Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases
Abstract
:1. Introduction
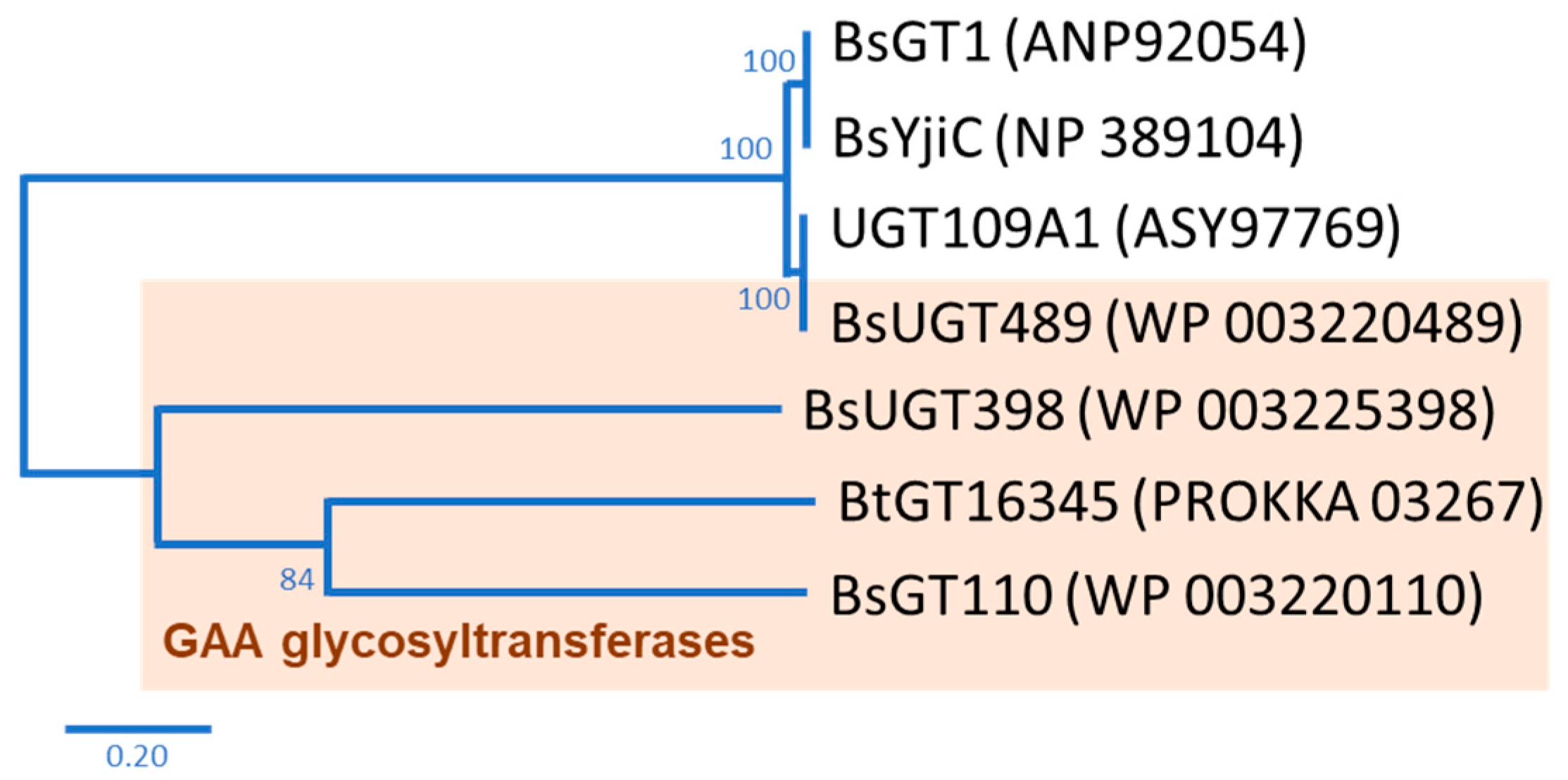
2. Results and Discussion
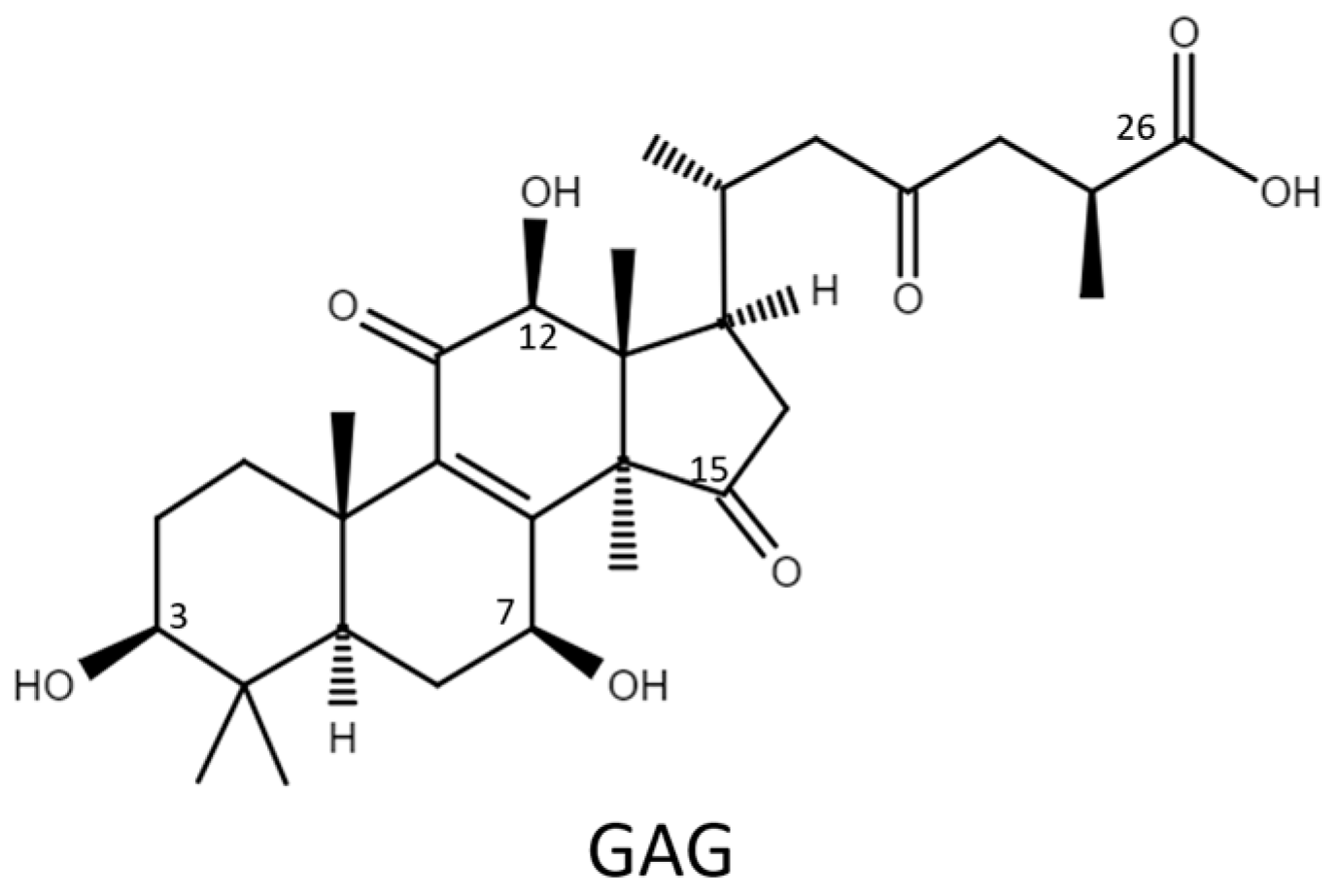
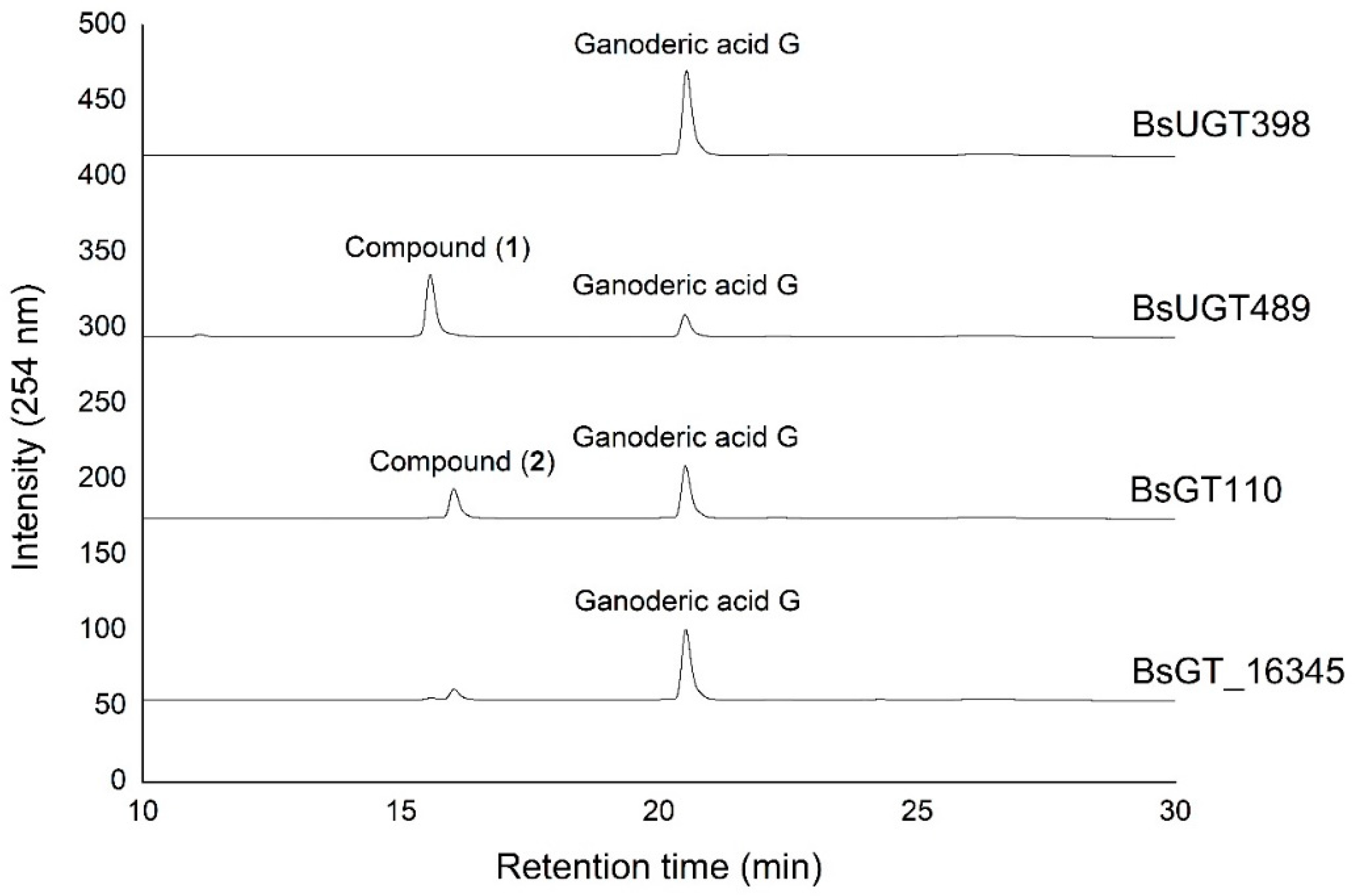
2.1. Biotransformation
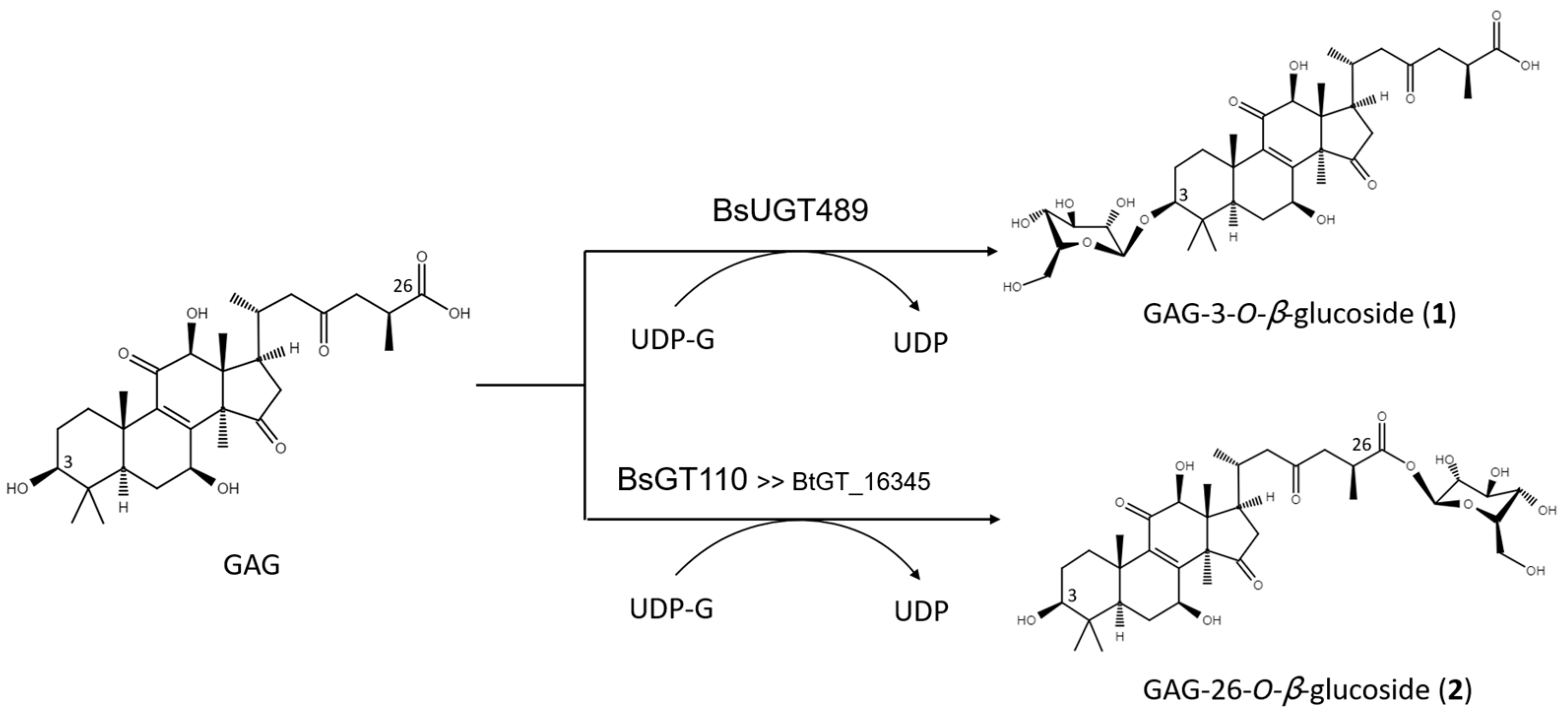
2.2. Purification and Identification of Biotransformed Products
2.3. Catalytic Specificity of the Bacillus GTs for the Glycosylation of Functional Groups on Ganoderic Acids
2.4. Determination of Solubility
2.5. Perspective
3. Materials and Methods
3.1. Enzymes and Chemicals
3.2. Biotransformation
3.3. HPLC Analysis
3.4. Purification and Identification of the Biotransformation Products
3.5. Determination of Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef]
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1113–1128. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Liang, C.Y.; Tian, D.N.; Liu, Y.Z.; Li, H.; Zhu, J.L.; Li, M.; Xin, M.H.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.Z.; Sun, X.F.; Zhao, H.J.; Wu, L.F.; Zhu, D.; Yang, G.H.; Shao, Y.Y.; Zhang, X.X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Li, T.; He, H.; Zhou, X.; Zhu, J. Ganoderic Acid A Inhibits Bleomycin-Induced Lung Fibrosis in Mice. Pharmacology 2020, 105, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Li, Y.; Sun, S.; Yang, Y.; Lv, Y.; Wang, L.; Song, M.; Chen, M.; Wu, C.; Pan, H.; et al. Ganoderic acid A attenuates lipopolysaccharide-induced lung injury in mice. Biosci. Rep. 2019, 39, BSR20190301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Wang, S.Z.; He, J.Z.; Zhu, S.Z.; Huang, B.Y.; Wang, S.Y.; Li, M.; Zhou, H.; Lin, S.Q.; Yang, B.X. Ganoderic acid A is the effective ingredient of Ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease. Acta Pharmacol. Sin. 2020, 41, 782–790. [Google Scholar] [CrossRef]
- Yu, Z.R.; Jia, W.H.; Liu, C.; Wang, H.Q.; Yang, H.G.; He, G.R.; Chen, R.Y.; Du, G.H. Ganoderic acid A protects neural cells against NO stress injury in vitro via stimulating β adrenergic receptors. Acta Pharmacol. Sin. 2020, 41, 516–522. [Google Scholar] [CrossRef]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzebski, A.B.; Szymanska, K.; Chrusciel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-beta-glucoside by Bacillus glycosyltransferases. J. Biosci. Bioeng. 2021, 131, 176–182. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Kao, Y.H.; Wu, J.Y.; Wu, Y.W.; Wang, T.Y. A new triterpenoid glucoside from a novel acidic glycosylation of ganoderic acid A via recombinant glycosyltransferase of Bacillus subtilis. Molecules 2019, 24, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.S.; Wu, J.Y.; Wang, T.Y.; Wu, K.Y.; Chiang, C.M. Uridine diphosphate-dependent glycosyltransferases from Bacillus subtilis ATCC 6633 catalyze the 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2018, 19, 3469. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.S.; Wang, T.Y.; Hsueh, T.Y.; Lee, Y.W.; Chuang, H.M.; Cai, W.X.; Wu, J.Y.; Chiang, C.M.; Wu, Y.W. A genome-centric approach reveals a novel glycosyltransferase from the GA A07 Strain of Bacillus thuringiensis responsible for catalyzing 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2019, 20, 5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulis, C.; Guieysse, D.; Morel, S.; Severac, E.; Remaud-Simeon, M. Natural and engineered transglycosylases: Green tools for the enzyme-based synthesis of glycoproducts. Curr. Opin. Chem. Biol. 2021, 61, 96–106. [Google Scholar] [CrossRef]
- Slamova, K.; Kapesova, J.; Valentova, K. “Sweet Flavonoids”: Glycosidase-catalyzedmodifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, Z.; Zhang, L. Clinical applications of the naturally occurring or synthetic glycosylated low molecular weight drugs. Progress Mol. Biol. Transl. Sci. 2019, 163, 487–522. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Bibby, B.M.; Frandsen, H.O.; Brown, L.D.; Nielsen, J.K.; Renwick, J.A. A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J. Chem. Ecol. 2003, 29, 1417–1433. [Google Scholar] [CrossRef]
- Christensen, S.; Enge, S.; Jensen, K.R.; Müller, C.; Kiær, L.P.; Agerbirk, N.; Heimes, C.; Hauser, T.P. Different herbivore responses to two co-occurring chemotypes of the wild crucifer Barbarea vulgaris. Arthropod Plant Interact. 2019, 13, 19–30. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Triterpenoid | Functional Groups in Ganoderic Acids | ||||
|---|---|---|---|---|---|
| C3-OH | C7-OH | C12-OH | C15-OH | C26-COOH | |
| GAA | N.A. 1 | N.D. 2 | N.A. | BsUGT489 > BtGT_16345 > BsUGT398 3 | BsGT110 |
| GAG | BsUGT489 | N.D. | N.D. | N.A. | BsGT110 >> BtBGT_16345 |
| Compound | Aqueous Solubility (mg/L) 1 | Fold 2 |
|---|---|---|
| GAG | 18.85 ± 1.36 | 1 |
| GAG-3-o-β-glucoside (1) | 1019.64 ± 9.54 | 54.0 |
| GAG-26-o-β-glucoside (2) | 1829.89 ± 51.21 | 97.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Zhang, Y.-R.; Chang, T.-S. Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases. Int. J. Mol. Sci. 2021, 22, 9744. https://doi.org/10.3390/ijms22189744
Wu J-Y, Ding H-Y, Wang T-Y, Zhang Y-R, Chang T-S. Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases. International Journal of Molecular Sciences. 2021; 22(18):9744. https://doi.org/10.3390/ijms22189744
Chicago/Turabian StyleWu, Jiumn-Yih, Hsiou-Yu Ding, Tzi-Yuan Wang, Yun-Rong Zhang, and Te-Sheng Chang. 2021. "Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases" International Journal of Molecular Sciences 22, no. 18: 9744. https://doi.org/10.3390/ijms22189744
APA StyleWu, J.-Y., Ding, H.-Y., Wang, T.-Y., Zhang, Y.-R., & Chang, T.-S. (2021). Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases. International Journal of Molecular Sciences, 22(18), 9744. https://doi.org/10.3390/ijms22189744








