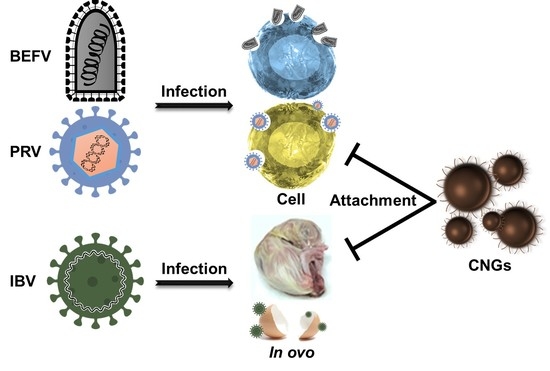
Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus
Abstract
1. Introduction
2. Results
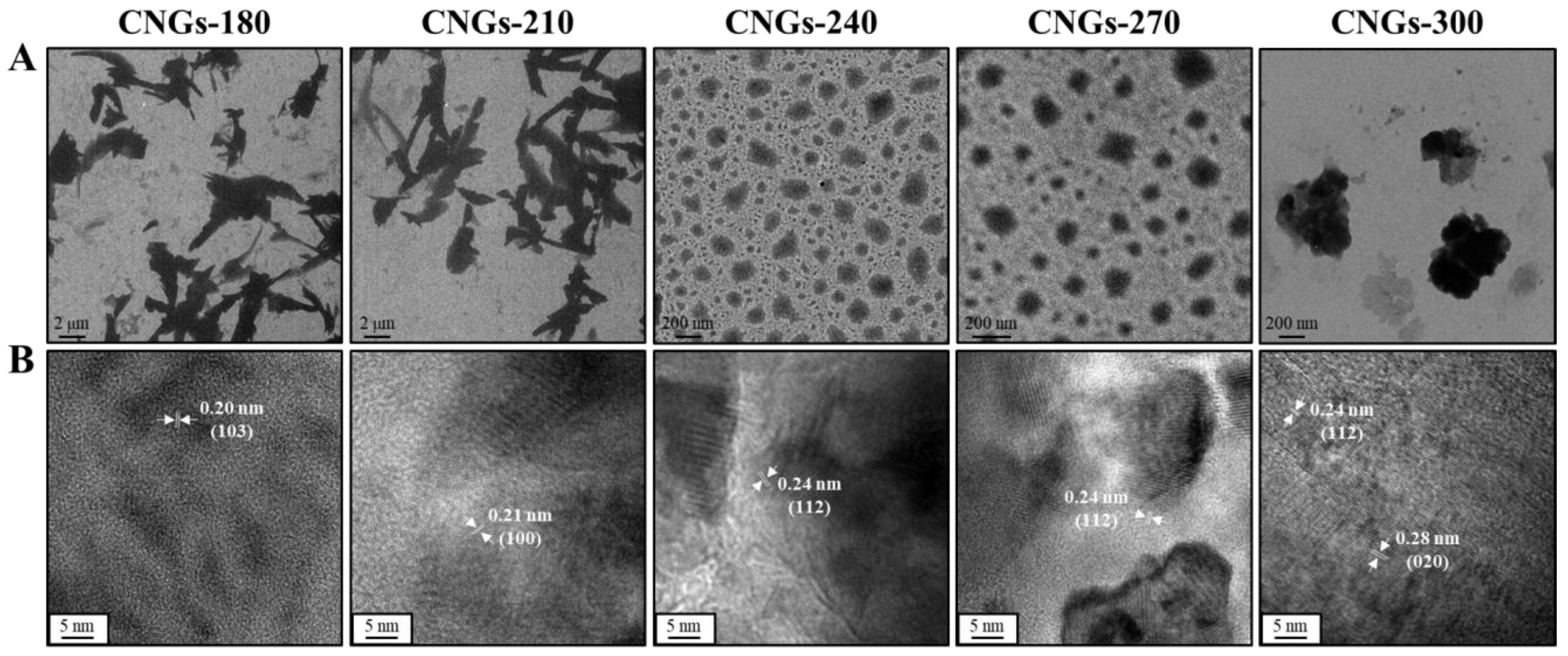
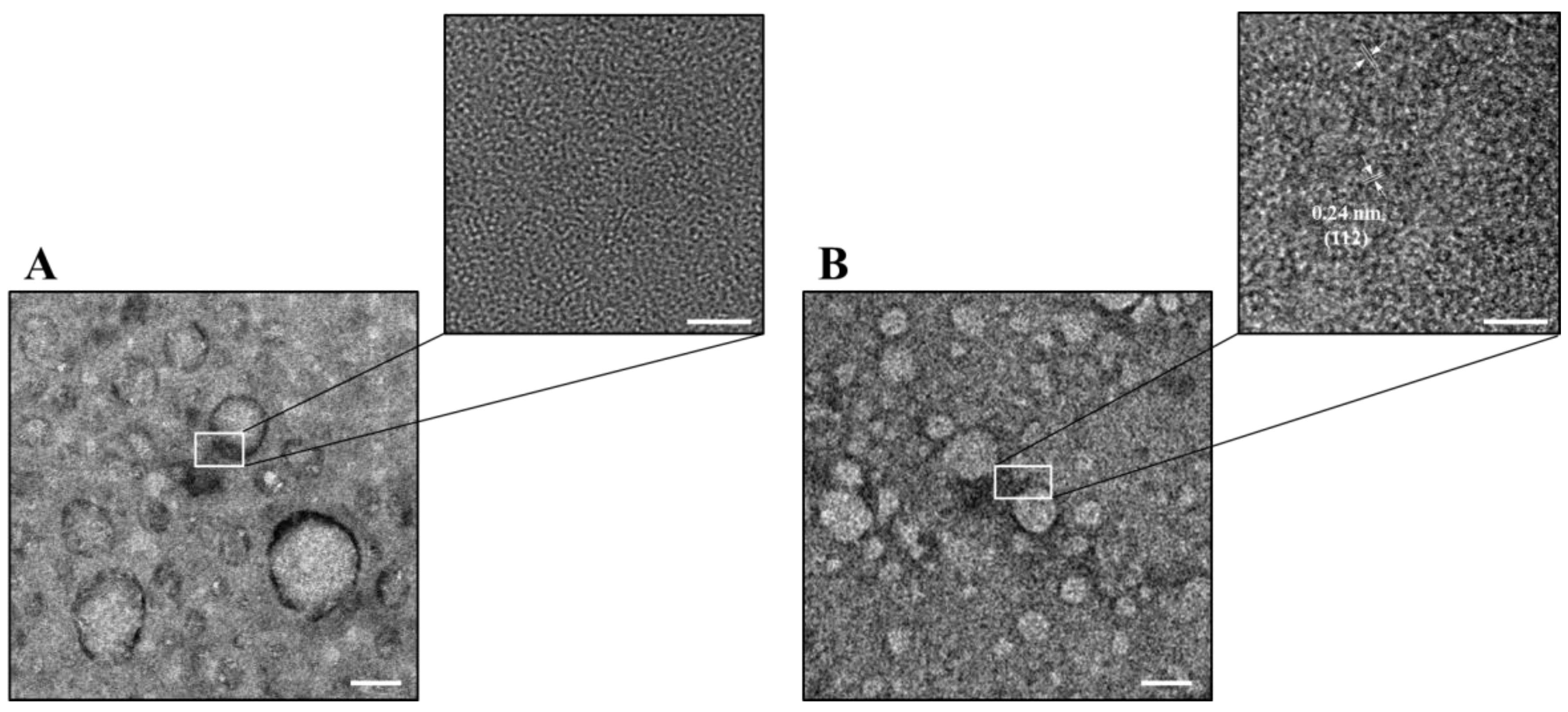
2.1. Characterization of CNGs
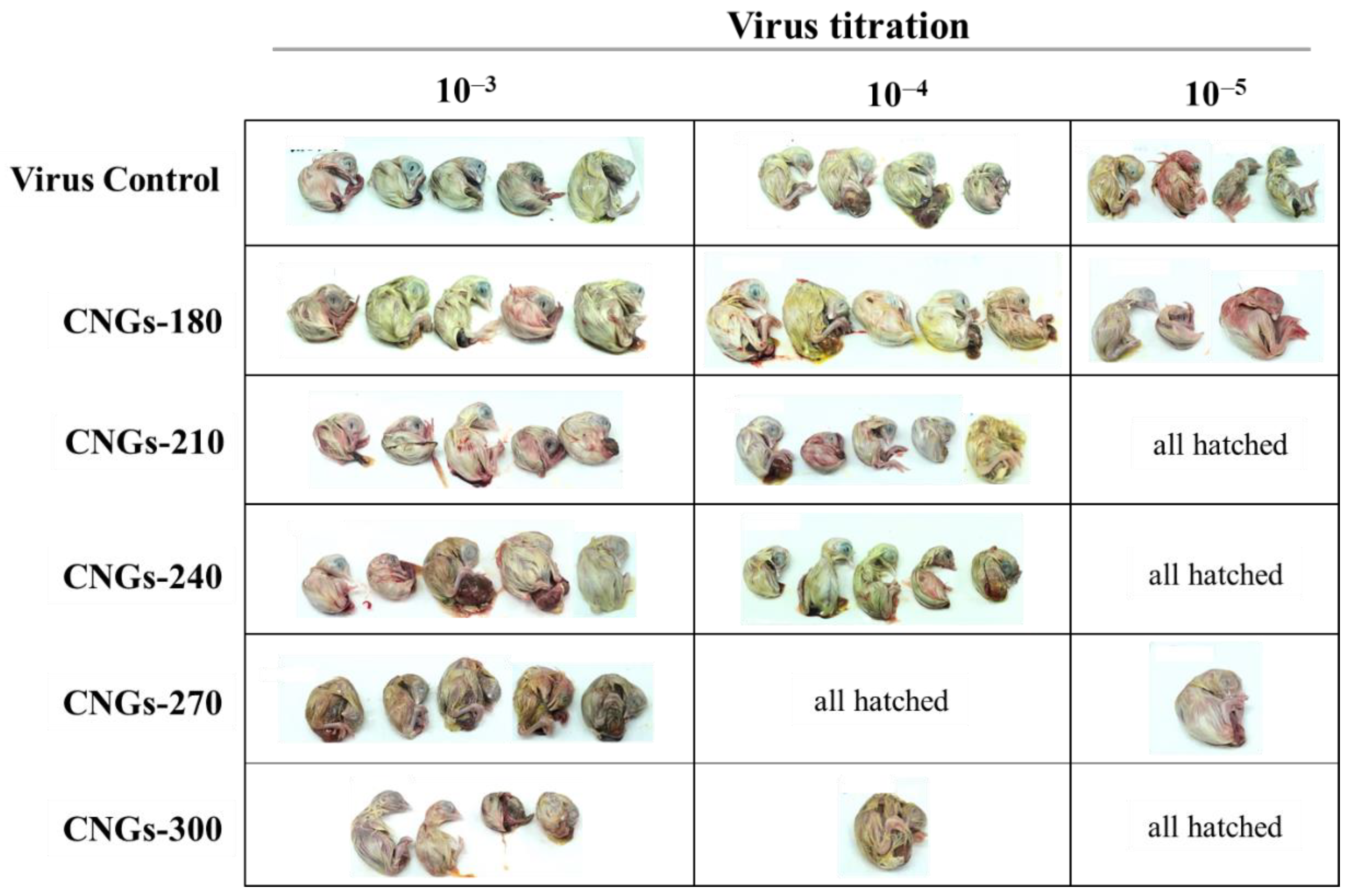
2.2. Antiviral Activity of CNGs
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of CNGs
4.2. Cytotoxicity Assays
4.3. Antiviral Assays
4.4. Hemolysis Assay
4.5. In Ovo Antiviral Test in Chicken Embryo against IBV Infection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ignjatovic, J.; Sapats, S. Avian infectious bronchitis virus. Rev. Sci. Tech. Off. Int. Epizoot. 2000, 19, 493–508. [Google Scholar] [CrossRef]
- Balestrin, E.; Fraga, A.P.; Ikuta, N.; Canal, C.W.; Fonseca, A.S.K.; Lunge, V.R. Infectious bronchitis virus in different avian physiological systems—A field study in Brazilian poultry flocks. Poult. Sci. 2014, 93, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Bijlenga, G.; Cook, J.K.A.; Gelb, J.; de Wit, J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: A review. Avian Pathol. 2004, 33, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lelešius, R.; Karpovaitė, A.; Mickienė, R.; Drevinskas, T.; Tiso, N.; Ragažinskienė, O.; Kubilienė, L.; Maruška, A.; Šalomskas, A. In vitro antiviral activity of fifteen plant extracts against avian infectious bronchitis virus. BMC Vet. Res. 2019, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 24. [Google Scholar] [CrossRef]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X.; et al. Antiviral activity against infectious bronchitis virus and bioactive components of Hypericum perforatum L. Front. Pharm. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, G.; Li, J.; Yang, Q.; Ren, X. In vitro and in vivo effects of Houttuynia cordata on infectious bronchitis virus. Avian Pathol. 2011, 40, 491–498. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Liu, H.; Wang, W.; Liu, X.; Li, X.; Wu, X. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb. Pathog. 2018, 114, 124–128. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small 2020, 16, 1906206. [Google Scholar] [CrossRef] [PubMed]
- Sametband, M.; Kalt, I.; Gedanken, A.; Sarid, R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, c-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Sukmayani, W.; Qamariyah Khairunisa, S.; Witaningrum, A.M.; Indriati, D.W.; Matondang, M.Q.Y.; Chang, J.-Y.; Kotaki, T.; Kameokaf, M. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016, 6, 92996–93002. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, C.M.; Li, Y.F.; Wang, J.; Huang, C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale 2017, 9, 16086–16092. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Carbon nanomaterials to combat virus: A perspective in view of COVID-19. Carbon Trends 2021, 2, 100019. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chang, L.; Chu, H.-W.; Lin, H.-J.; Chang, P.-C.; Wang, R.Y.L.; Unnikrishnan, B.; Mao, J.-Y.; Chen, S.-Y.; Huang, C.-C. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef]
- Huang, S.; Gu, J.; Ye, J.; Fang, B.; Wan, S.; Wang, C.; Ashraf, U.; Li, Q.; Wang, X.; Shao, L.; et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019, 542, 198–206. [Google Scholar] [CrossRef]
- Barras, A.; Pagneux, Q.; Sane, F.; Wang, Q.; Boukherroub, R.; Hober, D.; Szunerits, S. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces 2016, 8, 9004–9013. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Exploring the potential of carbon dots to combat COVID-19. Front. Mol. Biosci. 2020, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagasawa, T. ε-Poly-L-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-L-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Xia, Z.; Zhao, X.; Wu, Y.; An, M. Purification and structural analysis of the effective anti-TMV compound ε-poly-L-lysine produced by Streptomyces ahygroscopicus. Molecules 2019, 24, 1156. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 2018, 54, 5401–5406. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sharma, A.; Ghoshal, S.; Jain, S.; Hazra, M.K.; Nandi, C.K. Small molecular organic nanocrystals resemble carbon nanodots in terms of their properties. Chem. Sci. 2018, 9, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, D.J.; Nawar, W.W. Thermal decomposition of lysine. J. Agric. Food Chem. 1979, 27, 511–514. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Huang, Y.; Humphrey, M.G.; Zhang, C. Tunable carbon-dot-based dual-emission fluorescent nanohybrids for ratiometric optical thermometry in living cells. ACS Appl. Mater. Interfaces 2016, 8, 6621–6628. [Google Scholar] [CrossRef]
- Venerando, A.; Magro, M.; Baratella, D.; Ugolotti, J.; Zanin, S.; Malina, O.; Zboril, R.; Lin, H.; Vianello, F. Biotechnological applications of nanostructured hybrids of polyamine carbon quantum dots and iron oxide nanoparticles. Amino Acids 2020, 52, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Wang, S.-W.; Mao, J.-Y.; Chang, H.-T.; Harroun, S.G.; Lin, H.-J.; Huang, C.-C.; Lai, J.-Y. Carbonized nanogels for simultaneous antibacterial and antioxidant treatment of bacterial keratitis. Chem. Eng. J. 2021, 411, 128469. [Google Scholar] [CrossRef]
- Shmueli, U.; Traub, W. An X-ray diffraction study of poly-L-lysine hydrochloride. J. Mol. Biol. 1965, 12, 205–214. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, J.; Yao, Y.; Zhang, Y.; Ma, Y.; Wu, D.; Cao, Y.; Yang, M.; Zhang, Y.; Tang, M.; et al. N-doped carbon dots triggered the induction of ROS-mediated cytoprotective autophagy in Hepa1-6 cells. Chemosphere 2020, 251, 126440. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Guo, S.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Malik, S.A.; Mohanta, Z.; Srivastava, C.; Atreya, H.S. Modulation of protein−graphene oxide interactions with varying degrees of oxidation. Nanoscale Adv. 2020, 2, 1904–1912. [Google Scholar] [CrossRef]
- Ambepitiya Wickramasinghe, I.N.; van Beurden, S.J.; Weerts, E.A.W.S.; Verheije, M.H. The avian coronavirus spike protein. Virus Res. 2014, 194, 37–48. [Google Scholar] [CrossRef] [PubMed]

| Group | Control a | CNGs-180 | CNGs-210 | CNGs-240 | CNGs-270 | CNGs-300 |
|---|---|---|---|---|---|---|
| CEID50 mL–1 | 106.25 | 106.25 | 105.50 | 105.50 | 104.60 | 104.50 |
| Antiviral effect (%) c | NA b | 0% | 82.2% | 82.2% | 97.8% | 98.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, D.-L.; Mao, J.-Y.; Anand, A.; Lin, H.-J.; Lin, J.H.-Y.; Tseng, C.-P.; Huang, C.-C.; Wang, H.-Y. Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus. Int. J. Mol. Sci. 2021, 22, 5415. https://doi.org/10.3390/ijms22115415
Chou D-L, Mao J-Y, Anand A, Lin H-J, Lin JH-Y, Tseng C-P, Huang C-C, Wang H-Y. Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus. International Journal of Molecular Sciences. 2021; 22(11):5415. https://doi.org/10.3390/ijms22115415
Chicago/Turabian StyleChou, Ding-Li, Ju-Yi Mao, Anisha Anand, Han-Jia Lin, John Han-You Lin, Ching-Ping Tseng, Chih-Ching Huang, and Hsian-Yu Wang. 2021. "Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus" International Journal of Molecular Sciences 22, no. 11: 5415. https://doi.org/10.3390/ijms22115415
APA StyleChou, D.-L., Mao, J.-Y., Anand, A., Lin, H.-J., Lin, J. H.-Y., Tseng, C.-P., Huang, C.-C., & Wang, H.-Y. (2021). Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus. International Journal of Molecular Sciences, 22(11), 5415. https://doi.org/10.3390/ijms22115415