Nucleobindin-2/Nesfatin-1—A New Cancer Related Molecule?
Abstract
1. Introduction
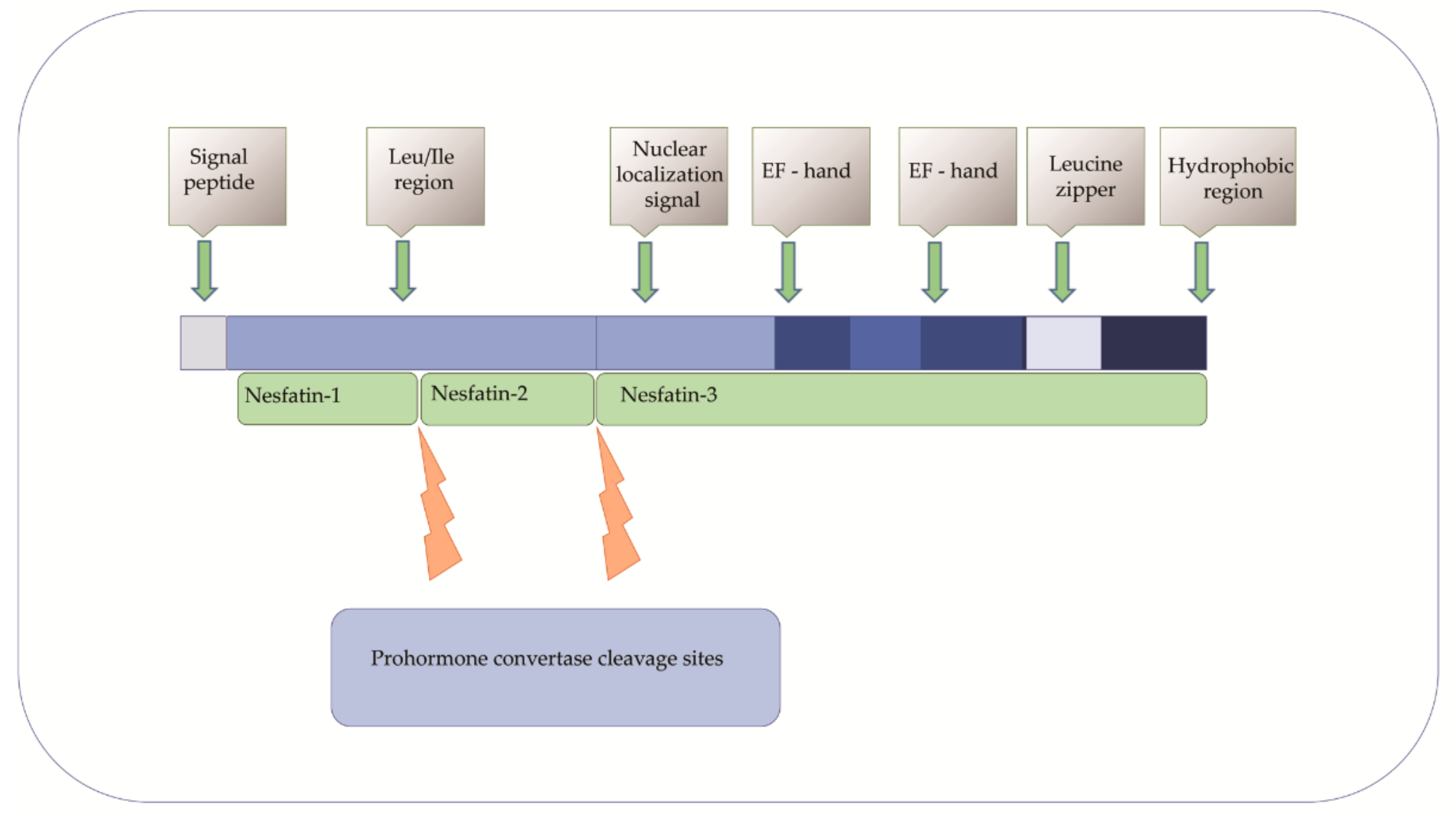
2. NUCB2/NESF-1 in Physiology
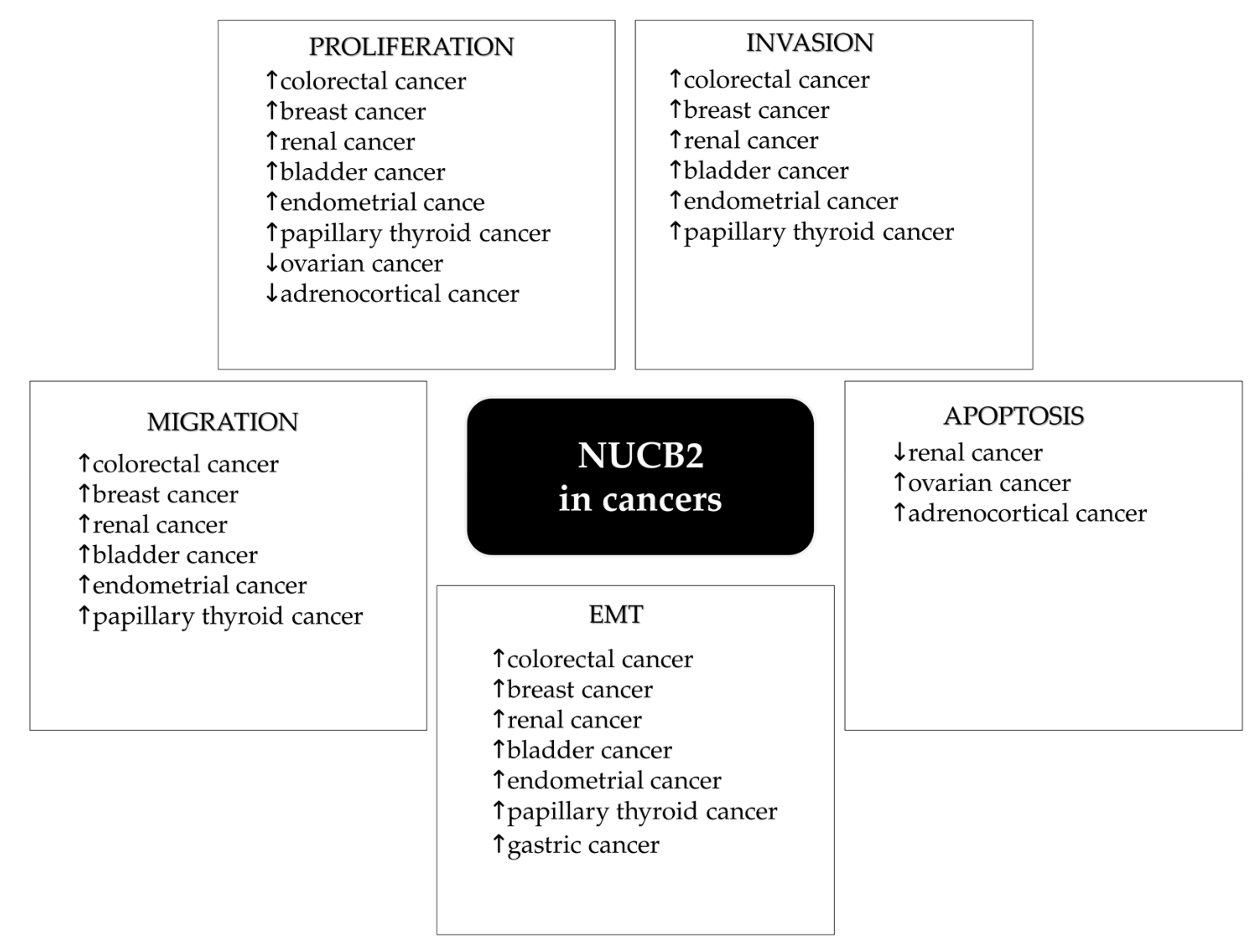
3. NUCB2—A Predictive/Prognostic Biomarker in Cancers?
3.1. Breast Cancer
3.2. Colon Cancer
3.3. Bladder Cancer
3.4. Prostate Cancer
3.5. Endometrial Cancer
3.6. Gastric Cancer
3.7. Papillary Thyroid Cancer
3.8. Renal Cell Carcinoma
3.9. Glioblastoma
4. Regulation of NUCB2 Expression in Cancers
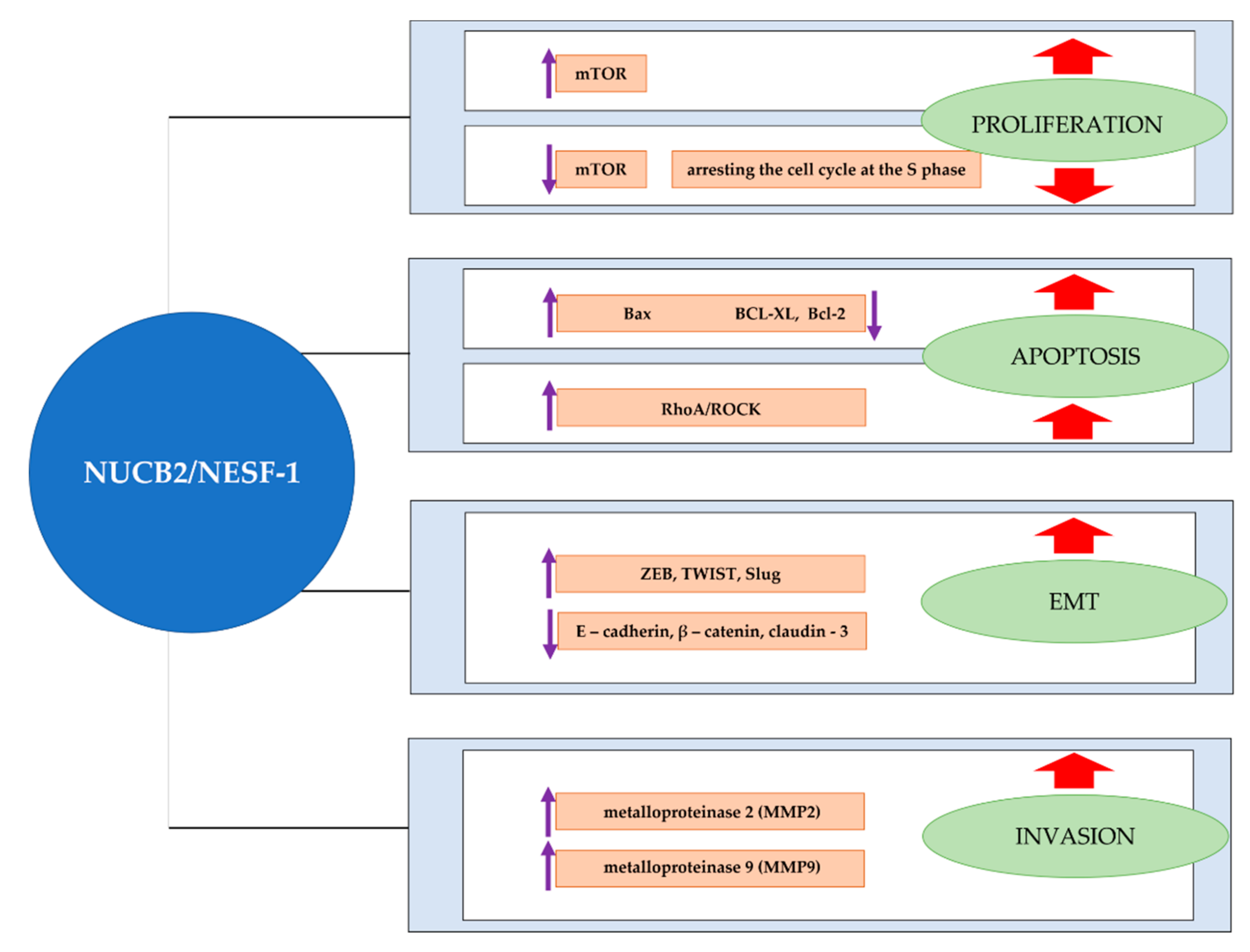
5. NUCB2 in Cancer Proliferation, Apoptosis, Migration and Invasion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skorupska, A.; Bystranowska, D.; Dąbrowska, K.; Ożyhar, A. Calcium ions modulate the structure of the intrinsically disordered Nucleobindin-2 protein. Int. J. Biol. Macromol. 2020, 154, 1091–1104. [Google Scholar] [CrossRef]
- Skorupska, A.; Ożyhar, A.; Bystranowska, D. The physiological role of nucleobindin-2/nesfatin-1 and their potential clinical significance. Postepy Hig. Med. Dosw. 2018, 72, 1084–1096. [Google Scholar] [CrossRef]
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Lembke, V.; Ahnis, A.; Elbelt, U.; Lambrecht, N.W.G.; Ordemann, J.; Klapp, B.F.; Kobelt, P. Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese subjects. Histochem. Cell Biol. 2013, 139, 909–918. [Google Scholar] [CrossRef]
- Foo, K.S.; Brauner, H.; Östenson, C.G.; Broberger, C. Nucleobindin-2/nesfatin in the endocrine pancreas: Distribution and relationship to glycaemic state. J. Endocrinol. 2010, 204, 255–263. [Google Scholar] [CrossRef]
- Angelone, T.; Filice, E.; Pasqua, T.; Amodio, N.; Galluccio, M.; Montesanti, G.; Quintieri, A.M.; Cerra, M.C. Nesfatin-1 as a novel cardiac peptide: Identification, functional characterization, and protection against ischemia/reperfusion injury. Cell. Mol. Life Sci. 2013, 70, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, H. Nesfatin-1 as a New Potent Regulator in Reproductive System. Dev. Reprod. 2012, 16, 253–264. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Dag, E.; Ozkan, Y.; Erman, F.; Dagli, A.F.; Kilic, N.; Sahin, I.; Karatas, F.; Yoldas, T.; Barim, A.O.; et al. Nesfatin-1 and ghrelin levels in serum and saliva of epileptic patients: Hormonal changes can have a major effect on seizure disorders. Mol. Cell. Biochem. 2009, 328, 49–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Shui, X.; Lian, X. Serum and synovial fluid nesfatin-1 concentration is associated with radiographic severity of knee osteoarthritis. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1078–1082. [Google Scholar] [CrossRef]
- Badillo-suárez, P.A.; Rodríguez-cruz, M.; Nieves-morales, X. Impact of Metabolic Hormones Secreted in Human Breast Milk on Nutritional Programming in Childhood Obesity. J. Mammary Gland. Biol. Neoplasia 2017, 22, 171–191. [Google Scholar] [CrossRef]
- Aydin, S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides 2010, 31, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Wang, H.Y.; Chen, X.; Guan, H.Z.; Jiang, Z.Y. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul. Pept. 2010, 159, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, C.; Wang, A.; Yao, B.; Li, L.; Wang, Y.; Xu, Y. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Horiguchi, K.; Umezawa, R.; Hashimoto, K.; Satoh, T.; Ozawa, A.; Shibusawa, N.; Monden, T.; Okada, S.; Shimizu, H.; et al. Troglitazone, a ligand of peroxisome proliferator-activated receptor-γ, stabilizes NUCB2 (nesfatin) mRNA by activating the ERK1/2 pathway: Isolation and characterization of the human NUCB2 gene. Endocrinology 2010, 151, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Gantulga, D.; Santoso, P.; Zhang, B.; Masuda, C.; Mori, M.; Okada, T.; Yada, T. Paraventricular NUCB2/nesfatin-1 supports oxytocin and vasopressin neurons to control feeding behavior and fluid balance in male mice. Endocrinology 2016, 157, 2322–2332. [Google Scholar] [CrossRef]
- Ayada, C.; Toru, Ü.; Korkut, Y. Nesfatin-1 and its effects on different systems. Hippokratia 2015, 19, 4–10. [Google Scholar] [PubMed]
- Miura, K.; Titani, K.; Kurosawa, Y.; Kanai, Y. Molecular cloning of nucleobindin, a novel DNA-binding protein that contains both a signal peptide and a leucine zipper structure. Biochem. Biophys. Res. Commun. 1992, 187, 375–380. [Google Scholar] [CrossRef]
- Taniguchi, N.; Taniura, H.; Niinobe, M.; Takayama, C.; Tominaga-Yoshino, K.; Ogura, A.; Yoshikawa, K. The postmitotic growth suppressor necdin interacts with a calcium-binding protein (NEFA) in neuronal cytoplasm. J. Biol. Chem. 2000, 275, 31674–31681. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; Juanatey, J.R.G.; Lago, F. Nesfatin-1: A new energy-regulating peptide with pleiotropic functions. Implications at cardiovascular level. Endocrine 2015, 52, 11–29. [Google Scholar] [CrossRef]
- Khalili, S.; Khaniani, M.S.; Afkhami, F.; Derakhshan, S.M. NUCB2/Nesfatin-1: A Potent Meal Regulatory Hormone and its Role in Diabetes. Egypt. J. Med. Hum. Genet. 2017, 18, 105–109. [Google Scholar] [CrossRef]
- García-Galiano, D.; Navarro, V.M.; Gaytan, F.; Tena-Sempere, M. Expanding roles of NUCB2/nesfatin-1 in neuroendocrine regulation. J. Mol. Endocrinol. 2010, 45, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Lago, F.; Pino, J.; Gómez-Reino, J.J.; Gualillo, O. NUCB2/nesfatin-1: A New Adipokine Expressed in Human and Murine Chondrocytes with Pro-Inflammatory Properties, An In Vitro Study. J. Orthop. Res. 2014, 32, 653–660. [Google Scholar] [CrossRef]
- Dore, R.; Levata, L.; Lehnert, H.; Schulz, C. Nesfatin-1: Functions and physiology of a novel regulatory peptide. J. Endocrinol. 2017, 232, R45–R65. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Catli, G.; Anik, A.; Kume, T.; Bober, E. The relation of serum nesfatin-1 level with metabolic and clinical parameters in obese and healthy children. Pediatr. Diabetes 2013, 14, 189–195. [Google Scholar] [CrossRef]
- Pałasz, A.; Krzystanek, M.; Worthington, J.; Czajkowska, B.; Kostro, K.; Wiaderkiewicz, R.; Bajor, G. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides 2012, 46, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. Current Understanding of the Role of Nesfatin-1. J. Endocr. Soc. 2018, 2, 1188–1206. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Wang, H.; Wang, G. NUCB2/nesfatin-1: Expression and functions in the regulation of emotion and stress. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 81, 221–227. [Google Scholar] [CrossRef]
- Atsuchi, K.; Asakawa, A.; Ushikai, M.; Ataka, K.; Tsai, M.; Koyama, K.; Sato, Y.; Kato, I.; Fujimiya, M.; Inui, A. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport 2010, 21, 1008–1011. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Nakata, M.; Nakae, J.; Mori, M.; Yada, T. Islet β-cell-produced NUCB2/nesfatin-1 maintains insulin secretion and glycemia along with suppressing UCP-2 in β-cells. J. Physiol. Sci. 2019, 69, 733–739. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Tang, Y.; Bi, F.; Liu, J.N. The novel function of nesfatin-1: Anti-hyperglycemia. Biochem. Biophys. Res. Commun. 2010, 391, 1039–1042. [Google Scholar] [CrossRef]
- Gonzalez, R.; Reingold, B.K.; Gao, X.; Gaidhu, M.P.; Tsushima, R.G.; Unniappan, S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet β- and MIN6 cells. J. Endocrinol. 2011, 208, R9–R16. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Fu, X.J.; Xu, X.L.; Wei, X.J.; Pan, H.S. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides 2012, 36, 39–45. [Google Scholar] [CrossRef]
- Islam, A.; Adamik, B.; Hawari, F.I.; Ma, G.; Rouhani, F.N.; Zhang, J.; Levine, S.J. Extracellular TNFR1 Release Requires the Calcium-dependent Formation of a Nucleobindin 2-ARTS-1 Complex. J. Biol. Chem. 2006, 281, 6860–6873. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; Otero, M.F.; Pereira, E.; Rubio, J.; Martínez, I.; Seoane, L.M.; Gualillo, O.; et al. Nesfatin-1 in human and murine cardiomyocytes: Synthesis, secretion, and mobilization of GLUT-4. Endocrinology 2013, 154, 4757–4767. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Li, X.; He, T.; Wang, Y.; Wang, Z.; Wang, S.; Xing, M.; Sun, W.; Ding, H. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides 2013, 46, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ayada, C.; Turgut, G.; Turgut, S.; Güçlü, Z. The effect of chronic peripheral nesfatin-1 application on blood pressure in normal and chronic restraint stressed rats: Related with circulating level of blood pressure regulators. Gen. Physiol. Biophys. 2015, 34, 81–88. [Google Scholar] [CrossRef]
- Galiano, D.G.; Pineda, R.; Ilhan, T.; Castellano, J.M.; Ruiz-Pino, F.; Sánchez-Garrido, M.A.; Vazquez, M.J.; Sangiao-Alvarellos, S.; Ruiz, A.R.; Pinilla, L.; et al. Cellular Distribution, Regulated Expression, and Functional Role of the Anorexigenic Peptide, NUCB2/Nesfatin-1, in the Testis. Endocrinology 2012, 153, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.; Stengel, A. Expression and regulation of peripheral NUCB2/nesfatin-1. Curr. Opin. Pharmacol. 2016, 31, 25–30. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Futur. Oncol. 2005, 1, 37–50. [Google Scholar] [CrossRef]
- Suzuki, S.; Takagi, K.; Miki, Y.; Onodera, Y.; Akahira, J.I.; Ebata, A.; Ishida, T.; Watanabe, M.; Sasano, H.; Suzuki, T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012, 103, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhong, J.; He, G.; Li, F.; Li, J.; Zhou, W.; Liu, W.; Zhang, Y.; Huang, S.; Liu, Z.; et al. Identification of nucleobindin-2 as a potential biomarker for breast cancer metastasis using iTRAQ-based quantitative proteomic analysis. J. Cancer 2017, 8, 3062–3069. [Google Scholar] [CrossRef]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Yen, M.C.; Wang, J.Y.; Wu, D.C.; Chiu, Y.J.; Ho, Y.W.; Kuo, P.L. Nesfatin-1/Nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget 2016, 7, 31336–31349. [Google Scholar] [CrossRef]
- Xie, J.; Chen, L.; Chen, W. High NUCB2 expression level is associated with metastasis and may promote tumor progression in colorectal cancer. Oncol. Lett. 2018, 15, 9188–9194. [Google Scholar] [CrossRef]
- Liu, G.-M.; Xu, Z.-Q.; Ma, H.-S. Nesfatin-1/Nucleobindin-2 Is a Potent Prognostic Marker and Enhances Cell Proliferation, Migration, and Invasion in Bladder Cancer. Dis. Markers 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Moon, K.T.; Lee, H.J.; Shin, S.C.; Choi, J.D.; Kang, J.Y.; Yoo, T.K. Nucleobindin 2 expression is an independent prognostic factor for bladder cancer. Medicine 2020, 99, e19597. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, C.; Li, L.; Luo, F.; Xu, Y. Clinical significance of NUCB2 mRNA expression in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, C.; Wang, A.; Li, L.; Xu, Y. High expression of nucleobindin 2 mRNA: An independent prognostic factor for overall survival of patients with prostate cancer. Tumor Biol. 2014, 35, 2025–2028. [Google Scholar] [CrossRef]
- Takagi, K.; Miki, Y.; Tanaka, S.; Hashimoto, C.; Watanabe, M.; Sasano, H.; Ito, K.; Suzuki, T. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: A potent prognostic factor associated with cell proliferation and migration. Endocr. J. 2016, 63, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Szarszewska, M.; Knapp, P.; Grybos, A.; Grybos, M.; Marszalek, A.; Filas, V.; Wojcik-krowiranda, K.; Swornik, M.; Markowska, J. The role of nesfatin and selected molecular factors in various types of endometrial cancer. Ginekol. Pol. 2019, 90, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Altan, B.; Kaira, K.; Okada, S.; Saito, T.; Yamada, E.; Bao, H.; Bao, P.; Takahashi, K.; Yokobori, T.; Tetsunari, O.; et al. High expression of nucleobindin 2 is associated with poor prognosis in gastric cancer. Tumor Biol. 2017, 39, 1–7. [Google Scholar] [CrossRef]
- Zhao, J.; Yun, X.; Ruan, X.; Chi, J.; Yu, Y.; Li, Y.; Zheng, X.; Gao, M. High expression of NUCB2 promotes papillary thyroid cancer cells proliferation and invasion. Onco. Targets. Ther. 2019, 12, 1309–1318. [Google Scholar] [CrossRef]
- Qi, C.; Ma, H.; Zhang, H.T.; Gao, J.D.; Xu, Y. Nucleobindin 2 expression is an independent prognostic factor for clear cell renal cell carcinoma. Histopathology 2015, 66, 650–657. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, Y.; Wang, Y.; Liu, Z.; Zhang, J.; Wang, Z.; Xie, H.; Dai, B.; Xu, J.; Ye, D. High NUCB2 expression level represents an independent negative prognostic factor in Chinese cohorts of non-metastatic clear cell renal cell carcinoma patients. Oncotarget 2017, 8, 35244–35254. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xu, H.; Shi, Q.; Li, L.; Zhou, J.; Yu, G.; Xu, B.; Liu, Y.; Wang, Z.; Li, W. Nucleobindin 2 ( NUCB2 ) in renal cell carcinoma: A novel factor associated with tumor development. Int. J. Clin. Exp. Med. 2019, 12, 8686–8693. [Google Scholar]
- Tao, R.; Niu, W.B.; Dou, P.H.; Ni, S.B.; Yu, Y.P.; Cai, L.C.; Wang, X.Y.; Li, S.Y.; Zhang, C.; Luo, Z.G. Nucleobindin - 2 enhances the epithelial - mesenchymal transition in renal cell carcinoma. Oncol. Lett. 2020, 19, 3653–3664. [Google Scholar] [CrossRef]
- Liu, Q.J.; Lv, J.X.; Liu, J.; Zhang, X.B.; Wang, L.B. Nucleobindin-2 promotes the growth and invasion of glioblastoma. Cancer Biother. Radiopharm. 2019, 34, 581–588. [Google Scholar] [CrossRef]
- Driscoll, M.D.; Sathya, G.; Muyan, M.; Klinge, C.M.; Hilf, R.; Bambara, R.A. Sequence requirements for estrogen receptor binding to estrogen response elements. J. Biol. Chem. 1998, 273, 29321–29330. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Chao, Y.; Zhang, L.; Jin, L.; Li, N.; He, R.; Ma, B.; Zhao, W.; Han, C. Regulation of the adaptation to ER stress by KLF4 facilitates melanoma cell metastasis via upregulating NUCB2 expression. J. Exp. Clin. Cancer Res. 2018, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of microRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Davis-Dusenbery, B.N.; Hata, A. MicroRNA in cancer: The involvement of aberrant microRNA biogenesis regulatory pathways. Genes Cancer 2010, 1, 1100–1114. [Google Scholar] [CrossRef]
- Huo, X.; Wang, H.; Huo, B.; Wang, L.; Yang, K.; Wang, J.; Wang, L.; Wang, H. FTX contributes to cell proliferation and migration in lung adenocarcinoma via targeting miR-335-5p/NUCB2 axis. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar]
- Xin, R.; Qu, D.; Xu, H.; Chen, D. circ_001504 promotes the development of renal cell carcinoma by sponging microRNA-149 to increase NUCB2. Cancer Gene Ther. 2020, 28, 667–678. [Google Scholar] [CrossRef]
- Su, M.; Xiao, Y.; Ma, J.; Tang, Y.; Tian, B.; Zhang, Y.; Li, X.; Wu, Z.; Yang, D.; Zhou, Y.; et al. Circular RNAs in Cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Zhao, Q.; Ma, W.; Liu, J. MiR-30a-5p inhibits proliferation, migration and invasion of nasopharyngeal carcinoma cells by targeting NUCB2. Hum. Exp. Toxicol. 2021, 40, 1274–1285. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneya, M.; Tan, B.K.; Rucinski, M.; Kawan, M.; Hu, J.; Kaur, J.; Patel, V.H.; Malendowicz, L.K.; Komarowska, H.; Lehnert, H.; et al. Nesfatin-1 inhibits proliferation and enhances apoptosis of human adrenocortical H295R cells. J. Endocrinol. 2015, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pang, X.; Dong, M.; Wen, F.; Zhang, Y. Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation in vitro. Biochem. Biophys. Res. Commun. 2013, 440, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hay, B.A. Cell proliferation and apoptosis. Curr. Opin. Cell Biol. 1999, 11, 745–752. [Google Scholar] [CrossRef]
- Letai, A. Apoptosis and cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Qi, K.A.I.; Zhou, J.; Gu, M.; Wang, Z. A novel function of NUCB2 in promoting the development and invasion of renal cell carcinoma. Oncol. Lett. 2018, 15, 2425–2430. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of Mcf7. Int. J. Cancer Res. Treat. 2015, 3154, 3147–3154. [Google Scholar] [CrossRef]
- Pereira, A.M.M.; Strasberg-Rieber, M.; Rieber, M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin. Exp. Metastasis 2005, 22, 285–295. [Google Scholar] [CrossRef]
- Son, H.; Moon, A. Epithelial-mesenchymal transition and cell invasion. Toxicol. Res. 2013, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Steele, N.G.; Carpenter, E.S.; Donahue, K.L.; Bushnell, G.G.; Morris, A.H.; The, S.; Orbach, S.M.; Sirihorachai, V.R.; Nwosu, Z.C.; et al. Pancreatic cancer is marked by complement-high blood monocytes and tumor-associated macrophages. Life Sci. Alliance 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Assi, M.; Dauguet, N.; Jacquemin, P. DIE-RNA: A reproducible strategy for the digestion of normal and injured pancreas, isolation of pancreatic cells from genetically engineered mouse models and extraction of high quality RNA. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tosti, L.; Hang, Y.; Debnath, O.; Tiesmeyer, S.; Trefzer, T.; Steiger, K.; Ten, F.W.; Lukassen, S.; Ballke, S.; Kühl, A.A.; et al. Single-Nucleus and In Situ RNA–Sequencing Reveal Cell Topographies in the Human Pancreas. Gastroenterology 2021, 160, 1330–1344. [Google Scholar] [CrossRef]
- Ham, B.; Fernandez, M.C.; D’Costa, Z.; Brodt, P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res. 2016, 11, 1–27. [Google Scholar] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Cui, X.; Hawari, F.; Alsaaty, S.; Lawrence, M.; Combs, C.A.; Geng, W.; Rouhani, F.N.; Miskinis, D.; Levine, S.J. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J. Clin. Investig. 2002, 110, 515–526. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Cancer Type | Evaluation Method | NUCB2/NESF-1 Expression/Function | Ref. |
|---|---|---|---|
| Breast cancer | IHC | NUCB2/NESF-1 was positively associated with the ER status of breast carcinoma patients, lymph node metastasis, clinical stage, and an increased risk of recurrence; NUCB2/NESF-1 was an independent prognostic factor for disease-free survival. | [41,42] |
| In vitro | Inhibition of NUCB2/NESF-1 expression in MCF-7 and SKBR-3 resulted in decreased proliferation, invasion, and migration properties; NUCB2 expression was upregulated by estradiol in ER-positive MCF-7 cells. | ||
| Colon cancer | IF | The expression of NUCB2/NESF-1 in cancer was higher than in non-tumor regions; NUCB2/NESF-1 was predominantly expressed in the cytoplasm and much less in the cancer cell membrane. The results also indicated a positive correlation between NUCB2/NESF-1 expression and lymph node metastasis and the TNM stage. | [45] |
| ELISA | There was no difference in serum nesfatin-1 concentration between healthy donors and colon cancer patients. | ||
| In vitro | NUCB2/NESF-1 enhanced EMT, migration, and invasion in colon cancer cells. | ||
| Bladder cancer | IHC | High expression of NUCB2/NESF-1 was associated with distant metastasis and vascular invasion. Patients with high NUCB2/NESF-1 had poor overall survival and progression-free survival rates | [46] |
| In vitro | Suppression of NUCB2/NESF-1 using shRNA in T24 and 5637 bladder cancer cell lines resulted in the inhibition of cell proliferation, migration, and invasion abilities compared to the control. NUCB2/NESF-1 knockdowned cells had a lower expression of MMP2 and MMP9. | ||
| In vivo | The growth of tumors from NUCB2/NESF-1knockdowned cells in BALB/c mice was slower compared to the control, and lung metastases were observed only in the control group. | ||
| Ovarian cancer | In vitro | ||
| Administration of recombinant human nesfatin-1 resulted in enhanced apoptosis and decreased ovarian cancer cell proliferation. | [70] | ||
| Prostate cancer | real time RT—PCR, IHC | Expression of NUCB2/NESF-1 was significantly higher in cancer cells than noncancerous control and correlated with higher Gleason scores, higher levels of preoperative PSA, positive lymph node metastasis and positive angiolymphatic invasion. High NUCB2/NESF-1 mRNA level was an independent predictor of shorter BCR-free survival. Multivariate Cox analysis indicated that NUCB2/NESF-1 mRNA was an independent prognostic factor for the overall survival of prostate cancer patients. | [47,48,49] |
| Gastric cancer | IHC | NUCB2 was localized in the nuclei, and its expression was higher in the tumor than in the adjacent normal tissues. NUCB2 was significantly associated with tumor depth, lymph node metastasis, lymphatic invasion, venous invasion, and clinical stage. NUC2/NESF-1 was an independent predictor of progression-free survival. A positive correlation was found between the expression of NUCB2/NESF-1 and EMT-related genes such as DSP, ITGAV, MMP3, TSPAN13, and CDH2. | [52] |
| Papillary thyroid cancer | IHC | Papillary thyroid cancer showed nucleus and cytoplasmic expression with no staining in the normal tissue adjacent to cancer. NUCB2/NESF-1 was significantly associated with extrathyroidal extension, TNM stage, and tumor size. | [53] |
| In vitro | Inhibition of NUCB2/NESF-1 expression in TPC-I and KI thyroid cell lines resulted in decreased proliferation, invasion, and migration properties and decreased expression of invasion-related proteins (MMP-2 and MMP). | ||
| Renal cell carcinoma | IHC | The expression of NUCB2/NESF-1 was significantly higher in the tumor compared to the control. NUCB2/NESF-1 expression was associated with the T stage and the presence of metastasis. High NUCB2/NESF-1 expression was associated with a shorter overall survival rate. NUCB2/NESF-1 was an independent prognostic factor for overall survival. NUCB2 was positively correlated with the Fuhrman grade (p < 0.002) and the presence of necrosis. | [55,56,57] |
| In vitro | Inhibition of NUCB2/NESF-1 expression in the 786-O renal cancer cell line resulted in an increased apoptosis rate and a decreased invasion rate. NUCB2/NESF-1 knockout in the SK-RC-52 cell line inhibited migration, invasion, and affected EMT-related proteins | ||
| Adreno- cortical cancer | In vitro | Treatment of H295R cells with nesfatin-1resulted in a decreased proliferative capacity. Nesfatin-1 induced a concentration-dependent increase in apoptosis of H295R cells compared to control cells | [69] |
| Glioblastoma | Real-time RT- PCR, IHC | mRNA expression level of NUCB2/NESF-1 in glioblastoma was significantly higher than in normal tissues. NUCB2/NESF-1 was predominantly expressed in the nucleus and was highly overexpressed in glioblastoma compared to the adjacent tissue. High expression of NUCB2/NESF-1 was related to recurrence. | [58] |
| In vitro | Inhibition of NUCB2/NESF-1 expression in U251 and U87 glioblastoma cell lines resulted in decreased proliferation, invasion, and migration properties | ||
| In vivo | The tumor volume of the NUCB2/NESF-1 ablation group was significantly smaller than the control; the occurrence of lung metastasis was decreased in mice injected with NUCB2/NESF-1 knockdowned U251 cells compared to the control. | ||
| Endometrial cancer | IHC | NUCB2/NESF-1 was positively correlated with the Ki-67 marker of proliferation. No association was found between NUCB2/NESF-1 expression and other clinicopathological parameters. NUCB2/NESF-1 status was significantly associated with an increased risk of recurrence (p = 0.004). NUCB2/NESF-1 status was an independent prognostic factor for disease-free survival and cancer-specific survival. | [50] |
| In vitro | Knockdown of NUCB2/NESF-1 using a specific siRNA significantly inhibited Ishikawa and Sawano endometrial cancer cell proliferation and migration. Treatment of Ishikawa cells with recombinant nesfatin-1 also promoted cell proliferation and migration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmiecik, A.M.; Dzięgiel, P.; Podhorska-Okołów, M. Nucleobindin-2/Nesfatin-1—A New Cancer Related Molecule? Int. J. Mol. Sci. 2021, 22, 8313. https://doi.org/10.3390/ijms22158313
Kmiecik AM, Dzięgiel P, Podhorska-Okołów M. Nucleobindin-2/Nesfatin-1—A New Cancer Related Molecule? International Journal of Molecular Sciences. 2021; 22(15):8313. https://doi.org/10.3390/ijms22158313
Chicago/Turabian StyleKmiecik, Alicja M., Piotr Dzięgiel, and Marzenna Podhorska-Okołów. 2021. "Nucleobindin-2/Nesfatin-1—A New Cancer Related Molecule?" International Journal of Molecular Sciences 22, no. 15: 8313. https://doi.org/10.3390/ijms22158313
APA StyleKmiecik, A. M., Dzięgiel, P., & Podhorska-Okołów, M. (2021). Nucleobindin-2/Nesfatin-1—A New Cancer Related Molecule? International Journal of Molecular Sciences, 22(15), 8313. https://doi.org/10.3390/ijms22158313







