Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change
Abstract
:1. Introduction
2. Results
2.1. Optimized Structures
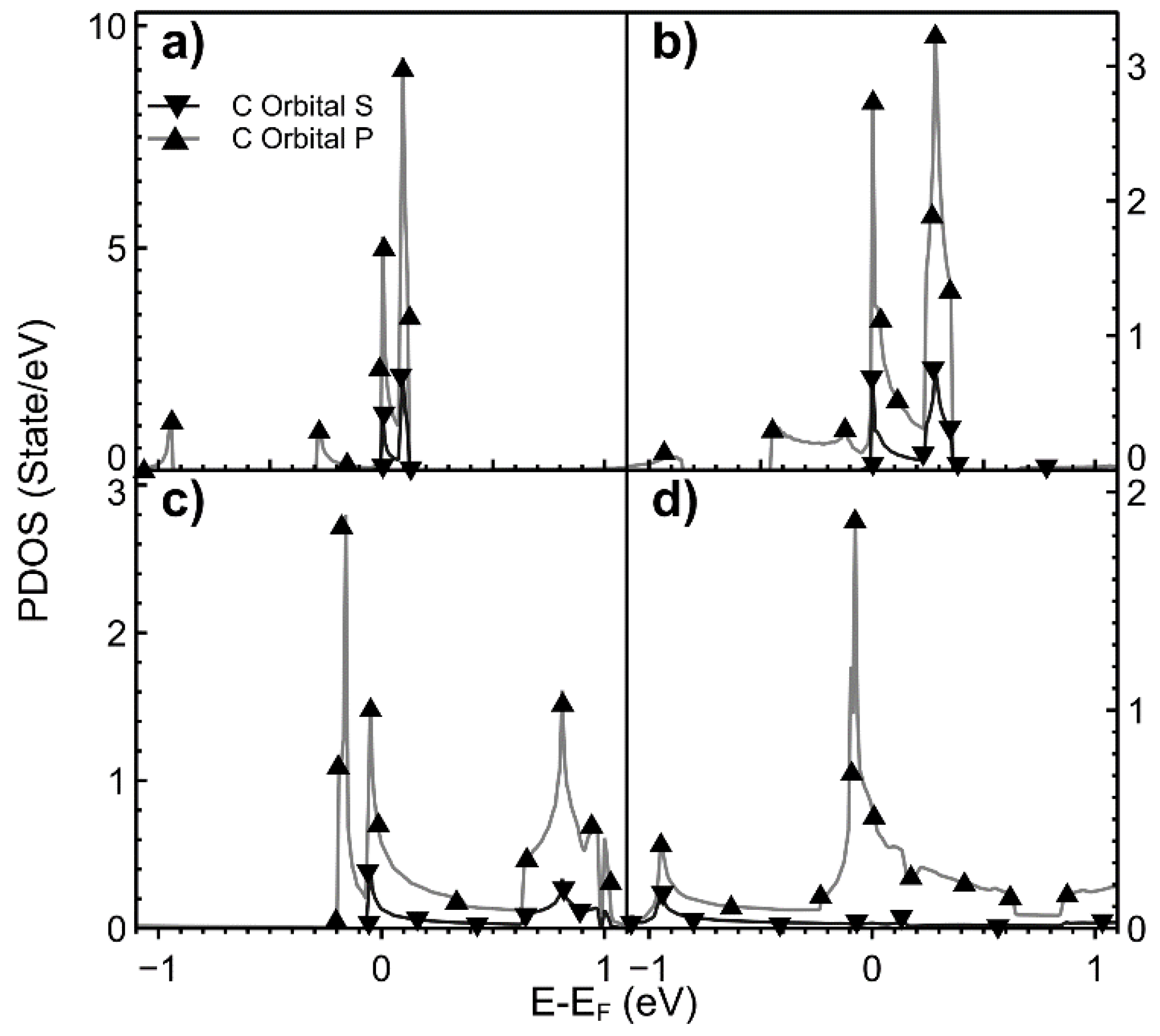
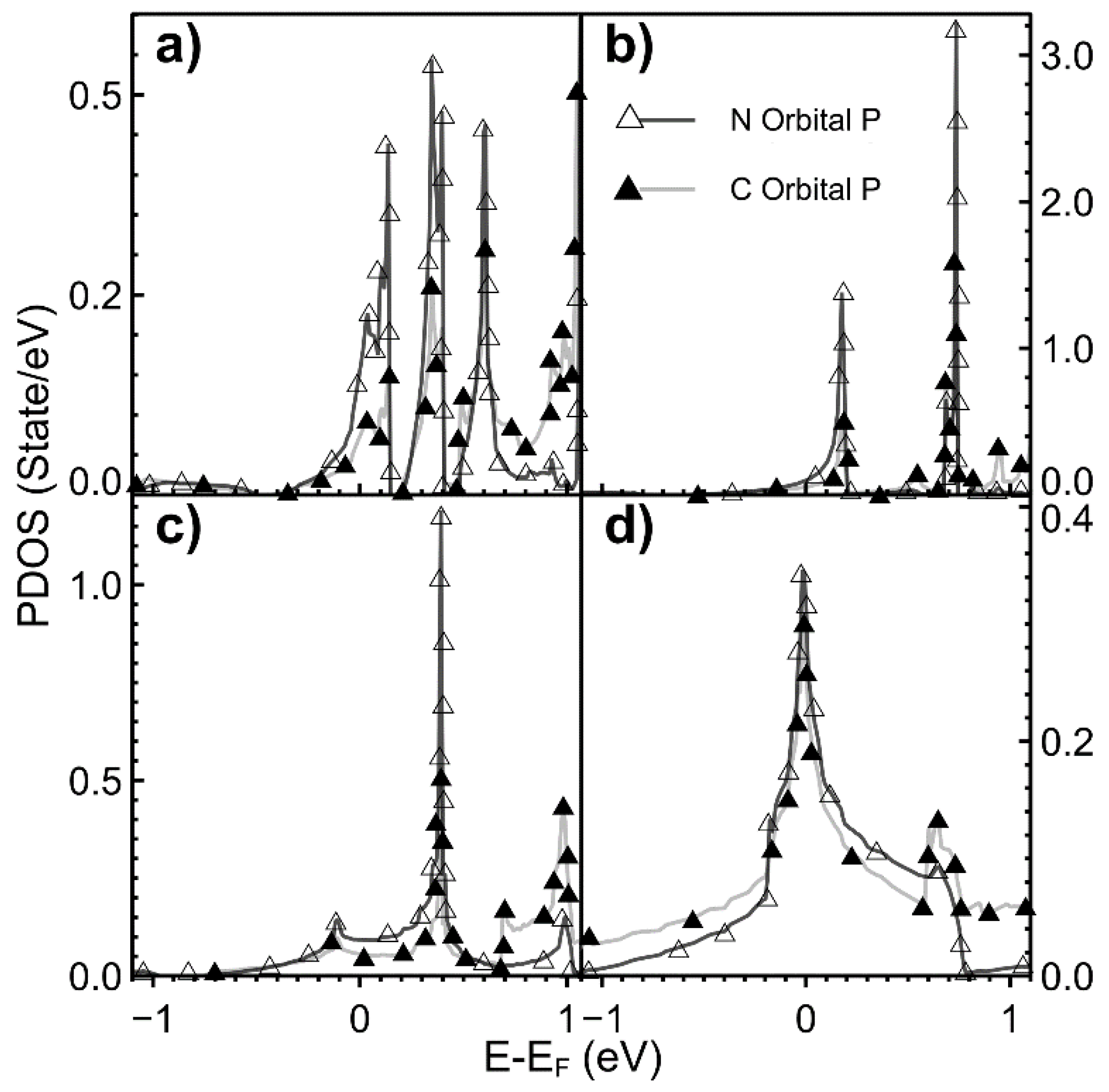
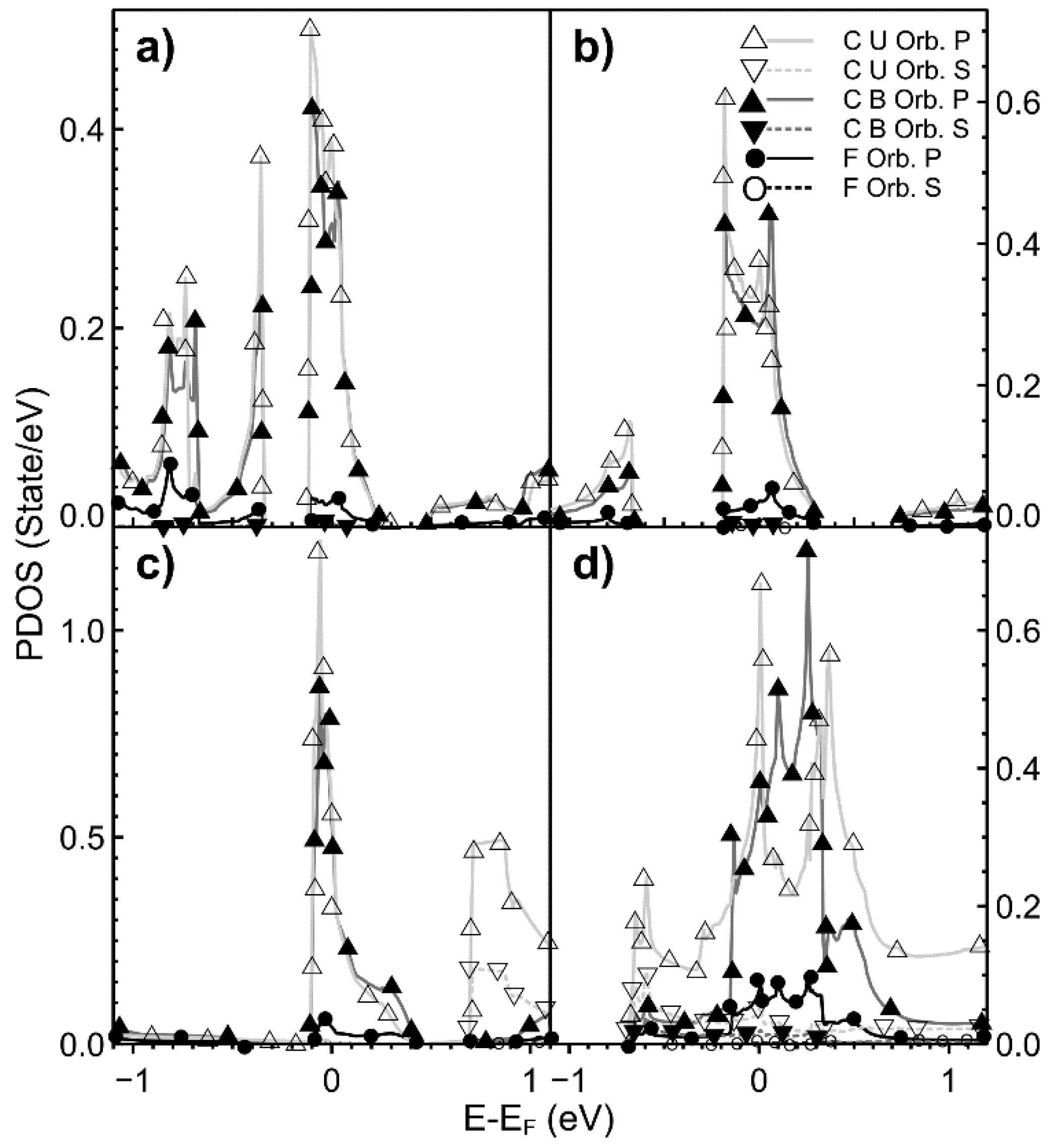
2.2. Projected Density of States (PDOS)
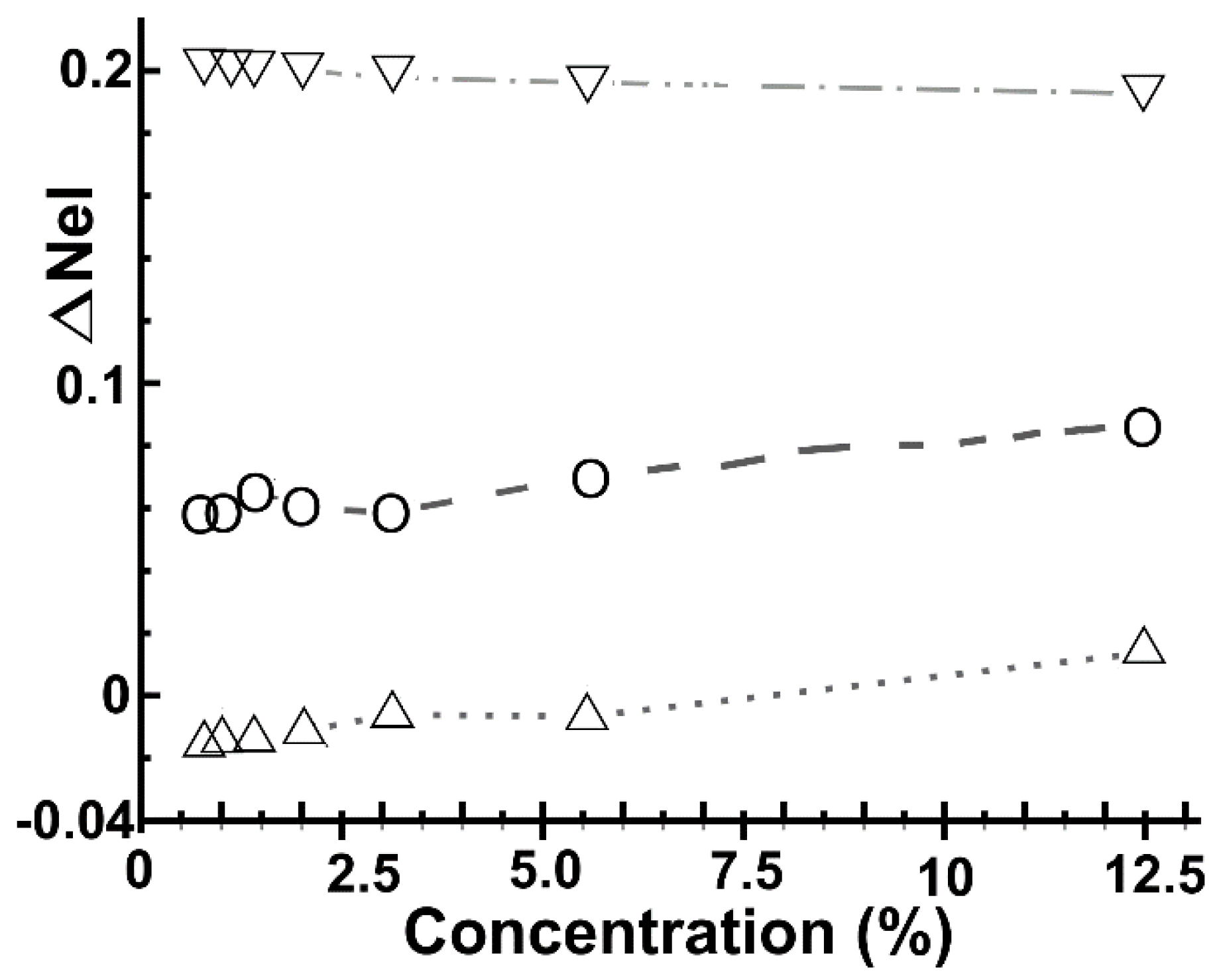
2.3. Electron Transfer
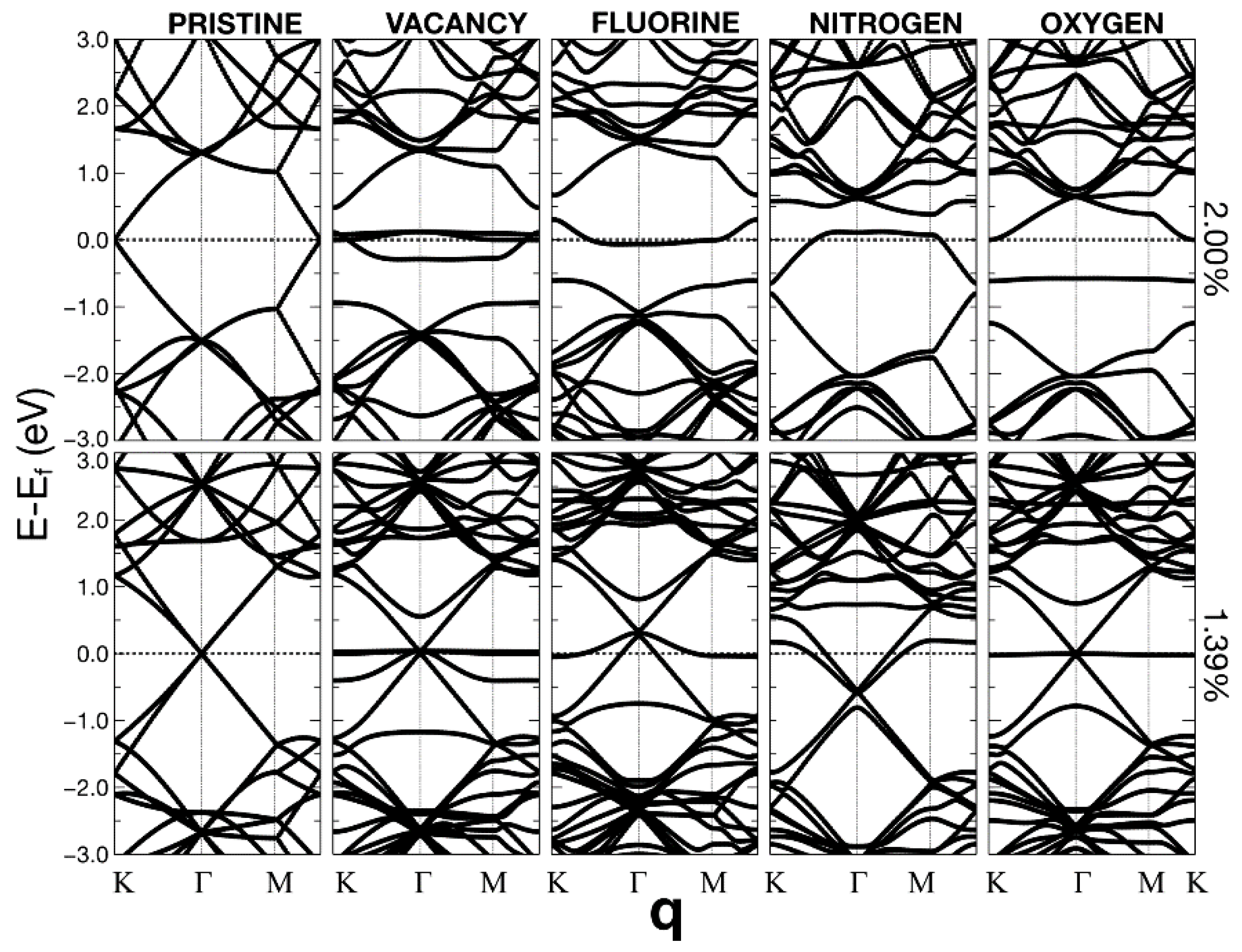
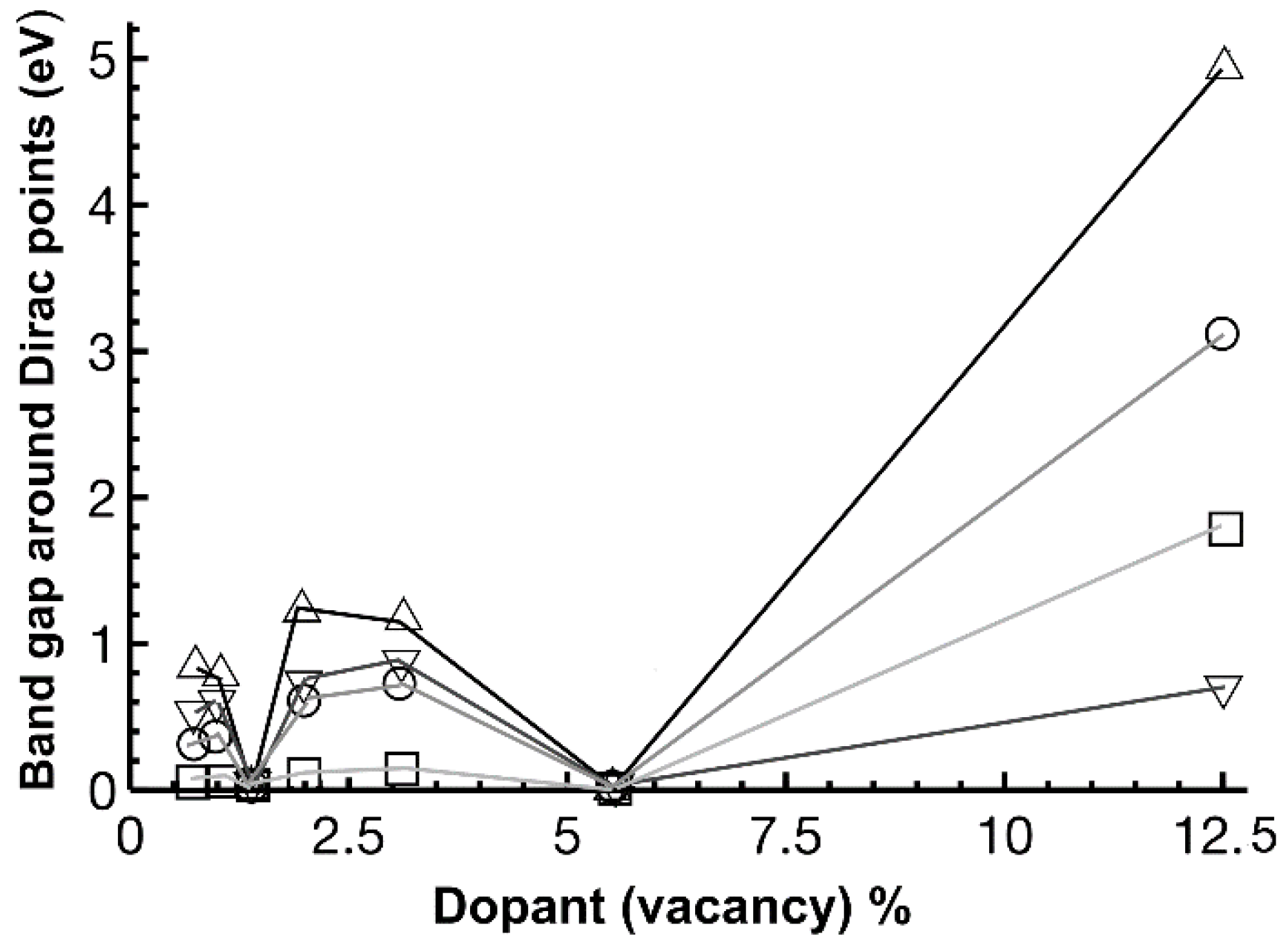
2.4. Band Structure
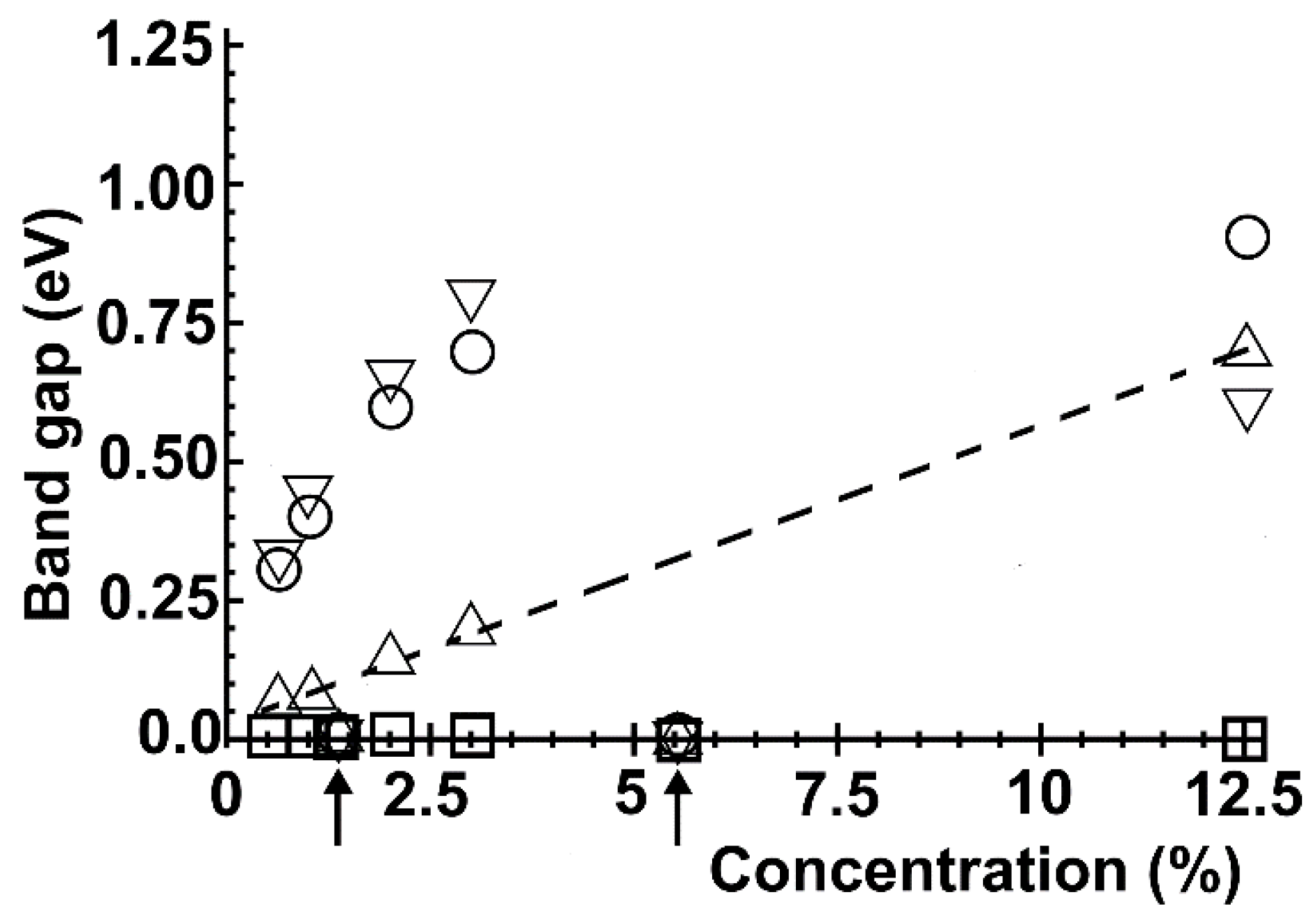
2.5. Optical Absorption and Reflectivity
2.5.1. Optical Absorption
2.5.2. Reflectivity
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaves, A.; Azadani, J.G.; Alsalman, H.; Da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap engineering of two-dimensional semiconductor materials. NPJ 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Angel, G.M.A.; Mansor, N.; Jervis, R.; Rana, Z.; Gibbs, C.; Seel, A.; Kilpatrick, A.F.R.; Shearing, P.R.; Howard, C.A.; Brett, D.J.L.; et al. Realising the electrochemical stability of graphene: Scalable synthesis of an ultra-durable platinum catalyst for the oxygen reduction reaction. Nanoscale 2020, 12, 16113–16122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Z.; Younas, W.; Zhu, Y.; Ma, X.; Cao, C. P-Type Boron-Doped Monolayer Graphene with Tunable Bandgap for Enhanced Photocatalytic H 2 Evolution under Visible-Light Irradiation. ChemCatChem 2019, 11, 5145–5153. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, C.; Qiao, C.; Wu, Y.; Yang, L.; Younas, W. Bandgap-tunable phosphorus-doped monolayer graphene with enhanced visible-light photocatalytic H2-production activity. J. Mater. Chem. C 2019, 7, 10613–10622. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, K.; Lu, Z.; Xu, Y.; Qi, S.; Zhang, G. Effect of oxygen atoms on graphene: Adsorption and doping. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 117, 113827. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H. Different elements doped graphene sensor for CO2 greenhouse gases detection: The DFT study. Chem. Phys. Lett. 2019, 721, 33–37. [Google Scholar] [CrossRef]
- Safardoust-Hojaghan, H.; Amiri, O.; Hassanpour, M.; Panahi-Kalamuei, M.; Moayedi, H.; Salavati-Niasari, M. S,N co-doped graphene quantum dots-induced ascorbic acid fluorescent sensor: Design, characterization and performance. Food Chem. 2019, 295, 530–536. [Google Scholar] [CrossRef]
- Xuab, X.; Zhangbc, S.; Tangd, J.; Pane, L.; Eguchib, M.; Nabd, J.; Yamauchid, Y. Nitrogen-doped nanostructured carbons: A new material horizon for water desalination by capacitive deionization. EnergyChem 2020, 2, 100043. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Z.; Han, F.; Kang, G.-J.; Liu, L.; Liu, J.; Wang, Q.-D. The identification of active N species in N-doped carbon carriers that improve the activity of Fe electrocatalysts towards the oxygen evolution reaction. RSC Adv. 2019, 9, 4806–4811. [Google Scholar] [CrossRef] [Green Version]
- Reda, M.; Hansen, H.A.; Vegge, T. DFT study of stabilization effects on N-doped graphene for ORR catalysis. Catal. Today 2018, 312, 118–125. [Google Scholar] [CrossRef]
- Laref, A.; Ahmed, A.; Bin-Omran, S.; Luo, S. First-principle analysis of the electronic and optical properties of boron and nitrogen doped carbon mono-layer graphenes. Carbon 2015, 81, 179–192. [Google Scholar] [CrossRef]
- Nath, P.; Chowdhury, S.; Sanyal, D.; Jana, D. Ab-initio calculation of electronic and optical properties of nitrogen and boron doped graphene nanosheet. Carbon 2014, 73, 275–282. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Feng, Y.; An, H.; Long, P.; Qin, C.; Feng, W. Nitrogen and fluorine co-doped graphene as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23095–23105. [Google Scholar] [CrossRef]
- Sofo, J.O.; Suarez, A.M.; Usaj, G.; Cornaglia, P.S.; Nieves, A.D.H.; Balseiro, C.A. Electrical control of the chemical bonding of fluorine on graphene. Phys. Rev. B 2011, 83, 081411. [Google Scholar] [CrossRef] [Green Version]
- Stine, R.; Lee, W.-K.; Whitener, K.; Robinson, J.T.; Sheehan, P. Chemical Stability of Graphene Fluoride Produced by Exposure to XeF2. Nano Lett. 2013, 13, 4311–4316. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Zhao, H.; Fang, Y.; Ming, K.; Huang, H.; Chen, J.; Wang, X. Fluorine-doped graphene with an outstanding electrocatalytic performance for efficient oxygen reduction reaction in alkaline solution. R. Soc. Open Sci. 2018, 5, 180925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, S.; Hussain, A.; Sato, F. Rectangular and hexagonal doping of graphene with B, N, and O: A DFT study. RSC Adv. 2017, 7, 16064–16068. [Google Scholar] [CrossRef] [Green Version]
- Nishidate, K.; Tanibayashi, S.; Matsukawa, M.; Hasegawa, M. Hybridization versus sublattice symmetry breaking in the band gap opening in graphene on Ni(111): A first-principles study. Surf. Sci. 2020, 700, 121651. [Google Scholar] [CrossRef]
- Goudarzi, M.; Parhizgar, S.; Beheshtian, J. Electronic and optical properties of vacancy and B, N, O and F doped graphene: DFT study. Opto-Electronics Rev. 2019, 27, 130–136. [Google Scholar] [CrossRef]
- Ismail, A.I.; Mubarak, A.A. Ab initio study of the electronic and magnetic properties of graphene with and without adsorption of m atom (M = C, N, O, F, Cl). Surf. Rev. Lett. 2018, 25, 1850069. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Zhang, H.-L.; Deng, W.-Q. A 3N rule for the electronic properties of doped graphene. Nanotechnology 2013, 24, 225705. [Google Scholar] [CrossRef]
- Zhang, S.; Hung, H.-H.; Wu, C. Proposed realization of itinerant ferromagnetism in optical lattices. Phys. Rev. A 2010, 82, 053618. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, S.; Kusuta, S.; Furukawa, N. BCS theory on a flat band lattice. Phys. C Supercond. 2007, 460–462, 1145–1146. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Wu, Y.-S. Exotic electronic states in the world of flat bands: From theory to material. Chin. Phys. B 2014, 23, 077308. [Google Scholar] [CrossRef]
- Rani, P.; Dubey, G.S.; Jindal, V. DFT study of optical properties of pure and doped graphene. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 62, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Thonhauser, T.; Cooper, V.; Li, S.; Puzder, A.; Hyldgaard, P.; Langreth, D.C. Van der Waals density functional: Self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 2007, 76, 125112. [Google Scholar] [CrossRef] [Green Version]
- Langreth, D.C.; Lundqvist, B.I.; Chakarova-Käck, S.D.; Cooper, V.; Dion, M.; Hyldgaard, P.; Kelkkanen, A.; Kleis, J.; Kong, L.; Li, S.; et al. A density functional for sparse matter. J. Phys. Condens. Matter 2009, 21, 084203. [Google Scholar] [CrossRef] [Green Version]
- Berland, K.; Cooper, V.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B.I. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef] [Green Version]
- Thonhauser, T.; Zuluaga, S.; Arter, C.A.; Berland, K.; Schröder, E.; Hyldgaard, P. Spin Signature of Nonlocal Correlation Binding in Metal-Organic Frameworks. Phys. Rev. Lett. 2015, 115, 136402. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—a new program for displaying crystalline structures and electron densities. J. Mol. Graph. Model. 1999, 17, 176–179. [Google Scholar] [CrossRef]
- Bohm, D.; Pines, D. A Collective Description of Electron Interactions: III. Coulomb Interactions in a Degenerate Electron Gas. Phys. Rev. 1953, 92, 609–625. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Maurya, T.K.; Auluck, S. Electronic and optical properties of ordered BexZn1−xSe alloys by the FPLAPW method. J. Physics: Condens. Matter 2008, 20, 075205. [Google Scholar] [CrossRef]


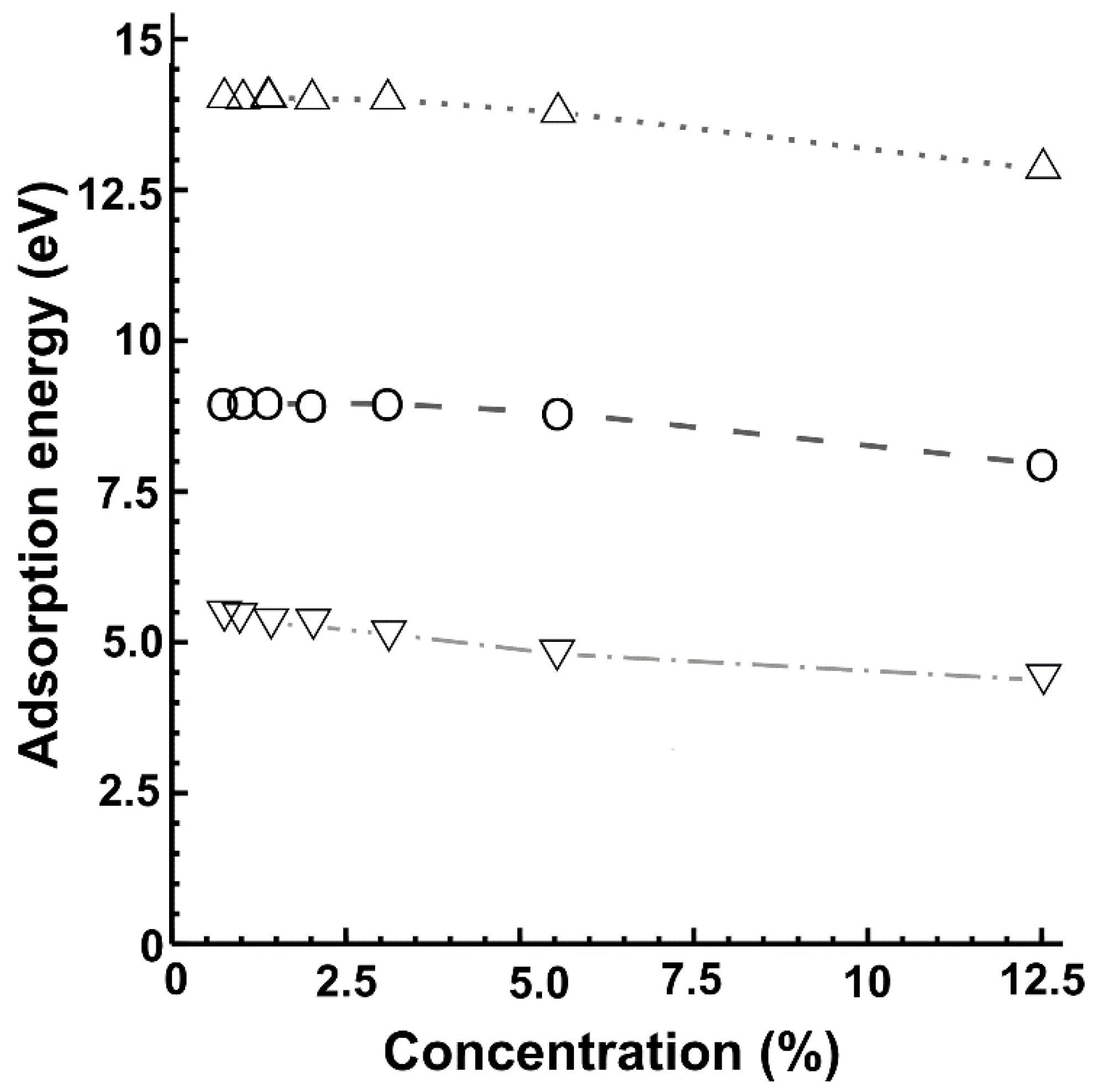




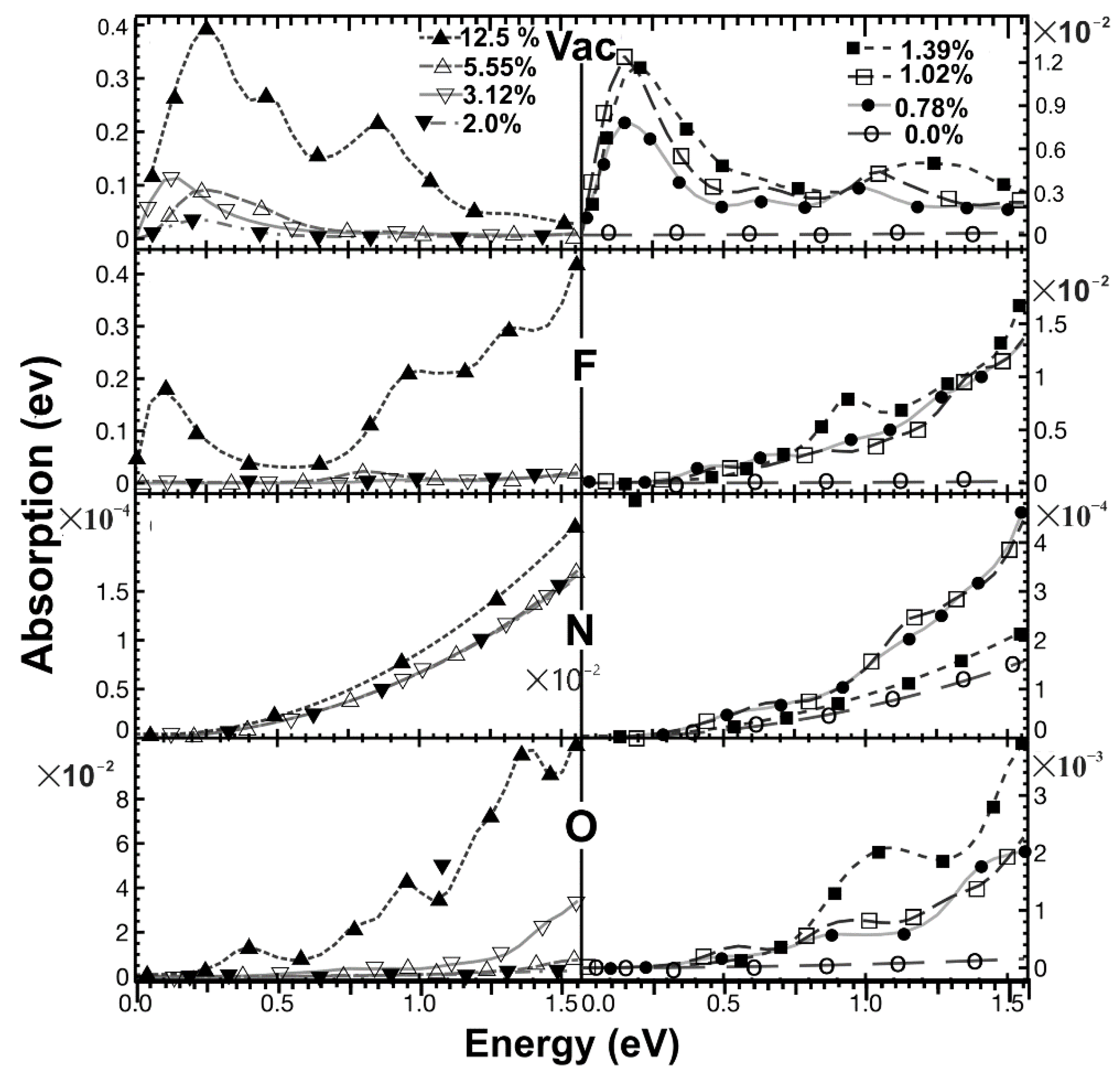
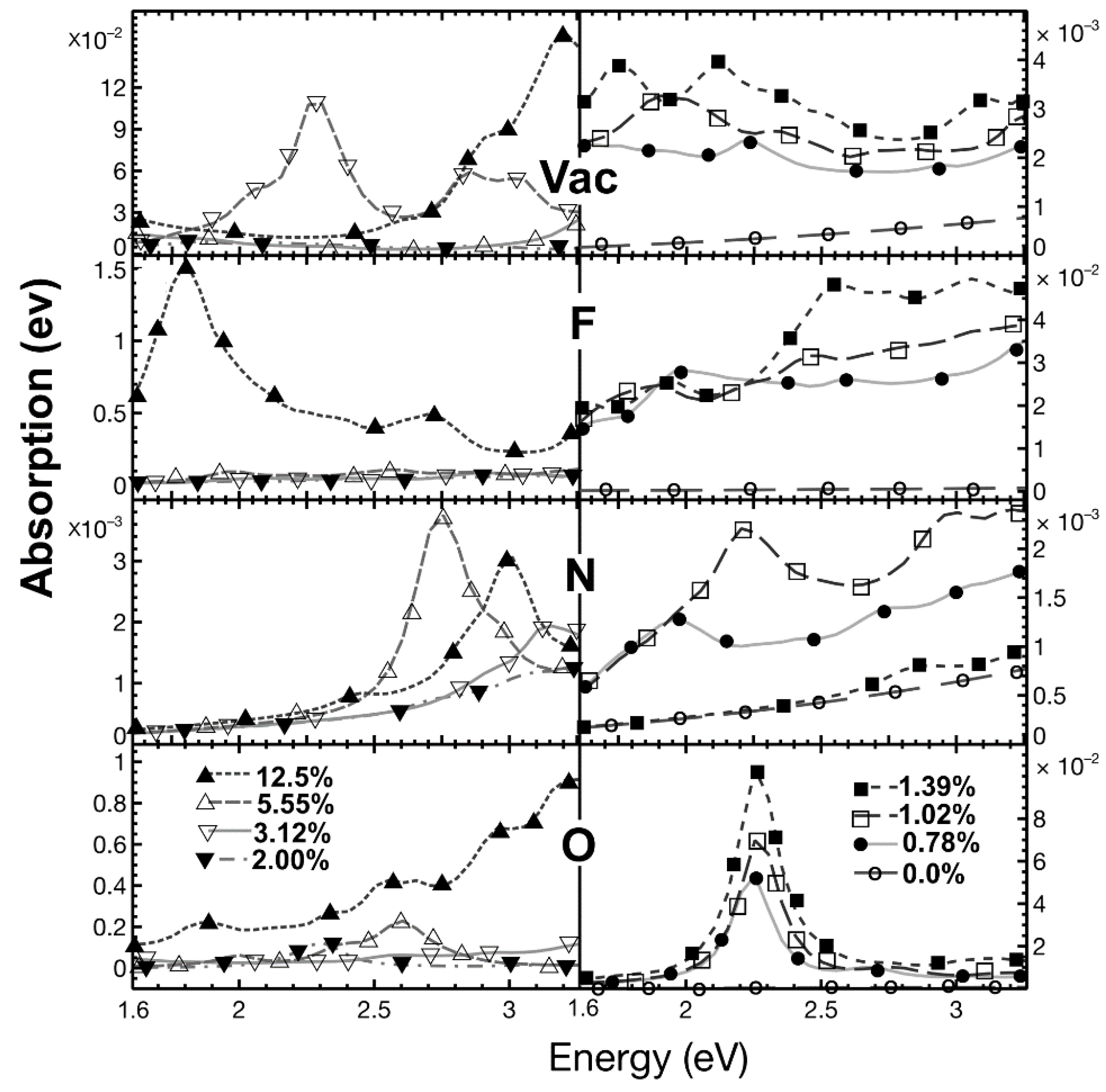
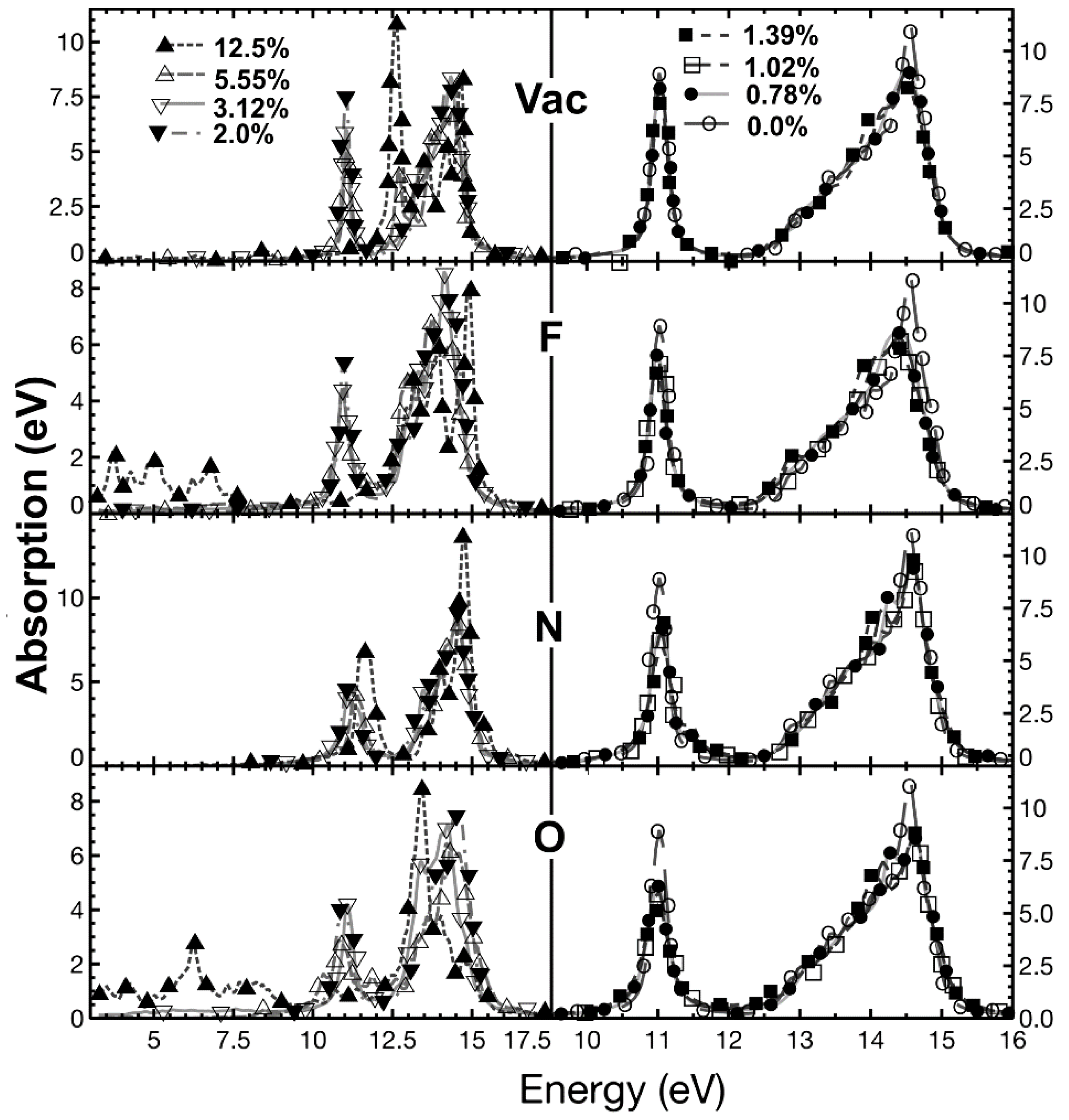
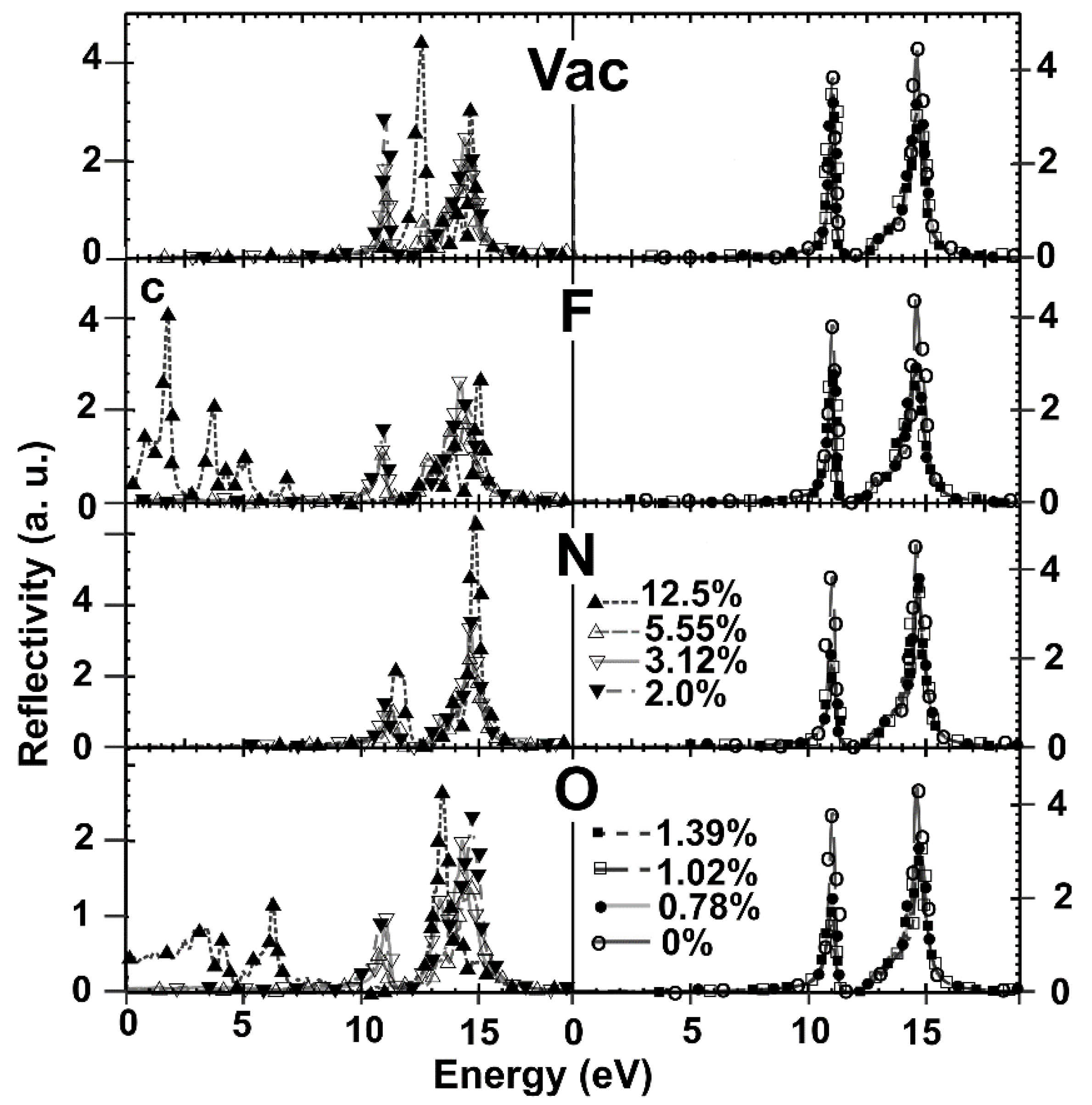
| % Doping | F-Graphene | N-Graphene | O-Graphene | Atoms in the Unit Cell |
|---|---|---|---|---|
| 12.5 | −4.373371287 | −12.83995707 | −7.955791861 | 8 |
| 5.55 | −4.806661273 | −13.78657355 | −8.806335029 | 18 |
| 3.12 | −5.137406876 | −13.99255507 | −8.953832994 | 32 |
| 2.00 | −5.266265993 | −14.01279697 | −8.967541945 | 50 |
| 1.39 | −5.323285282 | −14.02436461 | −8.939755112 | 72 |
| 1.02 | −5.403380587 | −14.03414828 | −8.964265388 | 98 |
| 0.78 | −5.439137917 | −14.04038286 | −8.967093484 | 128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-González, A.F.; Ramírez-de-Arellano, J.M.; Magaña, L.F. Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change. Int. J. Mol. Sci. 2021, 22, 6832. https://doi.org/10.3390/ijms22136832
Jiménez-González AF, Ramírez-de-Arellano JM, Magaña LF. Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change. International Journal of Molecular Sciences. 2021; 22(13):6832. https://doi.org/10.3390/ijms22136832
Chicago/Turabian StyleJiménez-González, Ali Fransuani, Juan Manuel Ramírez-de-Arellano, and Luis Fernando Magaña. 2021. "Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change" International Journal of Molecular Sciences 22, no. 13: 6832. https://doi.org/10.3390/ijms22136832
APA StyleJiménez-González, A. F., Ramírez-de-Arellano, J. M., & Magaña, L. F. (2021). Substantial Variations in the Optical Absorption and Reflectivity of Graphene When the Concentrations of Vacancies and Doping with Fluorine, Nitrogen, and Oxygen Change. International Journal of Molecular Sciences, 22(13), 6832. https://doi.org/10.3390/ijms22136832








