancer 2012, 12, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, S.J.; Jayachandran, P.; Keeling, C.A.; Stevens, K.J.; Sandhu, N.; Stamps-DeAnda, S.L.; Savic, R.; Shura, L.; Buyyounouski, M.K.; Grimes, K. Results from a Phase 1 Study of Sodium Selenite in Combination with Palliative Radiation Therapy in Patients with Metastatic Cancer. Transl. Oncol. 2019, 12, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Kumaran, M.N.; Gounder, M.; Gibbon, D.G.; Nieves-Neira, W.; Vaidya, A.; Hellmann, M.; Kane, M.P.; Buckley, B.; Shih, W.; et al. Phase I trial of selenium plus chemotherapy in gynecologic cancers. Gynecol. Oncol. 2018, 150, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).