Aquaporin-4 Mediates Permanent Brain Alterations in a Mouse Model of Hypoxia-Aged Hydrocephalus
Abstract
:1. Introduction
2. Results
2.1. Re-Normoxia Restores CSF Production to Baseline and Reverses Cognitive Decline in Aged wt Mice That Develop Hypoxia-Induced Hydrocephalus
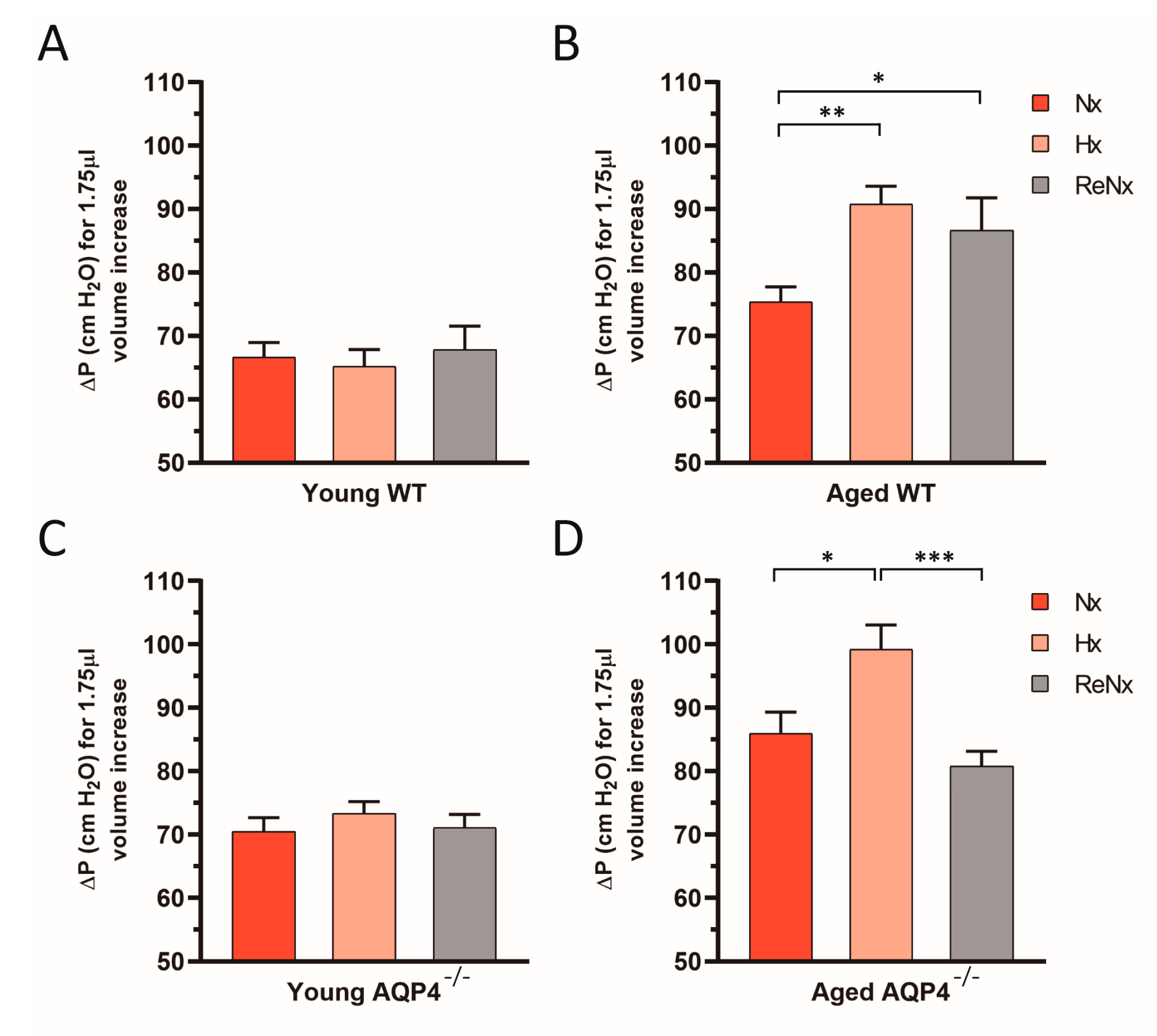
2.2. CSF Drainage Capacity Remains Impaired after Re-Normoxia in Aged Mice That Develop Hypoxia-Induced Hydrocephalus
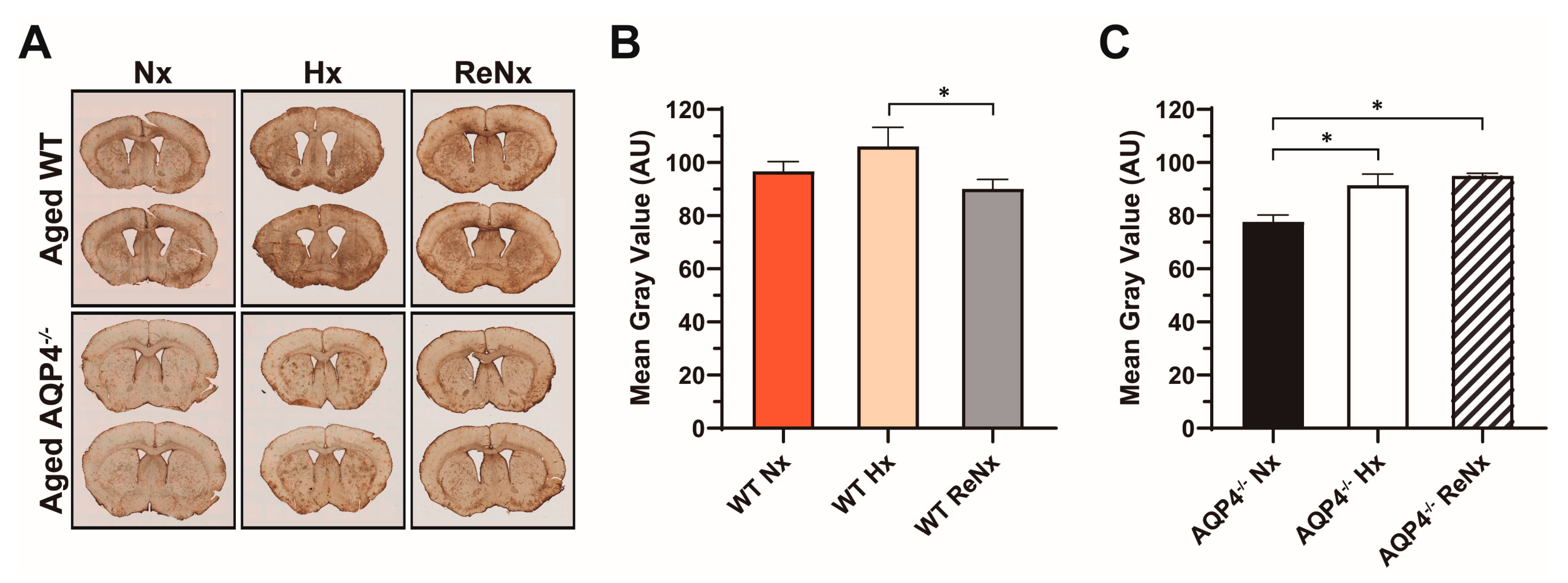
2.3. Diminished Ventricular Distensibility Is Not Recovered after Re-Normoxia in the Aged wt Mice
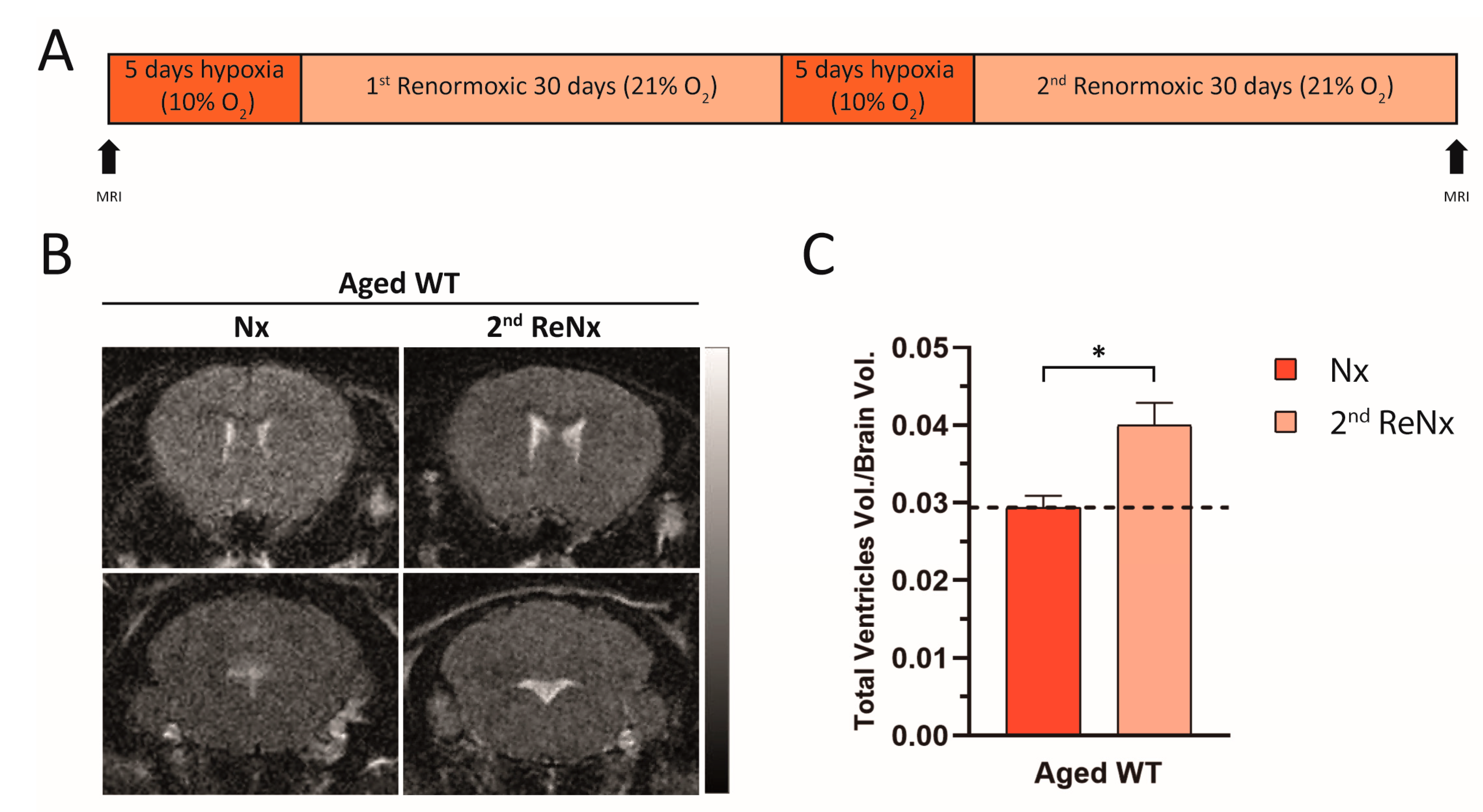
2.4. Repetitive Hypoxic Events Lead to Permanent and Irreversible Ventriculomegaly
3. Discussion
4. Materials and Methods
4.1. Animal Care and Hypoxic Treatments
4.2. Magnetic Resonance Imaging
4.3. Intraventricular Pressure Measurements
4.4. Novel Object Recognition Testing
4.5. CSF Outflow Dynamics and Ventricular Compliance Measurements
4.6. Histological Analyses and Densitometry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amiry-Moghaddam, M.; Ottersen, O.P. The molecular basis of water transport in the brain. Nat. Rev. Neurosci. 2003, 4, 991–1001. [Google Scholar] [CrossRef]
- Zelenina, M. Regulation of brain aquaporins. Neurochem. Int. 2010, 57, 468–488. [Google Scholar] [CrossRef] [PubMed]
- Venero, J.L.; Vizuete, M.L.; Ilundáin, A.A.; Machado, A.; Echevarria, M.; Cano, J. Detailed localization of aquaporin-4 messenger RNA in the CNS: Preferential expression in periventricular organs. Neuroscience 1999, 94, 239–250. [Google Scholar] [CrossRef]
- Igarashi, H.; Tsujita, M.; Kwee, I.L.; Nakada, T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. Neuroreport 2014, 25, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Trillo-Contreras, J.L.; Toledo-Aral, J.J.; Echevarria, M.; Villadiego, J. AQP1 and AQP4 Contribution to Cerebrospinal Fluid Homeostasis. Cells 2019, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid Transport in the Brain. Physiol. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jorgacevski, J.; Zorec, R.; Potokar, M. Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes. Cells 2020, 9, 2622. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [Green Version]
- Amiry-Moghaddam, M.; Otsuka, T.; Hurn, P.D.; Traystman, R.J.; Haug, F.M.; Froehner, S.C.; Adams, M.E.; Neely, J.D.; Agre, P.; Ottersen, O.P.; et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. USA 2003, 100, 2106–2111. [Google Scholar] [CrossRef] [Green Version]
- Tham, D.K.; Joshi, B.; Moukhles, H. Aquaporin-4 Cell-Surface Expression and Turnover Are Regulated by Dystroglycan, Dynamin, and the Extracellular Matrix in Astrocytes. PLoS ONE 2016, 11, e0165439. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Manley, G.T.; Binder, D.K.; Papadopoulos, M.C.; Verkman, A.S. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 2004, 129, 983–991. [Google Scholar] [CrossRef]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol. Med. 2020, 26, 285–295. [Google Scholar] [CrossRef]
- Grande, P.O.; Asgeirsson, B.; Nordstrom, C.H. Physiologic principles for volume regulation of a tissue enclosed in a rigid shell with application to the injured brain. J. Trauma 1997, 42, S23–S31. [Google Scholar] [CrossRef]
- Roales-Bujan, R.; Paez, P.; Guerra, M.; Rodriguez, S.; Vio, K.; Ho-Plagaro, A.; Garcia-Bonilla, M.; Rodriguez-Perez, L.M.; Dominguez-Pinos, M.D.; Rodriguez, E.M.; et al. Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 2012, 124, 531–546. [Google Scholar] [CrossRef] [Green Version]
- Del Puerto, A.; Pose-Utrilla, J.; Simon-Garcia, A.; Lopez-Menendez, C.; Jimenez, A.J.; Porlan, E.; Pajuelo, L.S.M.; Cano-Garcia, G.; Marti-Prado, B.; Sebastian-Serrano, A.; et al. Kidins220 deficiency causes ventriculomegaly via SNX27-retromer-dependent AQP4 degradation. Mol. Psychiatry 2021, 1–16. [Google Scholar] [CrossRef]
- Mao, X.; Enno, T.L.; Del Bigio, M.R. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur. J. Neurosci. 2006, 23, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Olive, M.M.; Enger, R.; Hansson, H.A.; Nagelhus, E.A.; Eide, P.K. Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia 2019, 67, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trillo-Contreras, J.L.; Ramirez-Lorca, R.; Hiraldo-Gonzalez, L.; Sanchez-Gomar, I.; Galan-Cobo, A.; Suarez-Luna, N.; Sanchez de Rojas-de Pedro, E.; Toledo-Aral, J.J.; Villadiego, J.; Echevarria, M. Combined effects of aquaporin-4 and hypoxia produce age-related hydrocephalus. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3515–3526. [Google Scholar] [CrossRef]
- Eide, P.K.; Sorteberg, W. Outcome of Surgery for Idiopathic Normal Pressure Hydrocephalus: Role of Preoperative Static and Pulsatile Intracranial Pressure. World Neurosurg. 2016, 86, 186–193 e181. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Hu, F.; Ding, J.; Wang, X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci. Ther. 2020, 26, 1230–1240. [Google Scholar] [CrossRef]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef]
- Hannocks, M.J.; Pizzo, M.E.; Huppert, J.; Deshpande, T.; Abbott, N.J.; Thorne, R.G.; Sorokin, L. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 2018, 38, 669–686. [Google Scholar] [CrossRef]
- Eide, P.K.; Hansson, H.A. Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol. Appl. Neurobiol. 2018, 44, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Vajda, Z.; Pedersen, M.; Fuchtbauer, E.M.; Wertz, K.; Stodkilde-Jorgensen, H.; Sulyok, E.; Doczi, T.; Neely, J.D.; Agre, P.; Frokiaer, J.; et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13131–13136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Duan, T.; Verkman, A.S. Aquaporin-4 reduces neuropathology in a mouse model of Alzheimer’s disease by remodeling peri-plaque astrocyte structure. Acta Neuropathol. Commun. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaraj, D.; Agerskov, S.; Rabiei, K.; Marlow, T.; Jensen, C.; Guo, X.; Kern, S.; Wikkelso, C.; Skoog, I. Vascular factors in suspected normal pressure hydrocephalus: A population-based study. Neurology 2016, 86, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelsson, H.; Carlberg, B.; Wikkelso, C.; Laurell, K.; Kahlon, B.; Leijon, G.; Eklund, A.; Malm, J. Vascular risk factors in INPH: A prospective case-control study (the INPH-CRasH study). Neurology 2017, 88, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Roman, G.C.; Jackson, R.E.; Fung, S.H.; Zhang, Y.J.; Verma, A.K. Sleep-Disordered Breathing and Idiopathic Normal-Pressure Hydrocephalus: Recent Pathophysiological Advances. Curr. Neurol. Neurosci. Rep. 2019, 19, 39. [Google Scholar] [CrossRef] [Green Version]
- Malm, J.; Graff-Radford, N.R.; Ishikawa, M.; Kristensen, B.; Leinonen, V.; Mori, E.; Owler, B.K.; Tullberg, M.; Williams, M.A.; Relkin, N.R. Influence of comorbidities in idiopathic normal pressure hydrocephalus—Research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, A.; Xiong, R.; Yu, J.; Liu, Y.; Liu, K.; Jin, G.; Xu, J.; Yan, J. Aquaporin-4 is a potential drug target for traumatic brain injury via aggravating the severity of brain edema. Burns Trauma 2021, 9, tkaa050. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Investig. 1997, 100, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galan-Cobo, A.; Ramirez-Lorca, R.; Toledo-Aral, J.J.; Echevarria, M. Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J. Cell. Physiol. 2016, 231, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Villadiego, J.; Romo-Madero, S.; Garcia-Swinburn, R.; Suarez-Luna, N.; Bermejo-Navas, A.; Echevarria, M.; Toledo-Aral, J.J. Long-term immunosuppression for CNS mouse xenotransplantation: Effects on nigrostriatal neurodegeneration and neuroprotective carotid body cell therapy. Xenotransplantation 2018, 25, e12410. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trillo-Contreras, J.L.; Toledo-Aral, J.J.; Villadiego, J.; Echevarría, M. Aquaporin-4 Mediates Permanent Brain Alterations in a Mouse Model of Hypoxia-Aged Hydrocephalus. Int. J. Mol. Sci. 2021, 22, 9745. https://doi.org/10.3390/ijms22189745
Trillo-Contreras JL, Toledo-Aral JJ, Villadiego J, Echevarría M. Aquaporin-4 Mediates Permanent Brain Alterations in a Mouse Model of Hypoxia-Aged Hydrocephalus. International Journal of Molecular Sciences. 2021; 22(18):9745. https://doi.org/10.3390/ijms22189745
Chicago/Turabian StyleTrillo-Contreras, José Luis, Juan José Toledo-Aral, Javier Villadiego, and Miriam Echevarría. 2021. "Aquaporin-4 Mediates Permanent Brain Alterations in a Mouse Model of Hypoxia-Aged Hydrocephalus" International Journal of Molecular Sciences 22, no. 18: 9745. https://doi.org/10.3390/ijms22189745
APA StyleTrillo-Contreras, J. L., Toledo-Aral, J. J., Villadiego, J., & Echevarría, M. (2021). Aquaporin-4 Mediates Permanent Brain Alterations in a Mouse Model of Hypoxia-Aged Hydrocephalus. International Journal of Molecular Sciences, 22(18), 9745. https://doi.org/10.3390/ijms22189745






