Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia
Abstract
1. Introduction
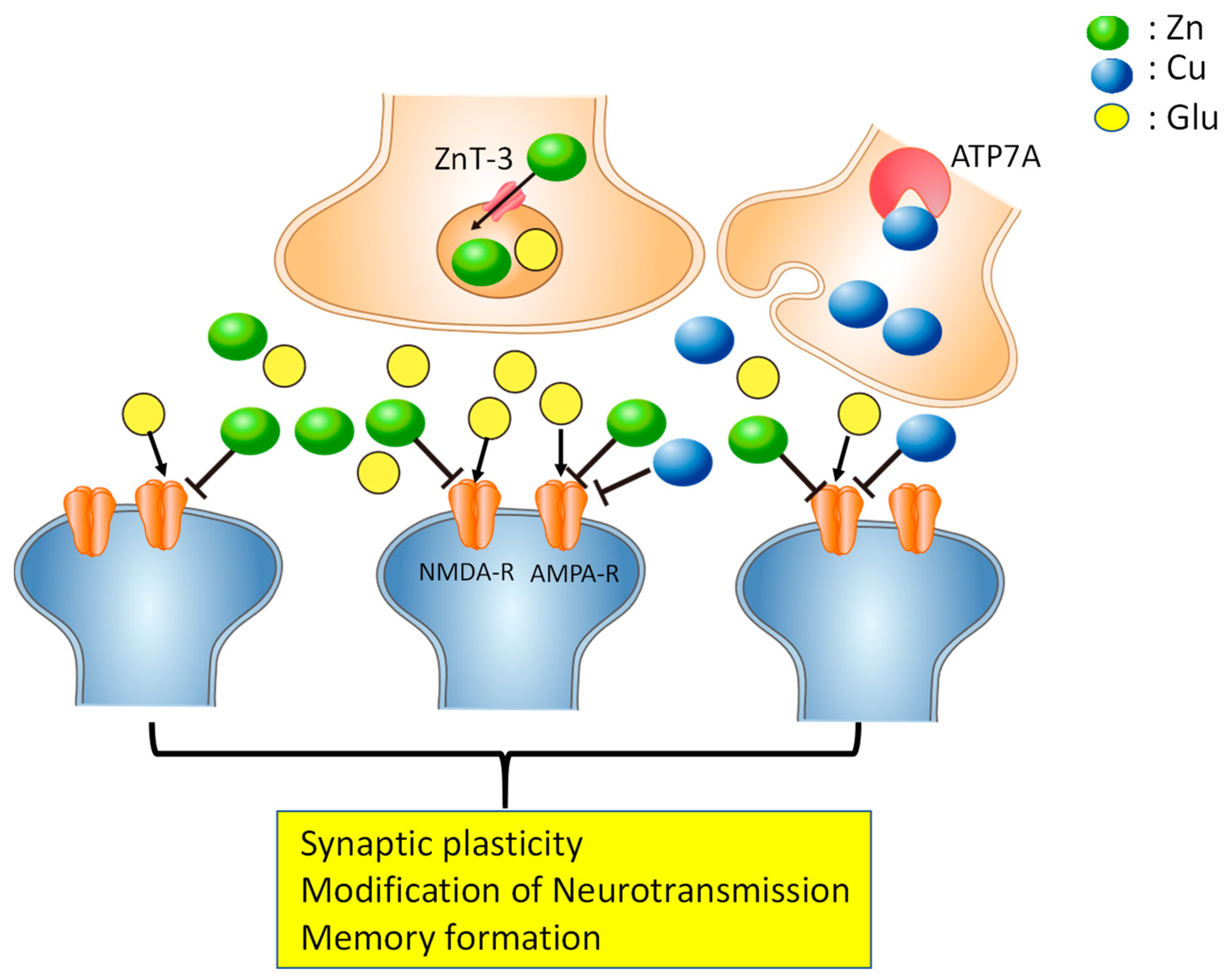
2. Copper in the Brain
3. Vascular Dementia and Zinc
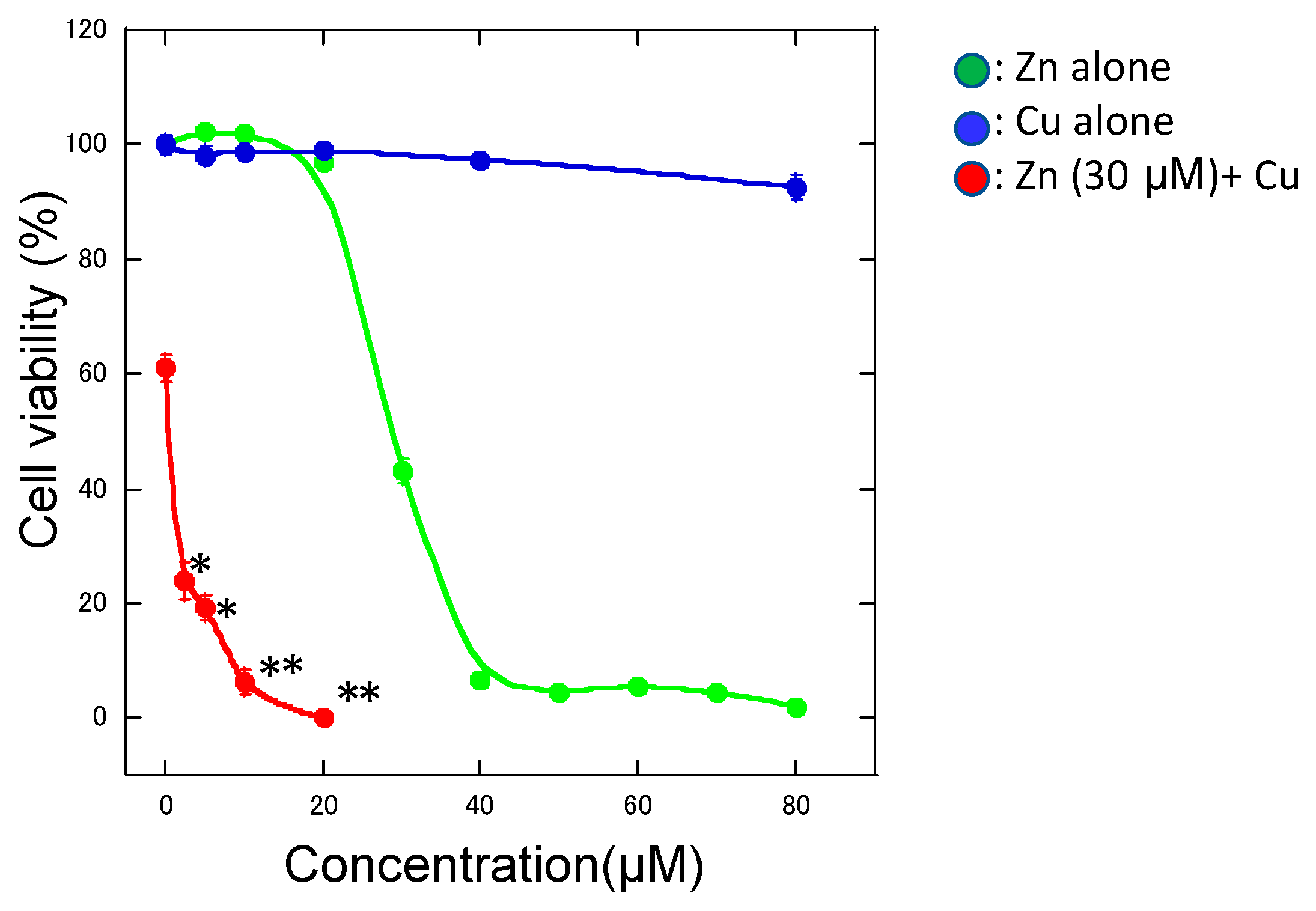
4. Copper Enhances Zinc-Induced Neurotoxicity
5. The Molecular Pathways of Copper-Enhanced Zinc-Induced Neurotoxicity
5.1. ER Stress Pathway
5.2. SAPK/JNK Pathway
5.3. Energy Production Pathway
5.4. Disruption of Ca2+ Homeostasis
5.5. ROS Production
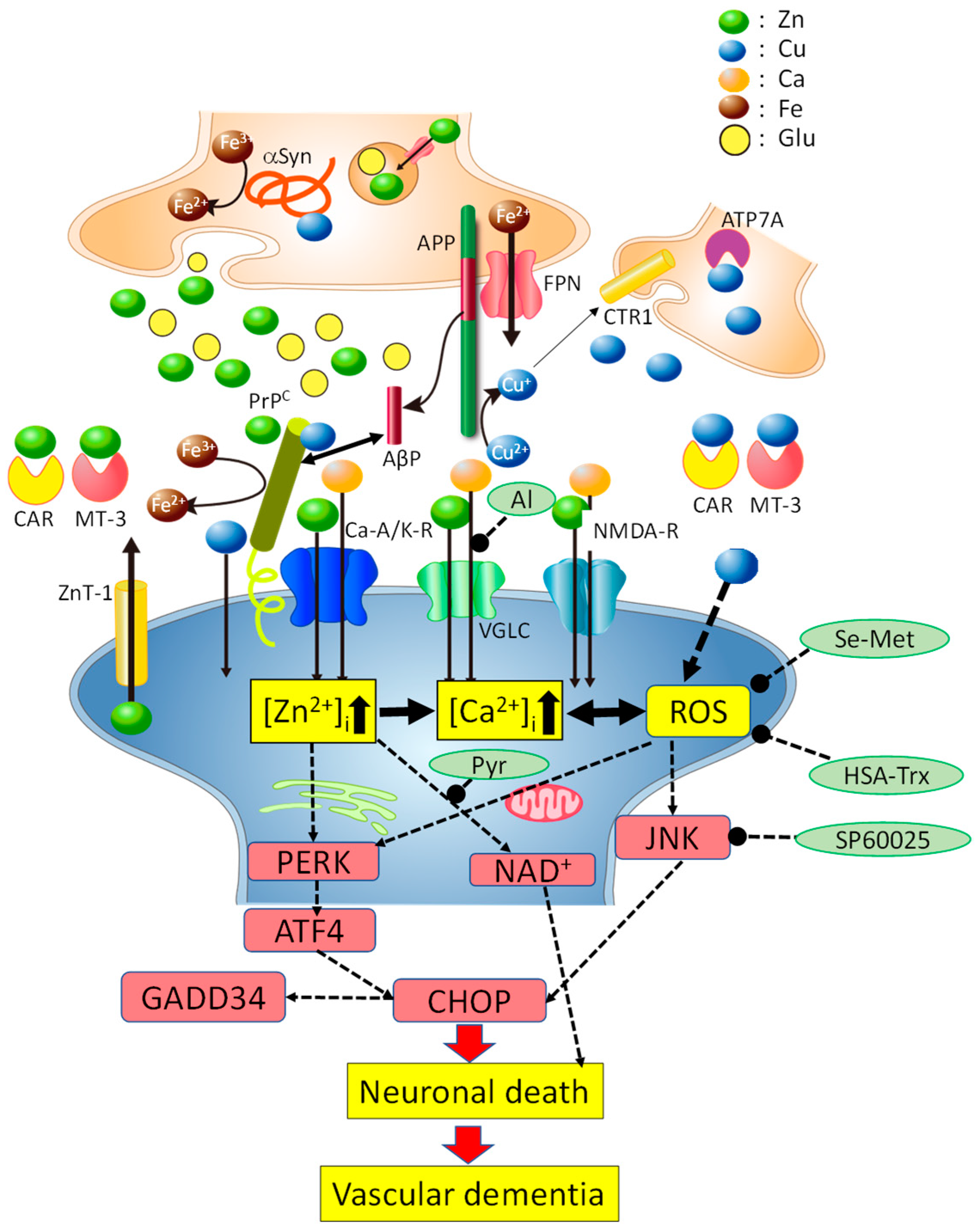
6. Hypothetical Scheme Regarding Cu/Zn Neurotoxicity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AβP | Alzheimer’s β-amyloid protein |
| ATF7A | copper-transporting ATPase 7A |
| AMPA | α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid |
| CSF | cerebrospinal fluid |
| CHOP | CCAAT-enhancer-binding protein homologous protein |
| CJD | Creutzfeldt–Jakob disease |
| CTR1 | copper transporter 1 |
| D-APV | 2-amino-5-phosphonovalerate |
| DLB | dementia with Lewy bodies |
| DMT1 | divalent metal transporter 1 |
| ER | endoplasmic reticulum |
| GABA | γ-aminobutyric acid |
| GADD34 | growth-arrest and DNA-damage-inducible gene 34 |
| [Ca2+]i | intracellular calcium levels |
| NMDA | N-methyl-d-aspartate |
| NAC | non-amyloid component |
| PD | Parkinson’s disease |
| PrP | prion protein |
| ROS | reactive oxygen species |
| SAPK/JNK | stress-activated protein kinases/c-Jun amino-terminal kinases |
| VD | vascular dementia |
| VGCC | voltage-gated Ca2+ channel |
| CSF | cerebrospinal fluid |
| DMT1 | divalent metal transporter-1 |
References
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, K.; Masaldan, S.; Opazo, C.M.; Bush, A.I. Redox active metals in neurodegenerative diseases. J. Biol. Inorg. Chem. 2019, 24, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato-Negishi, M.; Tanaka, K.-I. Neurometals in the Pathogenesis of Prion Diseases. Int. J. Mol. Sci. 2021, 22, 1267. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bea, F.J.; Aldanondo, G.; Lasa-Fernández, H.; de Munain, A.L.; Vallejo-Illarramendi, A. Insights into the mechanisms of copper dyshomeostasis in amyotrophic lateral sclerosis. Expert Rev. Mol. Med. 2017, 19, e7. [Google Scholar] [CrossRef]
- Lee, J.M.; Grabb, M.C.; Zipfel, G.Z.; Choi, D.W. Brain tissue responses to ischemia. J. Clin. Investig. 2000, 106, 723–731. [Google Scholar] [CrossRef]
- Pochwat, B.; Nowak, G.; Szewczyk, B. Relationship between Zinc (Zn2+) and glutamate receptors in the processes underlying neurodegeneration. Neural Plast. 2015, 591563. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Sadakane, Y.; Mizuno, K.; Kato-Negishi, M.; Tanaka, K.-I. Carnosine as a possible drug for zinc-induced neurotoxicity and vascular dementia. Int. J. Mol. Sci. 2020, 21, 2570. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Kawahara, M. Copper enhances zinc-induced neurotoxicity and the endoplasmic reticulum stress response in a neuronal model of vascular dementia. Front. Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Shimoda, M.; Kasai, M.; Ikeda, M.; Ishima, Y.; Kawahara, M. Involvement of SAPK/JNK signaling pathway in copper enhanced zinc-induced neuronal cell death. Toxicol. Sci. 2019, 169, 293–302. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Shimoda, M.; Chuang, V.T.G.; Nishida, K.; Kawahara, M.; Ishida, T.; Otagiri, M.; Maruyama, T.; Ishima, Y. Thioredoxin-albumin fusion protein prevents copper enhanced zinc-induced neurotoxicity via its antioxidative activity. Int. J. Pharm. 2018, 535, 140–147. [Google Scholar] [CrossRef]
- Nakano, Y.; Shimoda, M.; Okudomi, S.; Kawaraya, S.; Kawahara, M.; Tanaka, K.-I. Seleno-L-methionine suppresses copper-enhanced zinc-induced neuronal cell death via induction of glutathione peroxidase. Metallomics 2020, 12, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fujisawa, C.; Bhadhprasit, B. Inherited copper transport disorders: Biochemical mechanisms, diagnosis, and treatment. Curr. Drug Metab. 2012, 13, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.M.; Greenough, M.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Chang, J.; Kim, J. Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. J. Neurochem. 2016, 138, 918–928. [Google Scholar] [CrossRef]
- Meskini, R.E.; Kelli, J.; Crabtree, L.; Cline, L.B.; Mains, R.E.; Eipper, A.; Ronnett, G.V. ATP7A (Menkes protein) functions in axonal targeting and synaptogenesis. Mol. Cell. Neurosci. 2007, 34, 409–421. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H. The impact of synaptic Zn2+ dynamics on cognition and its decline. Int. J. Mol. Sci. 2017, 18, 2411. [Google Scholar] [CrossRef]
- Ueno, S.; Tsukamoto, M.; Hirano, T.; Kikuchi, K.; Yamada, M.K.; Nishiyama, N.; Nagano, N.; Matsuki, N.; Ikegaya, Y. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-d-aspartate receptor activity in hippocampal CA3 circuits. J. Cell Biol. 2002, 158, 215–220. [Google Scholar] [CrossRef]
- Huston, J.P.; Wagner, U.; Hasenöhrl, R.U. The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: Evidence for an inhibitory role. Behav. Brain Res. 1997, 83, 97–105. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Simon, A.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Gybina, A.A.; Tkac, I.; Prohaska, J.R. Copper deficiency alters the neurochemical profile of developing rat brain. Nutr. Neurosci. 2009, 12, 114–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roos, P.M.; Vesterberg, O.; Syversen, T.; Flaten, T.P.; Nordberg, M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013, 151, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Schikorski, T.; Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997, 17, 5858–5867. [Google Scholar] [CrossRef]
- Vogt, K.; Mellor, J.; Tong, G.; Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 2000, 26, 187–196. [Google Scholar] [CrossRef]
- Kardos, J.; Kovács, I.; Hajós, F.; Kálmán, N.; Simonyi, M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989, 103, 139–144. [Google Scholar] [CrossRef]
- Hopt, A.; Korte, S.; Fink, H.; Panne, U.; Niessner, R.; Jahn, R.; Herms, J. Methods for studying synaptosomal copper release. J. Neurosci. Methods 2003, 128, 159–172. [Google Scholar] [CrossRef]
- Román, G.C. Vascular dementia prevention: A risk factor analysis. Cerebrovasc. Dis. 2005, 20, 91–100. [Google Scholar] [CrossRef]
- De Haan, E.H.; Nys, G.M.; Van Zandvoort, M.J. Cognitive function following stroke and vascular cognitive impairment. Curr. Opin. Neurol. 2006, 19, 559–564. [Google Scholar]
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn2+: A novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000, 21, 395–401. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Hernandez, M.D.; Goik, S.A.; Morton, J.D.; McGinty, J.F. Loss of zinc staining from hippocampal mossy fibers during kainic acid induced seizures: A histofluorescence study. Brain Res. 1988, 446, 383–386. [Google Scholar]
- Koh, J.Y.; Choi, D.W. Zinc toxicity of cultured cortical neurons: Involvement of N-methyl-d-asparatate receptors. Neuroscience 1994, 4, 1049–1057. [Google Scholar] [CrossRef]
- Kim, A.H. L-type Ca2+ channel-mediated Zn2+ toxicity and modulation by ZnT-1 in PC12 cells. Brain Res. 2000, 886, 99–107. [Google Scholar] [CrossRef]
- Koh, J.Y.; Suh, S.W.; Gwag, B.J.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996, 272, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Canzoniero, L.M.; Yu, S.P.; Ying, H.S.; Koh, J.Y.; Kerchner, G.A.; Choi, D.W. Measurement of intracellular free zinc in living cortical neurons: Routes of entry. J. Neurosci. 1997, 17, 9554–9564. [Google Scholar]
- Pellegrini-Giampietro, D.E.; Gorter, G.A.; Bennett, M.V.; Zukin, R.S. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997, 20, 464–470. [Google Scholar]
- Calderone, A.; Jover, T.; Mashiko, T.; Noh, K.; Tanaka, H.; Bennett, M.V.L.; Zukin, R.S. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J. Neurosci. 2004, 24, 9903–9913. [Google Scholar]
- Kawahara, M.; Kato-Negishi, M.; Kuroda, Y. Pyruvate blocks zinc-induced neurotoxicity in immortalized hypothalamic neurons. Cell. Mol. Neurobiol. 2002, 22, 87–93. [Google Scholar] [CrossRef]
- Koyama, H.; Konoha, K.; Sadakane, Y.; Ohkawara, S.; Kawahara, M. Zinc neurotoxicity and the pathogenesis of vascular-type dementia: Involvement of calcium dyshomeostasis and carnosine. J. Clin. Toxicol. 2011, 3. [Google Scholar] [CrossRef]
- Mellon, P.L.; Windle, J.J.; Goldsmith, P.C.; Padula, C.A.; Roberts, J.L.; Weiner, R.I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990, 5, 1–10. [Google Scholar] [CrossRef]
- Mahesh, V.B.; Zamorano, P.; De Sevilla, L.; Lewis, D.; Brann, D.W. Characterization of ionotropic glutamate receptors in rat hypothalamus, pituitary and immortalized gonadotropin-releasing hormone (GnRH) neurons (GT1-7 cells). Neuroendocrinology 1999, 69, 397–407. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M.; Hosoda, R.; Kuroda, Y. Characterization of zinc-induced apoptosis of GT1-7 cells. Biomed. Res. Trace Elem. 2002, 13, 280–281. [Google Scholar]
- Kawahara, M.; Konoha, K.; Sadakane, Y. Neurotoxicity of zinc: The involvement of calcium homeostasis and carnosine. Biomed. Res. Trace Elem. 2007, 18, 26–34. [Google Scholar]
- Kawahara, M.; Sadakane, Y.; Koyama, H.; Konoha, K.; Ohkawara, S. D-histidine and L-histidine attenuate zinc-induced neuronal death in GT1-7 cells. Metallomics 2013, 5, 453–460. [Google Scholar] [CrossRef]
- Mizuno, D.; Konoha-Mizuno, D.; Mori, M.; Sadakane, Y.; Koyama, H.; Ohkawara, S.; Kawahara, M. Protective activity of carnosine and anserine against zinc-induced neurotoxicity: A possible treatment for vascular dementia. Metallomics 2015, 7, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Konoha, K.; Sadakane, Y.; Kawahara, M. Effects of gadolinium and other metal on the neurotoxicity of immortalized hypothalamic neurons induced by zinc. Biomed. Res. Trace Elem. 2004, 15, 275–277. [Google Scholar]
- Koksal, A.R.; Verne, G.N.; Zhou, Q. Endoplasmic reticulum stress in biological processing and disease. J. Investig. Med. 2021, 69, 309–315. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Kasai, M.; Shimoda, M.; Shimizu, A.; Kubota, M.; Kawahara, M. Nickel enhances zinc-induced neuronal cell death by priming the endoplasmic reticulum stress response. Oxid. Med. Cell. Longev. 2019, 2019, 9693726. [Google Scholar] [CrossRef]
- Kyosseva, S.V. Mitogen-activated protein kinase signaling. Int. Rev. Neurobiol. 2004, 59, 201–220. [Google Scholar] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Behrens, M.M.; Choi, D.W. Zinc-induced cortical neuronal death: Contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J. Neurosci. 2000, 20, 3139–3154. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.L.; Zipfel, G.J.; Sheline, C.T. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur. J. Neurosci. 2006, 24, 2169–2176. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.H.; Koh, J.Y. Protection by pyruvate against transient forebrain ischemia in rats. J. Neurosci. 2001, 21, RC171. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Shimoda, M.; Kawahara, M. Pyruvic acid prevents Cu2+/Zn2+-induced neurotoxicity by suppressing mitochondrial injury. Biochem. Biophys. Res. Commun. 2018, 495, 1335–1341. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chang, S.Y.; Chung, J.M.; Ryu, B.R.; Joo, C.K.; Moon, H.S.; Kang, K.; Yoon, S.H.; Han, P.L.; Gwag, B.J. Attenuation of Zn2+ neurotoxicity by aspirin: Role of N-type Ca2+ channel and the carboxyl acid group. Neurobiol. Dis. 2001, 8, 774–783. [Google Scholar]
- Gulati, P.; Muthuraman, A.; Jaggi, A.S.; Singh, N. Neuroprotective effect of gadolinium: A stretch-activated calcium channel blocker in mouse model of ischemia-reperfusion injury. Naunyn Schmiedeberg’s Arch. Pharmacol. 2013, 386, 255–264. [Google Scholar] [CrossRef]
- Platt, B.; Büsselberg, D. Combined actions of Pb2+, Zn2+, and Al3+ on voltage-activated calcium channel currents. Cell. Mol. Neurobiol. 1994, 14, 831–840. [Google Scholar] [CrossRef]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular sources of ROS/H2O2 in health and neurodegeneration: Spotlight on endoplasmic reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Contessa, J.; Caron, R.; Amorino, G.; Valerie, K.; Hagan, M.P.; Grant, S.; Schmidt-Ullrich, R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003, 159, 283–300. [Google Scholar] [CrossRef]
- Chang, C.C.; Kuan, C.P.; Lin, J.Y.; Lai, J.Y.; Ho, T.F. Tanshinone IIA facilitates TRAIL sensitization by up-regulating DR5 through the ROS-JNK-CHOP signaling axis in human ovarian carcinoma cell lines. Chem. Res. Toxicol. 2015, 28, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, G.; Fang, J. Association between serum copper and stroke risk factors in adults: Evidence from the National Health and Nutrition Examination Survey, 2011–2016. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Hu, L.; Bi, C.; Lin, T.; Liu, L.; Song, Y.; Wang, P.; Wang, B.; Fang, C.; Ma, H.; Huang, X.; et al. Association between plasma copper levels and first stroke: A community-based nested case-control study. Nutr. Neurosci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, W.; Wang, Y.; Wang, T.; Ma, M.; Tian, C. Association between the change of serum copper and ischemic stroke: A systematic review and meta-analysis. J. Mol. Neurosci. 2020, 70, 475–480. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M.; Tanaka, K.-I. Amyloids: Regulators of metal homeostasis in the synapse. Molecules 2020, 25, 1441. [Google Scholar] [CrossRef]
- Watt, N.T.; Griffiths, H.H.; Hooper, N.M. Neuronal zinc regulation and the prion protein. Prion 2013, 7, 203–208. [Google Scholar] [CrossRef]
- Mellone, M.; Pelucchi, S.; Alberti, L.; Genazzani, A.A.; Luca, M.D.; Gardoni, F. Zinc transporter-1: A novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 2015, 132, 159–168. [Google Scholar] [CrossRef]
- Singh, A.; Haldar, S.; Horback, K.; Tom, C.; Zhou, L.; Meyerson, H.; Singh, N. Prion protein regulates iron transport by functioning as a ferrireductase. J. Alzheimer’s Dis. 2013, 35, 541–552. [Google Scholar] [CrossRef]
- Multhaup, G.; Schlicksupp, A.; Hesse, L.; Beher, D.; Ruppert, T.; Masters, C.L.; Beyreuther, K. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I). Science 1996, 271, 1406–1409. [Google Scholar] [CrossRef]
- Wong, B.X.; Tsatsanis, A.; Lim, L.Q.; Adlard, P.A.; Bush, A.I.; Duce, J.A. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE 2014, 9, e114174. [Google Scholar] [CrossRef] [PubMed]
- Moons, R.; Konijnenberg, A.; Mensch, C.; Van Elzen, R.; Johannessen, C.; Maudsley, S.; Lambeir, A.-M.; Sobott, F. Metal ions shape α-synuclein. Sci. Rep. 2020, 10, 16293. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Moualla, D.; Brown, D.R. Alpha-synuclein is a cellular ferrireductase. PLoS ONE 2011, 6, e15814. [Google Scholar] [CrossRef]
- Rogers, J.T.; Cahill, C.M. Iron-responsive-like elements and neurodegenerative ferroptosis. Learn. Mem. 2020, 27, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Liu, G.; Zhao, Y.; Shi, Z.; Zheng, Q.; Bu, G.; Xu, H.; Zhang, Y. Role of copper and the copper-related protein CUTA in mediating APP processing and Aβ generation. Neurobiol. Aging 2015, 36, 1310–1315. [Google Scholar] [CrossRef]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Cerpa, W.; Varela-Nallar, L. Copper brain homeostasis: Role of amyloid precursor protein and prion protein. IUBMB Life 2005, 57, 645–650. [Google Scholar] [CrossRef]
- You, H.; Tsutsui, S.; Hameed, S.; Kannanayakal, T.J.; Chen, L.; Xia, P.; Engbers, J.D.T.; Lipton, S.A.; Stys, P.K.; Zamponi, G.W. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742. [Google Scholar] [CrossRef]
- Posadas, Y.; Parra-Ojeda, L.; Perez-Cruz, C.; Quintanar, L. Amyloid β perturbs Cu(II) binding to the prion protein in a site-specific manner: Insights into its potential neurotoxic mechanisms. Inorg. Chem. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhang, L.; Yu, W.; Wang, Y.; Chang, W. Cellular prion protein as a receptor of toxic amyloid-β42 oligomers is important for Alzheimer’s disease. Front. Cell. Neurosci. 2019, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Puig, B.; Yang, D.; Brenna, S.; Altmeppen, H.C.; Magnus, T. Show me your friends and I tell you who you are: The many facets of prion protein in stroke. Cells 2020, 9, 1609. [Google Scholar] [CrossRef]
- Black, S.A.G.; Stys, P.K.; Zamponi, G.W.; Tsutsui, S. Cellular prion protein and NMDA receptor modulation: Protecting against excitotoxicity. Front. Cell Dev. Biol. 2014, 2, 45. [Google Scholar] [CrossRef]
- Koh, J.Y.; Lee, S.J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Devyatov, A.A.; Lopacheva, O.M.; Kulikova, O.I.; Abaimov, D.A.; Fedorova, T.N. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids 2019, 51, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Trombley, P.Q.; Horning, M.S.; Blakemore, L.J. Interactions between carnosine and zinc and copper: Implications for neuromodulation and neuroprotection. Biochemistry 2000, 65, 807–816. [Google Scholar] [PubMed]
- Davis, C.K.; Laud, P.J.; Bahor, Z.; Rajanikant, G.K.; Majid, A. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J. Cereb. Blood Flow Metab. 2016, 36, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. [Google Scholar] [CrossRef]
- Kawahara, M.; Konoha, K.; Nagata, T.; Sadakane, Y. Drugs for Prevention or Treatment of Vascular Dementia. JP Patent 5,382,633, 11 October 2013. [Google Scholar]
- Kawahara, M.; Konoha, K.; Nagata, T.; Sadakane, Y. Drugs for Prevention or Treatment of Vascular Dementia. JP Patent 5,294,194, 21 June 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, M.; Tanaka, K.-i.; Kato-Negishi, M. Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia. Int. J. Mol. Sci. 2021, 22, 7242. https://doi.org/10.3390/ijms22147242
Kawahara M, Tanaka K-i, Kato-Negishi M. Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia. International Journal of Molecular Sciences. 2021; 22(14):7242. https://doi.org/10.3390/ijms22147242
Chicago/Turabian StyleKawahara, Masahiro, Ken-ichiro Tanaka, and Midori Kato-Negishi. 2021. "Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia" International Journal of Molecular Sciences 22, no. 14: 7242. https://doi.org/10.3390/ijms22147242
APA StyleKawahara, M., Tanaka, K.-i., & Kato-Negishi, M. (2021). Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia. International Journal of Molecular Sciences, 22(14), 7242. https://doi.org/10.3390/ijms22147242





