Decapitation Experiments Combined with the Transcriptome Analysis Reveal the Mechanism of High Temperature on Chrysanthemum Axillary Bud Formation
Abstract
1. Introduction
2. Results
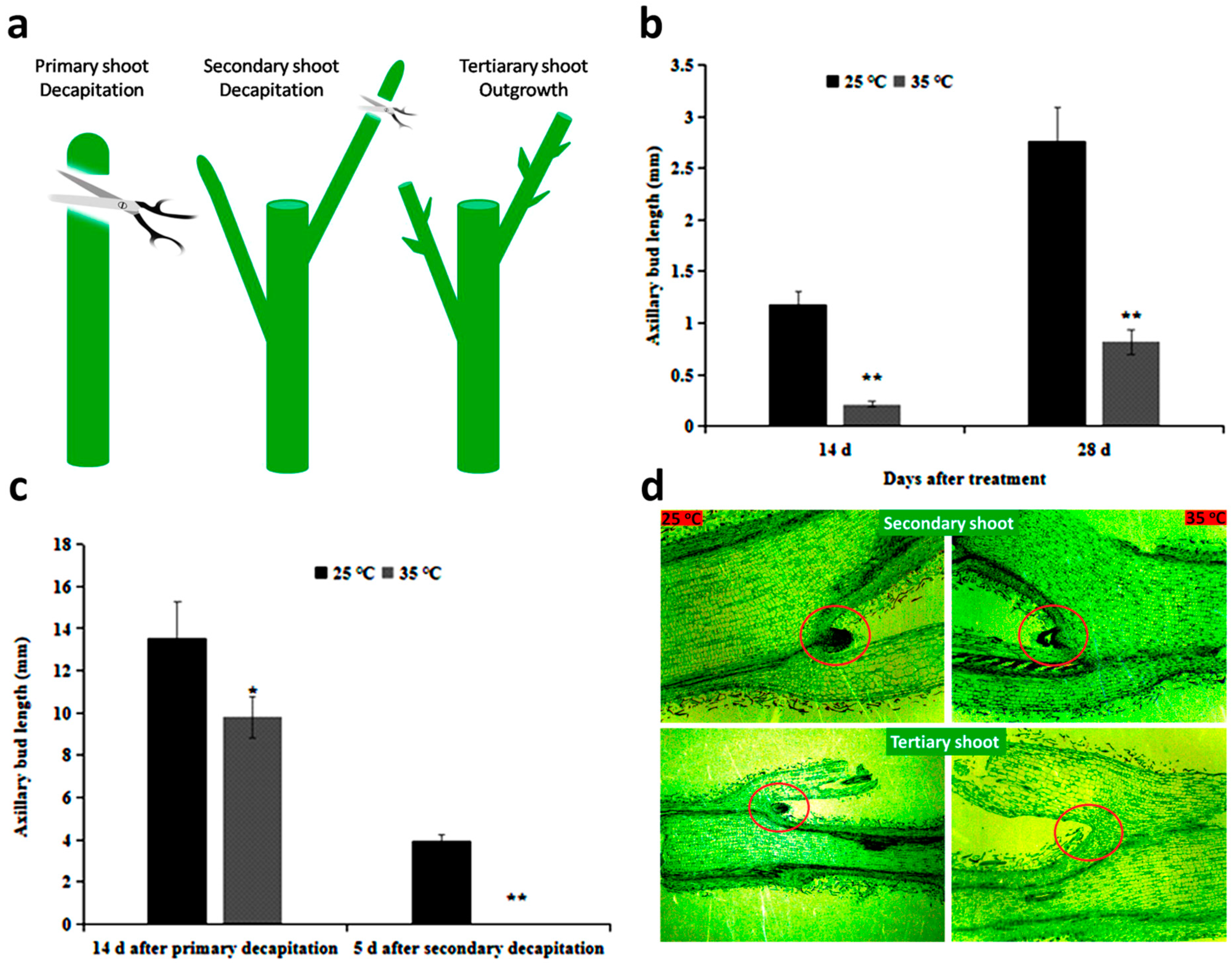
2.1. High Temperature Affects Axillary Bud Formation and Bud Outgrowth
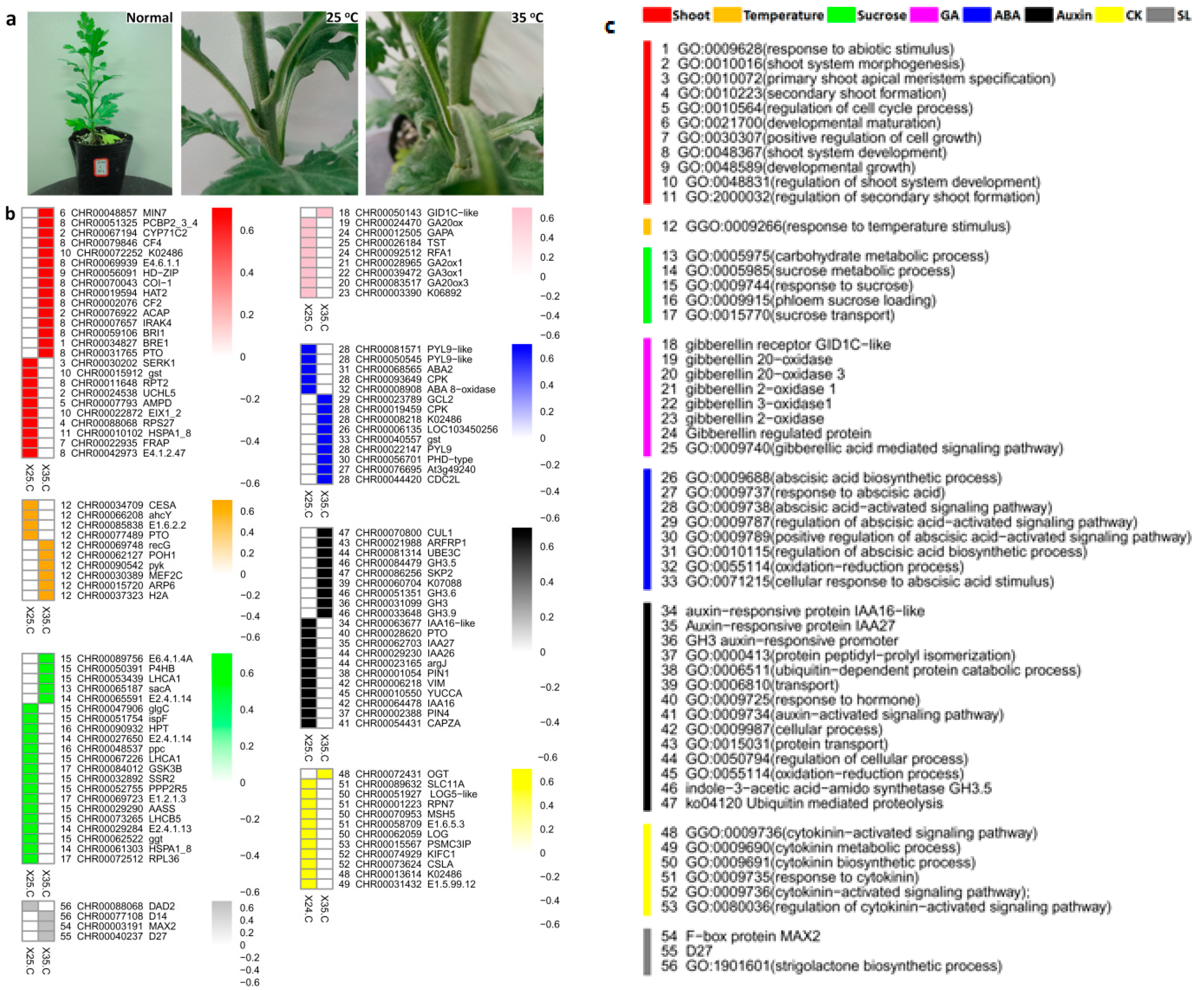
2.2. Comparative Analysis of Differentially Expressed Genes (DEGs) during Axillary Bud Formation at 25 °C and 35 °C Conditions
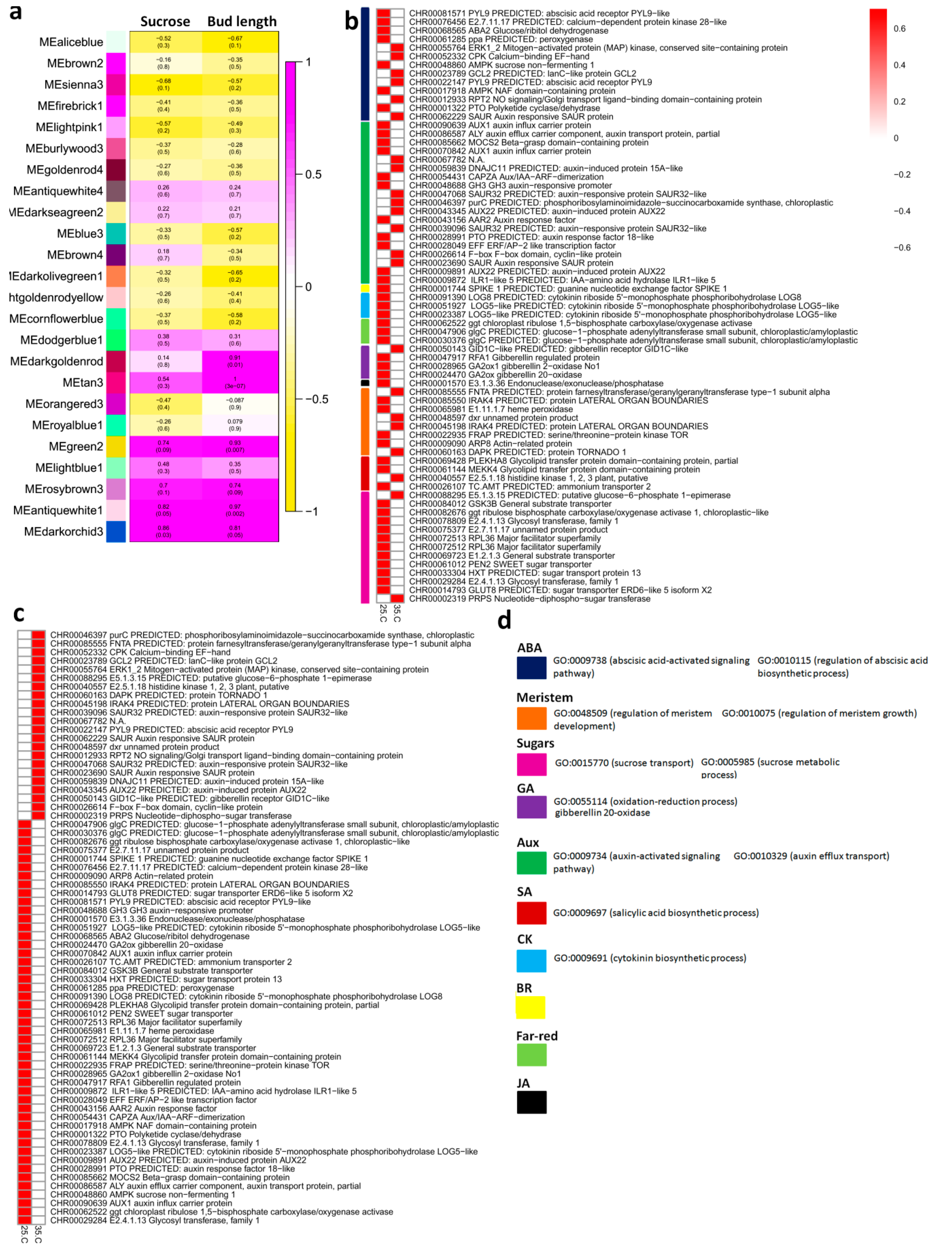
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA) for Sucrose Homeostasis and Bud Growth Dynamics
2.4. The Expression of Hormone-Related Core Genes Affected by High Temperature
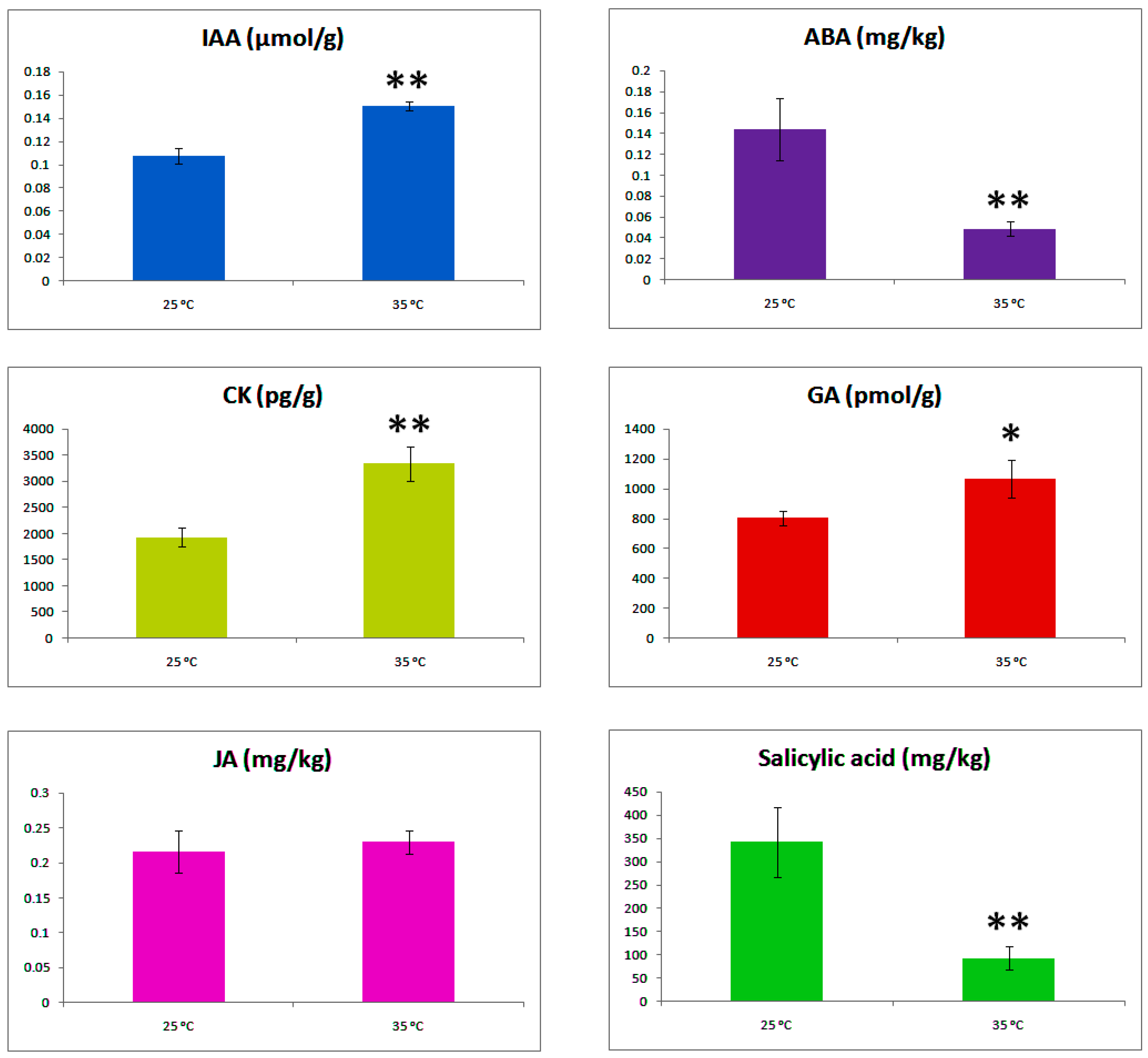
2.5. Hormonal Concentration Gradient and Axillary Bud Formation
3. Discussion
3.1. High Temperature Not Only Inhibited Axillary Bud Formation but Also Restricted Bud Outgrowth
3.2. Temperature Makes a Way to Arrest Bud Burst through Hormonal Network
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Plan
4.3. Microscopic Documentation of Axillary Buds under Different Temperature Regimes
4.4. Analysis of Hormones
4.5. RNA-Seq
4.6. Weighted Gene Coexpression Network Analysis (WGCNA)
4.7. Quantification of Gene Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Eunjoo, H.; Youngran, L.; Seongyoul, C.; Kyungran, D.; Chunho, P. Genotypic differences of axillary budding in response to temperature and ethephon treatment in non-branching chrysanthemums. Hortic. Environ. Biotechnol. 2007, 48, 370–375. [Google Scholar]
- Okamoto, A.; Suto, K. Morphological observation on viable and nonviable axillary bud formation in non-branching chrysanthemum ‘Iwanohakusen’. Jpn. Soc. Hortic. Sci. 2003, 75, 422–424. [Google Scholar] [CrossRef][Green Version]
- Li, J.X.; Wen, C.; Liu, F.L.; Yang, H.F.; Chen, X.L.; Xi, L.; Zhao, L.J. Effect of temperature on lateral bud formation of Chrysanthemum morifolium ‘fucashi’. J. China Agric. Univ. 2014, 19, 74–79. [Google Scholar] [CrossRef]
- Schoellhorn, R.K.; Barrett, J.E.; Bartuska, C.A.; Nell, T. Elevated temperature affects axillary meristem development in Dendranthema× grandiflorum‘Improved Mefo’. Hortscience Publ. Am. Soc. Hortic. Sci. 2001, 36, 1049–1052. [Google Scholar]
- Zhang, Q.Q.; Wang, J.G.; Wang, L.Y.; Wang, J.F.; Wang, Q.; Yu, P.; Bai, M.Y.; Fan, M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J. Integr. Plant Biol. 2020, 62, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Shi, B.; Yu, T.; Qi, J.; Meyerowitz, E.M.; Jiao, Y. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 2014, 26, 2055–2067. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, C.; Tian, C.; Wang, J.; Wang, Q. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet. 2016, 12, e1006168. [Google Scholar] [CrossRef]
- Wang, J.; Tian, C.; Cui, Z.; Shi, B.; Jiao, Y. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 2017, 29, 1373–1387. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Ottoline, L. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef]
- Mason, M.; Ross, J.; Babst, B.; Wienclaw, B.; Beveridge, C. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef]
- Sachs, T.; Thimann, K.V. The role of auxins and cytokinins in the release of buds from dominance. Am. J. Bot. 1967, 54, 136–144. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.; Dun, E.; Ferguson, B.; Rameau, C.; Beveridge, C. Strigolactone acts downstream of auxin to regulate bud outgrowth in Pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; Alexandre, D.S.G.; Catherine, R.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Braun, N.; De Saint Germain, A.; Luo, D.A.; Bendahmane, A.; Turnbull, C.; Rameau, C.; Pillot, J.-P.; Boutet-Mercey, S.; Dalmais, M.; Antoniadi, I.; et al. The Pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 2012, 158, 225–238. [Google Scholar] [CrossRef]
- Knox, J.P.; Wareing, P.F. Apical dominance in Phaseolus vulgaris L.: The possible roles of abscisic and indole-3-acetic acid. J. Exp. Bot. 1984, 35, 239–244. [Google Scholar] [CrossRef]
- Mader, J.C.; Emery, R.J.N.; Turnbull, C.G.N. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol. Plant. 2003, 119, 295–308. [Google Scholar] [CrossRef]
- Tamas, I.A.; Ozbun, J.L.; Wallace, D.H. Effect of fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol. 1979, 64, 615–619. [Google Scholar] [CrossRef]
- Gocal, G.F.W.; Pharis, R.P.; Yeung, E.C.; Pearce, D. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol. 1991, 95, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.E.; Cox, M.C.H.; Ross, J.J.; Krisantini, S.; Beveridge, C.A. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 2005, 138, 1665–1672. [Google Scholar] [CrossRef]
- Marquat, C.; Vandamme, M.; Gendraud, M.; Pétel, G. Dormancy in vegetative buds of peach: Relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Sci. Hortic. 1999, 79, 151–162. [Google Scholar] [CrossRef]
- Decourteix, M.; Alves, G.; Bonhomme, M.; Peuch, M.; Ben Baaziz, K.; Brunel, N.; Guilliot, A.; Rageau, R.; Améglio, T.; Pétel, G.; et al. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: Their potential role in early events of growth resumption. Tree Physiol. 2008, 28, 215–224. [Google Scholar] [CrossRef]
- Girault, T.; Abidi, F.; Sigogne, M.; Pelleschi-Travier, S.; Boumaza, R.; Sakr, S.; Leduc, N. Sugars are under light control during bud burst in Rosa sp: Photocontrol of sugars during bud burst. Plant Cell Environ. 2010, 33, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Rabot, A.; Laloi, M.; Mortreau, E.; Sigogne, M.; Leduc, N.; Lemoine, R.; Sakr, S.; Vian, A.; Pelleschi-Travier, S. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ. 2011, 34, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S.; et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef]
- Hall, C.R.; Dickson, M.W. Economic, environmental, and health/well-being benefi ts associated with green industry products and services: A review. J. Environ. Hortic. 2011, 29, 96–103. [Google Scholar] [CrossRef]
- Da Silva, J.A.T. Chrysanthemum: Advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol. Adv. 2003, 21, 715–766. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, L.; Challis, R.; Leyser, O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J. Exp. Bot. 2010, 61, 3069. [Google Scholar] [CrossRef]
- Beveridge, C.A. Axillary bud outgrowth: Sending a message. Curr. Opin. Plant Biol. 2006, 9, 35–40. [Google Scholar] [CrossRef]
- Janssen, B.J.; Drummond, R.S.; Snowden, K.C. Regulation of axillary shoot development. Curr. Opin. Plant Biol. 2014, 17, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dierck, R.; De Keyser, E.; De Riek, J.; Dhooghe, E.; Van Huylenbroeck, J.; Prinsen, E.; Van Der Straeten, D. Change in auxin and cytokinin levels coincides with altered expression of branching genes during axillary bud outgrowth in Chrysanthemum. PLoS ONE 2016, 11, e0161732. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.C.; Timpte, C.; Tan, S.; Callis, J.; Estelle, M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 1998, 280, 1760–1763. [Google Scholar] [CrossRef]
- Hellmann, H.; Hobbie, L.; Chapman, A.; Dharmasiri, S.; Dharmasiri, N.; Del Pozo, C.; Reinhardt, D.; Estelle, M. Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J. 2003, 22, 3314–3325. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; Chatfield, S.P.; Leyser, H.M. AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. Plant Physiol. 1999, 121, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Petrásek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef] [PubMed]
- Soundappan, I.; Bennett, T.; Morffy, N.; Liang, Y.; Stanga, J.P.; Abbas, A.; Leyser, O.; Nelson, D.C. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 2015, 27, 3143. [Google Scholar] [CrossRef]
- Nelson, D.C.; Adrian, S.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Beveridge, C.A.; Ghisalberti, E.L.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Plant Signal. Behav. 2011, 108, 8897–8902. [Google Scholar] [CrossRef]
- Gonzalez-Grandio, E.; Poza-Carrion, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 2013, 25, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, X.; Su, J.; Guan, Z.; Jiang, J.; Chen, F.; Fang, W.; Zhang, F. The genetics of planting density-dependent branching in chrysanthemum. Sci. Hortic. 2019, 256, 108598. [Google Scholar] [CrossRef]
- Dierck, R.; Leus, L.; Dhooghe, E.; Van Huylenbroeck, J.; De Riek, J.; Van Der Straeten, D.; De Keyser, E. Branching gene expression during chrysanthemum axillary bud outgrowth regulated by strigolactone and auxin transport. Plant Growth Regul. 2018, 86, 23–36. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Mullet, J.E. Transcriptome profiling of tiller buds provides new insights into PhyB regulation of tillering and indeterminate growth in Sorghum. Plant Physiol. 2016, 170, 2232. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Spielmeyer, W.; Finnegan, E.J. Grasses provide new insights into regulation of shoot branching. Trends Plant Sci. 2013, 18, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xi, L.; Kou, Y.; Zhao, Y.; Zhao, L. Current perspectives on shoot branching regulation. Front. Agric. Sci. Eng. 2015, 2, 38–52. [Google Scholar] [CrossRef]
- Faust, J.E.; Heins, R.D. High night temperatures do not cause poor lateral branching of chrysanthemum. HortScience 1992, 27, 981–982. [Google Scholar] [CrossRef]
- Bredmose, N.; Kristiansen, K.; Nørbæk, R.; Christensen, L.P.; Hansen-Møller, J. Changes in concentrations of cytokinins (CKs) in root and axillary bud tissue of miniature rose suggest that local CK biosynthesis and zeatin-type CKs play important roles in axillary bud growth. J. Plant Growth Regul. 2005, 24, 238. [Google Scholar] [CrossRef]
- Talanova, V.V.; Akimova, T.V.; Titov, A.F. Effect of whole plant and local heating on the ABA content in cucumber seedling leaves and roots and on their heat tolerance. Russ. J. Plant Physiol. 2003, 50, 90–94. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Waldie, T.; Hayward, A.; Beveridge, C.A. Axillary bud outgrowth in herbaceous shoots: How do strigolactones fit into the picture? Plant Mol. Biol. 2010, 73, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Holalu, S.V.; Finlayson, S.A. The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J. Exp. Bot. 2017, 68, 943–952. [Google Scholar] [PubMed]
- Liu, X.; Huang, B. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 442–447. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef]
- Bennett, T.; Hines, G.; Leyser, O. Canalization: What the flux? Trends Genet. 2014, 30, 41–48. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- Lilley, J.L.S.; Gee, C.W.; Sairanen, I.; Ljung, K.; Nemhauser, J.L. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012, 160, 2261–2270. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Mullet, J.E. Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ. 2015, 38, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Y.; Song, A.; Dong, G.; Zhao, H.; Sun, W.; Ramakrishnan, S.; Wang, Y.; Wang, S.; Li, T.; et al. The Chrysanthemum nankingense genome provides insights into the evolution and diversification of Chrysanthemum flowers and medicinal traits. Mol. Plant 2018, 11, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 1–45. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 1–13. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ahmad, S.; Yang, Q.; Yuan, C.; Zhang, Q. Decapitation Experiments Combined with the Transcriptome Analysis Reveal the Mechanism of High Temperature on Chrysanthemum Axillary Bud Formation. Int. J. Mol. Sci. 2021, 22, 9704. https://doi.org/10.3390/ijms22189704
Yang Y, Ahmad S, Yang Q, Yuan C, Zhang Q. Decapitation Experiments Combined with the Transcriptome Analysis Reveal the Mechanism of High Temperature on Chrysanthemum Axillary Bud Formation. International Journal of Molecular Sciences. 2021; 22(18):9704. https://doi.org/10.3390/ijms22189704
Chicago/Turabian StyleYang, Yujie, Sagheer Ahmad, Qingqing Yang, Cunquan Yuan, and Qixiang Zhang. 2021. "Decapitation Experiments Combined with the Transcriptome Analysis Reveal the Mechanism of High Temperature on Chrysanthemum Axillary Bud Formation" International Journal of Molecular Sciences 22, no. 18: 9704. https://doi.org/10.3390/ijms22189704
APA StyleYang, Y., Ahmad, S., Yang, Q., Yuan, C., & Zhang, Q. (2021). Decapitation Experiments Combined with the Transcriptome Analysis Reveal the Mechanism of High Temperature on Chrysanthemum Axillary Bud Formation. International Journal of Molecular Sciences, 22(18), 9704. https://doi.org/10.3390/ijms22189704






